Abstract
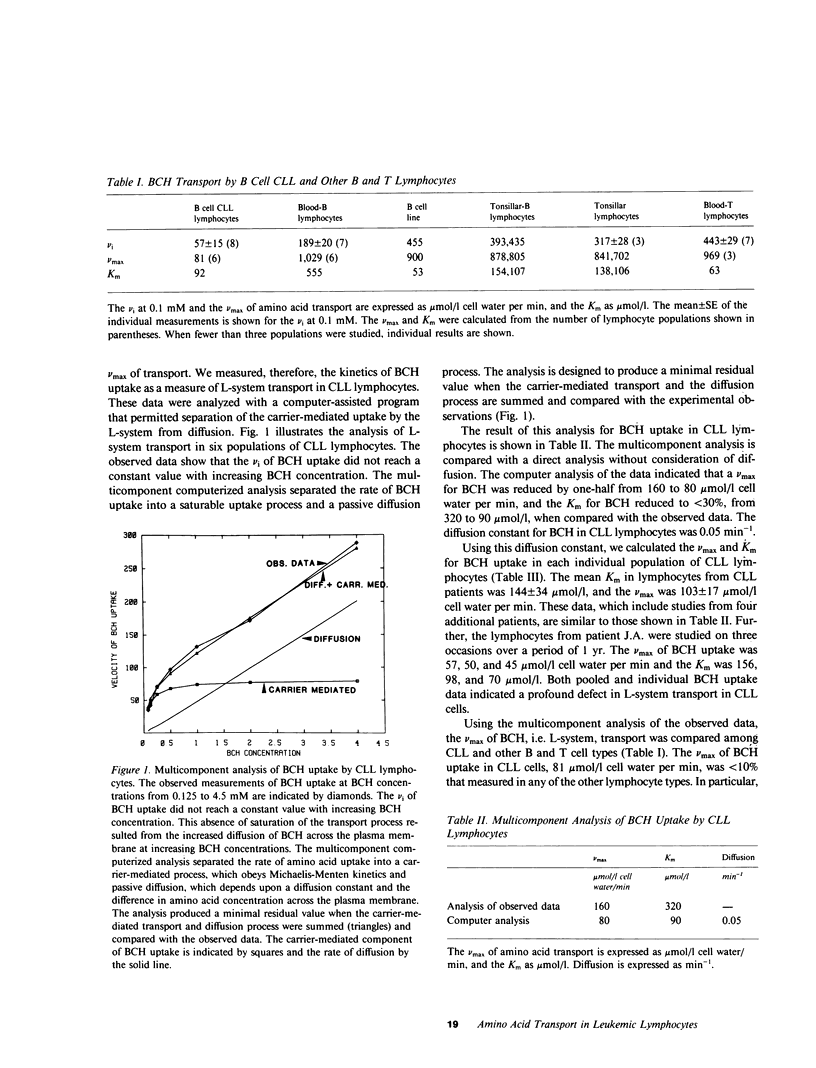
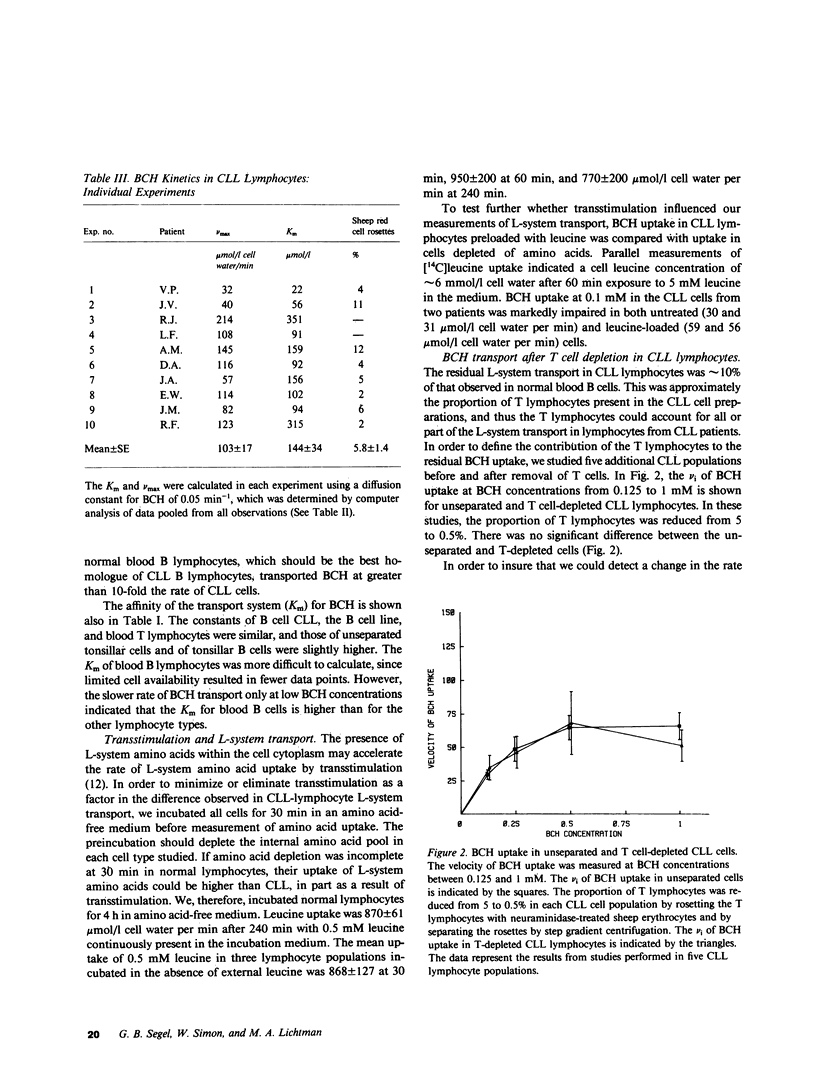
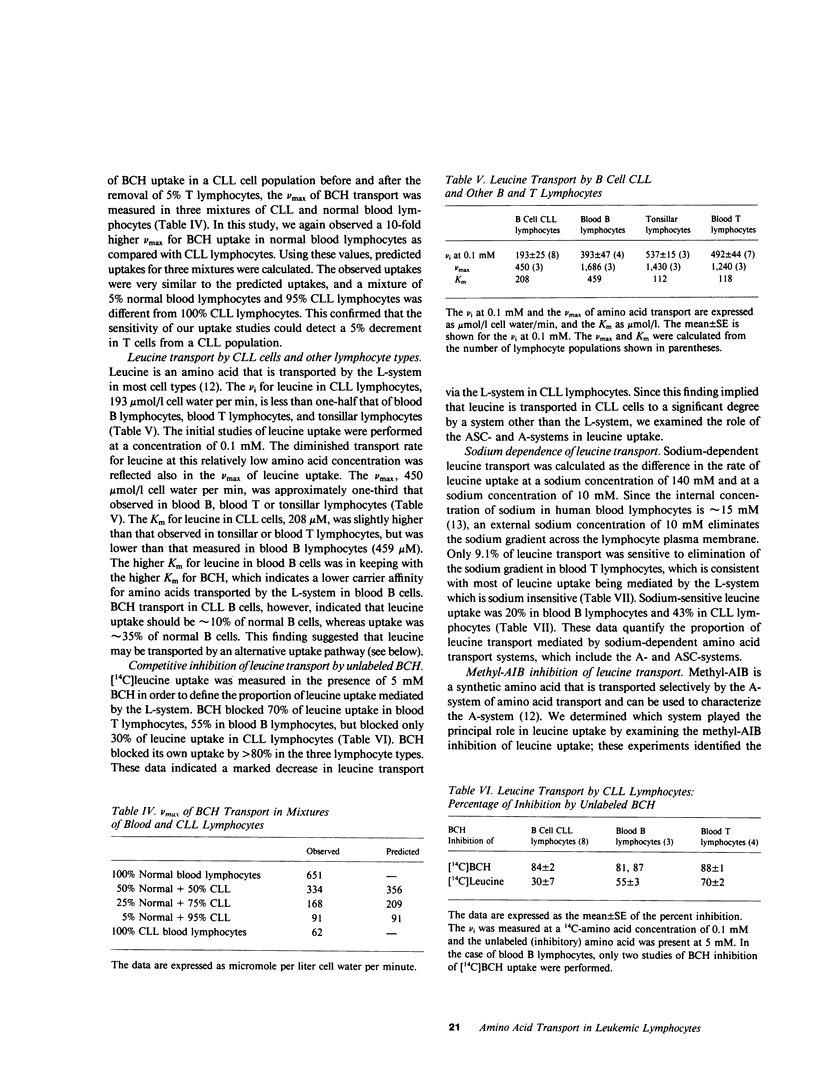
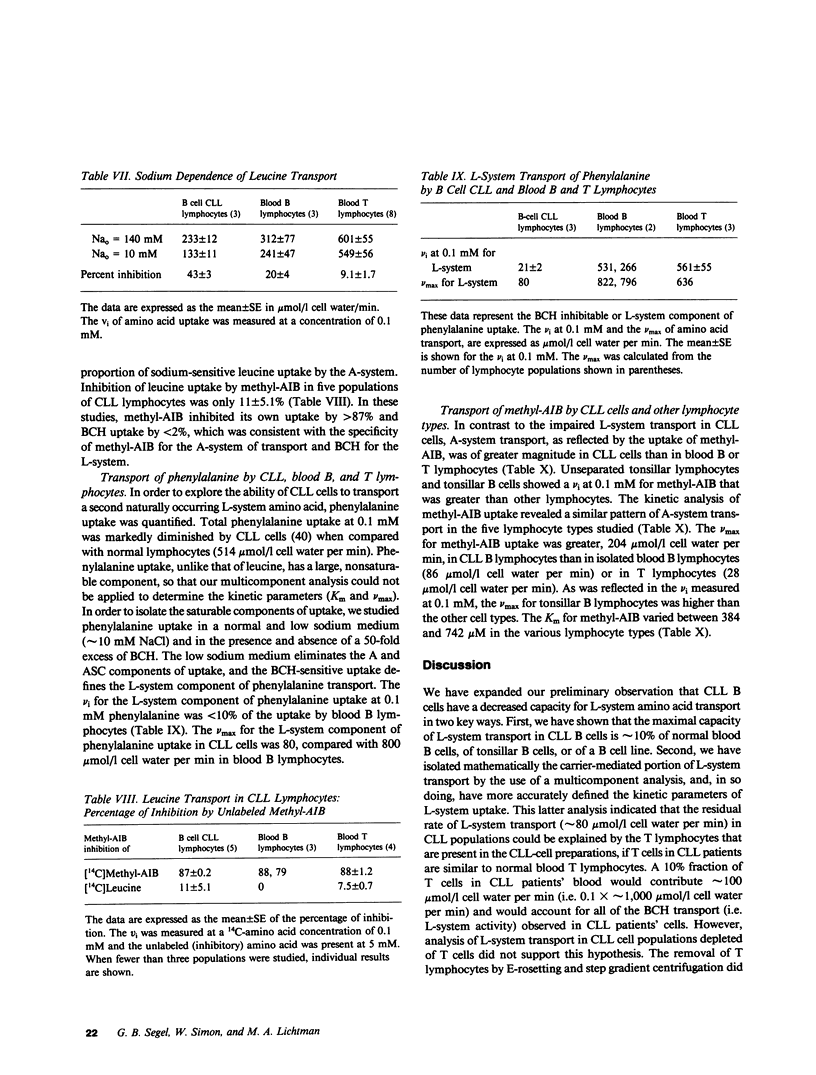
We have examined the amino acid transport in B cell chronic lymphocytic leukemia and compared it with the amino acid transport in isolated B lymphocytes from human blood and tonsils. L-system transport was measured with 2-amino-2-carboxy-bicyclo (2,2,1)-heptane, which is a synthetic amino acid whose transport is limited to the L-system. Amino acid uptake was subjected to a multicomponent analysis that partitioned the total uptake into the saturable carrier-mediated transport system and the uptake by diffusion. The maximal velocity of L-system transport in chronic lymphocytic leukemia cells, 81 mumol/1 cell water per min, was less than 10% that of blood B lymphocytes, which was 1,029 mumol/1 cell water per min. The uptake of 2-amino-2-carboxy-bicyclo (2,2,1)-heptane by tonsillar B cells, by a B lymphocyte cell line, and by blood T-lymphocytes was also 10-fold greater than that observed in chronic lymphocytic leukemic cells. Similarly, the L-system uptake of leucine and phenylalanine, which are naturally occurring amino acids usually transported primarily by the L-system, was reduced in chronic lymphocytic leukemic B cells to 15 and 10% of normal B cells, respectively. Total leucine uptake by chronic lymphocytic leukemic cells, however, was sustained at 30% of that expected because of transport via an alternative transport system. The A- or ASC-systems, the other major amino acid transport pathways, were not defective in chronic lymphocytic leukemic cells. These data indicate that there is a specific, profound decrease in L-system carrier-mediated amino acid transport in chronic lymphocytic leukemic B cells, as judged by the system-specific synthetic amino acid, 2-amino-2-carboxy-bicyclo (2,2,1)-heptane. This defect was confirmed by studies with two naturally occurring L-system amino acids, leucine and phenylalanine. This specific abnormality of membrane transport by chronic lymphocytic leukemic B lymphocytes is not shared by other B lymphocyte types, and thus appears to be related to the neoplastic nature of the leukemic B cells rather than to their immunologic subtype.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autio K., Turunen O., Erämaa E., Penttilä O., Schröder J. Human chronic lymphocytic leukaemia. Surface markers and activation of lymphocytes. Scand J Haematol. 1979 Oct;23(4):265–271. doi: 10.1111/j.1600-0609.1979.tb02860.x. [DOI] [PubMed] [Google Scholar]
- Christensen H. N., Handlogten M. E., Lam I., Tager H. S., Zand R. A bicyclic amino acid to improve discriminations among transport systems. J Biol Chem. 1969 Mar 25;244(6):1510–1520. [PubMed] [Google Scholar]
- Dantzig A. H., Adelberg E. A., Slayman C. W. Properties of two mouse lymphocyte cell lines genetically defective in amino acid transport. J Biol Chem. 1979 Sep 25;254(18):8988–8993. [PubMed] [Google Scholar]
- Davis S. The variable pattern of circulating lymphocyte subpopulations in chronic lymphocytic leukemia. N Engl J Med. 1976 May 20;294(21):1150–1153. doi: 10.1056/NEJM197605202942104. [DOI] [PubMed] [Google Scholar]
- Frengley P. A., Lichtman M. A., Peck W. A. Adaptive enhancement of amino acid transport in human leukemia leukocytes: studies with alpha-aminoisobutyric acid. J Lab Clin Med. 1975 Dec;86(6):984–986. [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Handwerger B. S., Schwartz R. H. Separation of murine lymphoid cells using nylon wool columns. Recovery of the B cell-enriched population. Transplantation. 1974 Dec;18(6):544–548. doi: 10.1097/00007890-197412000-00013. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Decreased phytohemagglutinin receptor sites in chronic lymphocytic leukemia. Biochim Biophys Acta. 1969 Dec 30;192(3):542–545. doi: 10.1016/0304-4165(69)90409-7. [DOI] [PubMed] [Google Scholar]
- LaMantia K., Conklyn M., Quagliata F., Silber R. Lymphocyte 5'-nucleotidase: absence of detectable protein in chronic lymphocytic leukemia. Blood. 1977 Oct;50(4):683–689. [PubMed] [Google Scholar]
- Segel G. B., Lichtman M. A. Amino acid transport in human lymphocytes: distinctions in the enhanced uptake with PHA treatment or amino acid deprivation. J Cell Physiol. 1981 Feb;106(2):303–308. doi: 10.1002/jcp.1041060217. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Lichtman M. A. Decreased L-system for amino acid transport in chronic lymphocytic leukemic lymphocytes. J Biol Chem. 1982 Aug 25;257(16):9255–9257. [PubMed] [Google Scholar]
- Segel G. B., Lichtman M. A. Decreased membrane potassium permeability and transport in human chronic leukemic and tonsillar lymphocytes. J Cell Physiol. 1977 Nov;93(2):277–284. doi: 10.1002/jcp.1040930213. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Lichtman M. A., Gordon B. R., MacPherson J. L., Nusbacher J. Plateletpheresis residues: a source of large quantities of human blood lymphocytes. Transfusion. 1976 Sep-Oct;16(5):455–459. doi: 10.1046/j.1537-2995.1976.16577039302.x. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Simon W., Lichtman M. A. A multicomponent analysis of amino acid transport systems in human lymphocytes. 1. Kinetic parameters of the A and L systems and pathways of uptake of naturally occurring amino acids in blood lymphocytes. J Cell Physiol. 1983 Sep;116(3):372–378. doi: 10.1002/jcp.1041160315. [DOI] [PubMed] [Google Scholar]
- Segel G. B., Simon W., Lichtman M. A. Regulation of sodium and potassium transport in phytohemagglutinin-stimulated human blood lymphocytes. J Clin Invest. 1979 Sep;64(3):834–841. doi: 10.1172/JCI109531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds M. A., Sobczak G., Hauptman S. P. Chronic lymphocytic leukemia cells lack the 185,000-dalton macromolecular insoluble cold globulin present on normal B lymphocytes. J Clin Invest. 1981 Mar;67(3):624–631. doi: 10.1172/JCI110076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Knowlton R. P., Koons L. S. Immunologic studies in chronic lymphocytic leukemia: defective stimulation of T-cell proliferation in autologous mixed lymphocyte culture. J Natl Cancer Inst. 1977 Mar;58(3):579–585. doi: 10.1093/jnci/58.3.579. [DOI] [PubMed] [Google Scholar]
- Speckart S. F., Boldt D. H., MacDermott R. P. Chronic lymphatic leukemia (CLL): cell surface changes detected by lectin binding and their relation to altered glycosyltransferase activity. Blood. 1978 Oct;52(4):681–695. [PubMed] [Google Scholar]
- Stark R., Liebes L. F., Nevrla D., Conklyn M., Silber R. Decreased actin content of lymphocytes from patients with chronic lymphocytic leukemia. Blood. 1982 Mar;59(3):536–541. [PubMed] [Google Scholar]



