Abstract
IL-13 is expressed in lesions of atopic dermatitis (AD) and has been associated with increased disease severity. IL-13 has two cognate receptors: IL-13Rα1 and IL-13Rα2. Although IL-13Rα2 expression is known to be induced in response to IL-13 in keratinocytes, its function in AD has never been evaluated. We characterized the loss of skin barrier function and the development of cutaneous inflammation in IL-13Rα2–null versus wild-type BALB/c mice following an epicutaneous allergen-sensitization/challenge model that shares similarities with human AD. Mice lacking IL-13Rα2 had significantly increased transepidermal water loss, cutaneous inflammation, peripheral eosinophilia, and IgG1 and IgE levels compared with wild-type mice. The rate of resolution of the cutaneous inflammation was not significantly altered in the IL-13Rα2–null mice. IL-13 induced expression of IL-13Rα2 in keratinocyte cell lines and primary human keratinocytes. Depletion of IL-13Rα2 in a keratinocyte cell line resulted in increased STAT6 signaling in response to IL-13. In conclusion, IL-13Rα2 serves a protective role in the pathogenesis of allergic inflammation and loss of skin barrier function in a mouse model of AD, suggesting that it may be an important endogenous regulator of IL-13–induced cutaneous inflammation in humans.
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disease whose prevalence in industrialized countries has nearly tripled in the past 30 y (1). This disorder results from complex interactions between host factors (genetic susceptibility, immunologic responses, and skin barrier dysfunction) and environmental factors (allergens, irritants, and infectious agents). Although Th1 and Th2 cytokines play a role in the development of AD, there is mounting evidence that IL-13, specifically, is central to its pathogenesis.
IL-13 is a critical mediator of allergic inflammation (2–5), is expressed in acute and chronic lesions of AD (6), and its production from cord blood mononuclear cells at birth has been associated with subsequent development of AD (7, 8). The percentage of peripheral blood CD4+IL-13+ T lymphocytes was also shown to correlate with AD disease severity in children (9). Furthermore, studies demonstrated a distinct and critical role of IL-13 versus IL-4 in AD, despite the 20–25% sequence homology (10) and shared effector functions between these Th2 cytokines. Investigators have identified significant upregulation of IL-13 message in subacute and chronic AD lesions in 27 of 28 patients, whereas IL-4 expression was seen in only 3 patients (11). Studies using a skin-specific IL-13 transgenic mouse demonstrated increased inflammation and fibrosis in the skin, supporting a critical role for IL-13–signaling pathways in AD (12).
IL-13 mediates its effects via a complex receptor system that includes IL-4Rα, IL-13Rα1, and IL-13Rα2 (13–18). IL-13Rα1 and IL-4Rα form a heterodimeric IL-13R that signals through the Jak-stat pathway (16). In contrast, IL-13Rα2 was postulated to be a decoy receptor, which was supported by the characterization of IL-13Rα2 knockout (KO) mice (19, 20) and by our in vitro studies demonstrating that upregulation of surface IL-13Rα2 is associated with decreased IL-13 responsiveness (21) and contradicted by one study demonstrating a positive role for IL-13Rα2 in fibrosis (22).
Keratinocytes are the principal cells in the epidermis and produce myriad inflammatory cytokines and chemokines, underscoring their importance in the pathogenesis of AD (1, 23). IL-13 acts directly on keratinocytes to enhance production of CCL26 (eotaxin-3) and, thereby, contributes to the recruitment of eosinophils to eczematous skin (23). IL-13–treated keratinocytes also increase migration of CD4+CCR4+ T cells. The expression of IL-13Rα2 is regulated by Th2 cytokines in keratinocytes (24, 25), and microarray analysis of keratinocytes from skin lesions of patients with AD revealed elevated IL-13Rα2 levels (26); however, the biologic role of IL-13Rα2 in the skin during inflammation is not known. We used a murine model that shares many similarities with AD to investigate the role of IL-13Rα2 in cutaneous allergic inflammation.
Materials and Methods
Mice
IL-13Rα2–deficient mice (27) and BALB/c mice (The Jackson Laboratory, Bar Harbor, ME) were kept in a specific pathogen-free environment. All procedures were performed in accordance with the ethical guidelines in the Guide for the Care and Use of Laboratory Animals of the Institutional Animal Care and Use Committee approved by the Veterinary Services Department of the Cincinnati Children’s Hospital Medical Center Research Foundation.
Epicutaneous Ag sensitization and challenge
The protocol followed was described previously (24), with the following changes. The mice were treated epicutaneously with 200 μg Aspergillus fumigatus extract (Greer Laboratories, Lenoir, NC) in 200 μl sterile saline or saline alone as control. The allergen was applied to a 2-cm2 gauze patch and taped to the back of the mice with Tegaderm, an adhesive bandage, and water-resistant tape. On day 7, the patch was removed, and eosinophil counts were measured by Discombe’s analysis using 5 μl blood obtained from the lateral tail vein, as previously described (28). Twenty-four hours after removing the patch, transepidermal water loss (TEWL) was measured, and the patch was reapplied. This was repeated two more times. Twenty-four hours after the last patch, TEWL was measured, and the mice were euthanized following standard procedures.
To assess the resolution of inflammation, mice were subjected to the same protocol above. After the third patch was removed on day 21, TEWL was measured every other day for 8 d (days 23, 25, 27, and 29).
Measurement of TEWL
TEWL was measured using the Dermalab instrument DermaLab USB module (Cortex Technology, Hadsund, Denmark). The temperature and relative humidity of the rooms in which measurements were made did not differ significantly during the measurements. Measurements were recorded as g/m2/h after the rate of TEWL had stabilized, usually after 60 s, and when the SD became ≤0.2. TEWL was assessed on the back of the mice, over the surface where the patch containing allergen was applied. The probe was placed against the skin surface exposed to allergen, and the readings were recorded for 1 min. The probe was removed from the skin, replaced, and a second measurement was taken. An average of the two readings was used as the TEWL for each mouse. The same investigator measured TEWL on all of the mice for each of the experiments.
Skin-scoring system
Mice were visually assessed for excoriations, erythema, and skin thickening in the 2-cm2 area covered by the patch. Scores for erythema were 0 (no visible redness) or 1 (redness present). Skin thickening was scored as 0 (thickness comparable to wild-type [WT] mouse skin), 1 (slight thickening of skin), or 2 (significant thickening of the skin). Excoriations were scored as 0 (no scratches), 1 (up to three scratches), 2 (four to eight scratches), 3 (one third of the back), 4 (two thirds of the back), or 5 (the entire back). The measurements were made by two independent investigators, and the average of the scores was recorded for each parameter. The total score from the excoriations, redness, and thickening is presented as the skin score for each mouse.
Tissue processing, measurement of epidermal thickness, and eosinophil quantification
Skin tissues were fixed in 10% formalin immediately after mice were euthanized. Paraffin-embedded tissues were cut into 5-μm sections and stained with H&E, per the manufacturer’s protocol. Epidermal thickness was quantified using the video-assistant integrated computer software program Image Pro Plus 4.1 (Media Cybernetics, Silver Spring, MD). Skin eosinophil numbers were measured by staining skin sections for MBP (major basic protein), as previously described (29). The MBP Ab was a kind gift of Dr. Jamie Lee (Mayo Clinic, Scottsdale, AZ).
IgG1 and IgE ELISAs
Plasma was diluted 1:100 for IgE and 1:10,000 for IgG1. ELISAs were performed using the appropriate kit from BD Biosciences (San Jose, CA). Briefly, ELISA wells were coated with 2 μg/ml anti-mouse IgE (or IgG1) mAb (BD Biosciences) overnight, blocked with 1% BSA for 1 h, washed with PBS/0.05% Tween-20, and then incubated with diluted plasma along with appropriate standards (BD Biosciences) for 1 h. Biotin-conjugated anti-mouse Ig mAb (2 μg/ml; BD Biosciences) was used for detection, followed by incubation with avidin-peroxidase (1 μg/ml; Pierce, Rockford, IL), and development with 2,2′-azino-di(3-ethyl-benzthiazone sulfonate) (Sigma-Aldrich, St. Louis, MO). Absorbance at 405 nm was measured within 30 min.
For measurement of Aspergillus-specific IgE and IgG1 levels, wells were coated with 0.01% Aspergillus extract (Greer Laboratories) overnight, and the rest of the protocol was carried out as described above.
Cell culture
Neonatal and adult primary human keratinocytes were purchased from Cambrex (East Rutherford, NJ) and cultured in keratinocyte basal medium (KGM; Cambrex) supplemented with bovine pituitary extract, human epidermal growth factor, insulin, hydrocortisone, gentamicin, and amphotericin-B (SingleQuot; Cambrex) at 37°C in 5% CO2. HaCaT cells were kindly provided by Dr. T. Bowden (University of Arizona, Tucson, AZ) and cultured in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin from Invitrogen (Carlsbad, CA). Cells were seeded onto 12-well culture plates or T-75 or T-175 flasks; after confluence reached 80%, they were treated with IL-4 (10 ng/ml), IL-13 (50 ng/ml), TNF-α (50 ng/ml), and IFN-γ (10 ng/ml), alone or in combination.
Supernatants and cell lysates
Cells were nonenzymatically detached with Versene (Invitrogen), washed twice in PBS, and resuspended at 5 million cells in 250 μl EMSA lysis buffer (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 1.5 mM MgCl2, 0.2% Nonidet P-40, 1.0 mM DTT, and 0.5 mM PMSF) for 10 min at 4°C, followed by centrifugation and collection of supernatants (cytoplasmic lysates) that were subsequently used for ELISA. STAT6 EMSAs were performed as previously described (30).
IL-13Rα2 ELISA
Human IL-13Rα2 was detected by ELISA, as previously described (31), modified only by the use of polyclonal goat anti-human IL-13Rα2 (1 μg/ml; R&D Systems, Minneapolis, MN) for the capture Ab. The standard curve was linear from 100–8000 pg/ml. Results are presented as IL-13Rα2 pg/ml of supernatant or pg/mg total protein for cell lysates (total protein determined by Bradford assay).
RT-PCR
Total RNA was isolated from cells using TRIzol reagent (Invitrogen), according to the manufacturer’s instructions, and were treated with DNase (Qiagen, Valencia, CA) before being reverse transcribed with a First-Strand Superscript Synthesis kit (Invitrogen). Conventional PCR analysis was performed using previously published primers for IL-13Rα2 (32) and the following primers: 5′-CCC TGG TGT TCT TCC TGA TAC TTT G-3′ forward and 5′-CAC TAC AGA GTC GGT TTC CTC CTT G-3′ reverse for human IL-13Rα1 and 5′-AAG GTG AAG GTC GGA GTC AAC G-3′ forward and 5′-TGG AAG ATG GTG ATG GGA TTT C-3′ reverse for human GAPDH. Quantitative analysis of human mRNA expression was done by real-time PCR using the LightCycler 480 and TaqMan Probes Master kit (both from Roche Applied Science, Indianapolis, IN). The primers and hydrolysis probes for IL-13Rα1 (forward 5′-GTG CCT TTA ACT TCC CGT GT-3′ and reverse 5′-CCC ATT GCA CAT ATA GGT CAT C-3′ : probe #12), IL-13Rα2 (forward 5′-GCA ATG CAC AAA TGG ATC AG-3′ and reverse 5′-CGC AAT CCA TAT CCT GAA CTT-3′ : probe #113), and hypoxanthine phosphoribosyltransferase (HPRT) (forward 5′-TGATAG ATC CAT TCC TAT GAC TGT AG-3′ and reverse 5′-CGATAC CTT TCC AGT TAA AGT TGA G-3′ : probe #22) control were selected using the manufacturer’s online software (http://www.roche-applied-science.com/sis/rtpcr/upl/adc.jsp) and based on the published sequences of each mRNA (National Center for Biotechnology Information). Relative amounts of gene expression were normalized using HPRT expression.
Small interfering RNA-mediated knockdown of IL-13Rα2
The small interfering RNA (siRNA) sequence and protocol used were described previously (33). Briefly, 72 h after transfection with a control siRNA or IL-13Rα2 siRNA, HaCaT cells were treated for 30 min with 10 ng/ml IL-13 and harvested for RT-PCR (to measure the extent of knockdown) and EMSA (to measure STAT6 activity).
Statistical analysis
Reported values are expressed as mean ± SD. All statistical analysis was done using Prism software (GraphPad, San Diego, CA). For all studies, with the exception of that shown in Fig. 1B, statistical significance was assessed using one-way ANOVA, followed by the Tukey–Kramer posttest (for statistical significance between groups). Significance was set at a p value of 0.05. For Fig. 1B, because the score distribution was non-parametric, statistical significance was assessed using the Kruskal–Wallis test, followed by the Dunn posttest for comparison between groups.
FIGURE 1.
IL-13Rα2–null mice demonstrate enhanced TEWL and inflammation following ASP challenge. A, TEWL measurements. B, Scoring of AD-like phenotypes (excoriations, redness, and thickening). Results are combined data from three independent experiments. **p < 0.01; ***p < 0.001. ASP, Aspergillus.
Results
IL-13Rα2 protects against increased TEWL in mouse models of AD
Previous studies by our group and other investigators (34) established TEWL as a measure of skin barrier function. Increased TEWL is associated with AD in children. We also observed an increase in TEWL in the skin of WT mice treated with Aspergillus compared with saline-treated mice, and this increase was exacerbated in the IL-13Rα2–null mice treated with Aspergillus after the third patch (Fig. 1A).
The skin was visually evaluated for AD-like phenotypes, including redness, thickening, and excoriations. WT Aspergillus-treated mice showed an increase in all three phenotypes compared with saline-treated mice. Although the average skin scores do not appear different between the WT and IL-13Rα2–null mice treated with Aspergillus, there was a modest increase in the number of mice with higher skin scores among the Aspergillus-treated IL-13Rα2–null mice (Fig. 1B). Together, the data indicate an increased disruption in the skin barrier following allergen treatment in the absence of IL-13Rα2.
Increased sensitization to Aspergillus in the absence of IL-13Rα2
We next tested whether sensitization to the allergen was affected by the absence of IL-13Rα2. Consistent with previous data (19), IL-13Rα2–null mice had elevated levels of total IgG1 and IgE at baseline. We did not observe a significant increase in IgG1 or IgE levels in the WT Aspergillus-treated mice (Fig. 2A, 2B). This could be due to the acute nature of our model; we predict that we will observe increased sensitization in more chronic models of AD, as demonstrated by other studies (24). Following Aspergillus treatment, we observed significant increases in total and Aspergillus-specific IgG1 and IgE levels in the IL-13Rα2–null mice, indicating that they are more easily sensitized than are WT BALB/c mice (Fig. 2).
FIGURE 2.
IL-13Rα2–null mice show increased sensitization following ASP challenge. Total serum IgG1 (A) and IgE (B) levels. ASP-specific serum IgG1 (C) and IgE (D) levels. Results are combined data from three independent experiments. **p < 0.01; ***p < 0.001. ASP, Aspergillus.
IL-13Rα2 mice have increased eosinophils in the blood but not in the skin
On histological examination, WT and IL-13Rα2–null mice exhibited epidermal thickening and dermal infiltration of inflammatory cells (Fig. 3A). We measured the increase in epidermal thickness. Although there was a trend toward an increase in the thickness in Aspergillus-treated WT mice compared with the saline group, the increase was statistically significant in the Aspergillus-treated null mice compared with the saline group (Fig. 3B). To address the possibility that the skin from the IL-13Rα2–null mice had a weakened epidermal barrier, we measured the epidermal thickness in naive WT and IL-13Rα2–null mice. The epidermal thickness for the naive WT and KO mice was 17 ± 2.1 μm and 16.3 ± 2.7 μm, respectively; it was 26.1 ± 9.5 μm and 25.9 ± 5 μm for the saline-treated WT and KO mice, respectively. Although the saline patch caused an increase in the epidermal thickness, there was no difference between the two genotypes, indicating that the absence of IL-13Rα2 does not affect the epidermal barrier in the absence of allergen.
FIGURE 3.
Changes in cutaneous cellular infiltrate, epidermal thickening, and peripheral blood eosinophilia in IL-13Rα2–null mice. A, Representative H&E-stained sections of patched skin (original magnification ×100). B, Quantification of epidermal thickness (μm) after the third weekly topical ASP application. C, Number of peripheral blood eosinophils. D, Number of eosinophils (MBP+ cells) in the skin. Results are combined data from three independent experiments. *p < 0.05; ***p < 0.001. ASP, Aspergillus.
Because we observed an increase in inflammatory cells in the skin, we measured eosinophil numbers in the blood and skin following Aspergillus treatment. Eosinophil numbers were increased in the blood in WT and IL-13Rα2–null mice following Aspergillus treatment, with a much greater magnitude of increase in the null mice (Fig. 3C). Eosinophil numbers were also elevated in the skin following Aspergillus treatment (not statistically significant by ANOVA). However, the increase in eosinophil numbers in the skin was comparable in both groups of Aspergillus-treated mice (Fig. 3D). Together, the data suggest that the eosinophilic component of the local inflammatory response was not affected by the absence of IL-13Rα2 but that IL-13Rα2 does play a critical role in the epidermal barrier function. We also measured the number of CD3+ cells in the skin sections and did not observe a significant difference in the WT and IL-13Rα2–null mice (30.8 ± 7.3 and 40.3 ± 17.4 cells/field for WT + Aspergillus and KO + Aspergillus mice, respectively).
IL-13Rα2 is not involved in resolution of changes to the skin barrier
Taken together, the above experiments indicate a clear role for IL-13Rα2 in attenuating the induction of the immune response and skin-barrier function in response to A. fumigatus extract. We next asked whether the resolution of skin-barrier disruption would be different in the IL-13Rα2–null mice. We measured TEWL every other day for 8 d following removal of the third allergen patch applied to the skin. In the WT and IL-13Rα2–null mice, the TEWL returned to baseline by day 8 after removal of allergen, suggesting that the absence of IL-13Rα2 does not result in a sustained inflammatory response (Fig. 4). Levels of IgG1 and IgE remained elevated in the IL-13Rα2–null mice after 8 d (data not shown), suggesting that the increase in sensitization is the consequence, not the cause, of impaired skin-barrier function.
FIGURE 4.
No differences in the resolution of inflammation between the WT and IL-13Rα2–null ASP-treated mice. TEWL measurements were taken on the days indicated. Results are presented as the average of three independent experiments. *p < 0.05; WT ASP versus KO ASP. Other significant p values were found between WT SAL and WT ASP at days 14 (p < 0.05), 21 (p < 0.001), and 23 (p < 0.001) and between KO SAL and KO ASP at days 14 (p < 0.01), 21 (p < 0.001), 23 (p < 0.001), and 25 (p < 0.01). ASP, Aspergillus.
Expression of IL-13Rs is induced in primary neonatal keratinocytes and transformed keratinocytes in response to IL-13
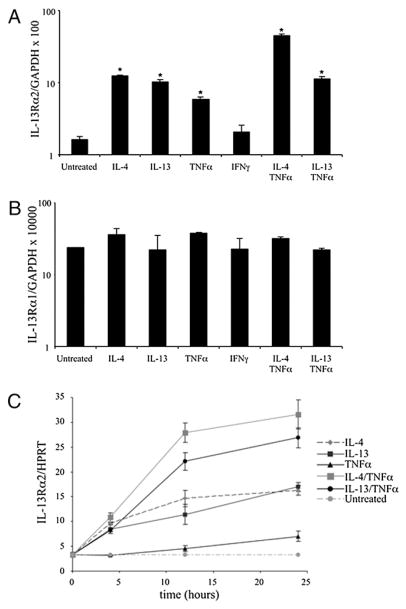
To evaluate a role for IL-13α2 in the skin, we focused our attention on keratinocytes, the critical cell type in the skin that produces proinflammatory cytokines and chemokines (1, 25). Because AD is most prevalent in early childhood, we examined IL-13Rα1 and IL-13Rα2 expression in human primary neonatal keratinocytes (HPKs), as well as a transformed keratinocyte cell line (HaCaT). Cells were treated with IL-4, IL-13, TNF-α, or IFN-γ, individually or in combination, and IL-13Rα1 and IL-13Rα2 mRNA expression was determined by quantitative PCR. Consistent with studies by Purwar et al. (35), expression of IL-13Rα2, but not IL-13Rα1, was dependent upon the cytokine milieu (Fig. 5A, 5B) in the HPKs. IL-4, IL-13, and TNF-α, but not IFN-γ, led to significant increases in IL-13Rα2 mRNA (Fig. 5A). TNF-α acted synergistically with IL-4 and IL-13. Strikingly, IL-4 and TNF-α together induced a 25-fold greater IL-13Rα2 message compared with that of untreated neonatal keratinocytes (p < 0.001). Similar results were seen in the HaCaT cells (Fig. 6A) and primary adult keratinocytes (data not shown).
FIGURE 5.
Cytokines regulate expression of IL-13Rα2 but not IL-13Rα1 in primary keratinocytes. Quantitative real-time PCR for IL-13Rα2 (A) and IL-13Rα1 (B) in primary neonatal keratinocytes incubated with the stated cytokines for 40 h. Expression was quantified relative to GAPDH or HPRT control. *Incubation with IL-4 (p < 0.001), IL-13 (p < 0.002), TNF-α (p = 0.002), IL-4 plus TNF-α (p = 0.001) and IL-13 plus IFN-γ (p < 0.002). C, Kinetics of IL-13Rα2 expression.
FIGURE 6.
IL-4 and IL-13 induce IL-13Rα2 expression in HaCaT cells. A, Quantitative PCR in stimulated and control HaCaT cells after 24 h of incubation with the stated cytokines. *Incubation with IL-4 (p < 0.004), IL-13 (p < 0.004), TNF-α (p = 0.008), IL-4+TNFα (p = 0.0004) and IL-13+TNF-α (p < 0.0002). B, Flow cytometry after 48 h. Representative graphs of cells without Ab staining (shaded histogram) or stained with irrelevant goat IgG (thin gray line) or goat anti–IL-13Rα2 (thick gray line). C, IL-13Rα2 protein in HaCaT cell lysates (representative of three independent experiments done in triplicate). All p values <0.05, except between IL-13–stimulated and IL-4+TNF-α–stimulated lysates (p < 0.06). The untreated sample was used as comparison for p value calculations.
We also examined the kinetics of IL-13Rα1 and IL-13Rα2 induction in neonatal keratinocytes (Fig. 5C). IL-13Rα2 mRNA was induced by 4 h of stimulation with IL-4 or IL-13, individually or in combination with TNF-α. By 12 h, the combination of IL-4 or IL-13 plus TNF-α resulted in greater IL-13Rα2 expression compared with the individual cytokines alone. Incubation with TNF-α alone also led to slightly elevated expression, but only at 24 h. There was no effect observed with IFN-γ alone (data not shown). Similar trends were observed for adult keratinocytes, and there was no significant effect on IL-13Rα1 expression at any time point (data not shown).
We confirmed that IL-13Rα2 protein was induced on the surface of HaCaT cells after IL-4 and IL-13 stimulation by flow cytometry (Fig. 6B). IL-13 stimulation increased surface expression compared with IL-4. TNF-α synergized with IL-4 and IL-13 to increase IL-13Rα2 surface expression. Cytokine stimulation with IL-4 or IL-13, alone or in combination with TNF-α, did not alter surface expression of IL-13Rα1 (data not shown).
Having demonstrated IL-13Rα2 surface induction on HaCaT cells after cytokine stimulation, we confirmed that this was due to an increase in total IL-13Rα2 protein and was not simply the result of increased mobilization from intracellular stores to the surface. To this end, we measured total IL-13Rα2 protein in HaCaT cell lysates after incubation with or without cytokines. As depicted in Fig. 6C, IL-4 and IL-13, individually or in combination with TNF-α, increased total IL-13Rα2 protein.
Depletion of IL-13Rα2 results in increased STAT6 activation following IL-13 treatment
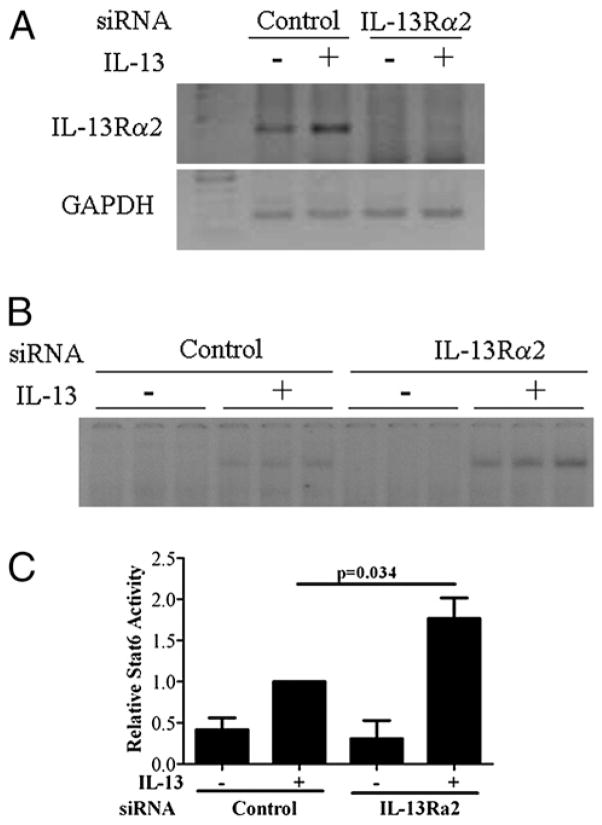
To evaluate the role of increased IL-13 Rα2 expression in keratinocytes, we assayed STAT6 activity following IL-13 treatment in HaCaT cells in which IL-13Rα2 had been depleted using siRNA. We verified the decrease in mRNA levels by RT-PCR (Fig. 7A). We observed an increase in STAT6 activation in the control siRNA-treated cells 30 min following addition of IL-13 and a further increase in the absence of IL-13Rα2, supporting its role as a decoy receptor in the skin (Fig. 7B, 7C).
FIGURE 7.
Depletion of IL-13Rα2 in HaCaT cells increases IL-13 signaling. RT-PCR analysis of IL-13Rα2 and GAPDH (A) and representative STAT6 EMSA (B) following 72 h of siRNA transfection and 30 min of treatment with IL-13 (10 ng/ml). C, Quantitation of STAT6 EMSA. Data shown are combined from three separate experiments done in triplicate.
Discussion
AD results in significant morbidity for patients and families, with associated health care costs ranging from $0.9–3.8 billion (36). Current therapeutics broadly target allergic inflammatory cascades, but it would be advantageous to develop agents specifically targeting the key mediators in AD. IL-13 plays a central role in allergic inflammation and has been implicated in AD pathogenesis, yet little is known regarding the regulation of IL-13 responses in the skin. This gap in understanding has hindered the development of novel therapeutic interventions for this disease targeting IL-13 or its signaling pathways. In this study, we demonstrated that Th2 cytokines, as well as allergic cutaneous inflammation in vivo, result in a preferential induction in the expression and distribution of IL-13Rα2 compared with IL-13Rα1. Because IL-13Rα2 binds IL-13 with high affinity and can impact IL-13 responses, even small variations in expression or distribution are likely to have biological significance. Elucidating the mechanisms that govern these changes in IL-13Rα2 in the skin may yield new insights into the regulation of Th2 inflammation in AD and potentially lead to new pharmacotherapeutic options.
To examine the role of IL-13Rs in vivo, we adapted a well-described murine model of epicutaneous sensitization and challenge that shares many characteristics with AD, including gross cutaneous inflammation, epidermal thickening, dermal inflammatory cell infiltrate, and peripheral eosinophilia. In this article, we demonstrate that, in the absence of IL-13Rα2, each of the characteristics of AD described above is exacerbated, supporting a role for IL-13Rα2 as a negative regulator of cutaneous allergic inflammation. Consistent with this mechanism, STAT6 activation by IL-13 is significantly enhanced in keratinocytes in which IL-13Rα2 is depleted. Our findings are consistent with a recent report that IL-13Ra2 deletion exacerbates the effect of IL-13 overexpression on the development of experimental eosinophilic esophagitis, a disease that shares several features with AD, including hyperproliferation and fibrosis of squamous epithelium (37).
HaCaT keratinocytes and HPKs express functional IL-13Rs, and we observed that IL-4 and IL-13 increase IL-13Rα2 without altering IL-13Rα1 expression. These findings support previous studies in HaCaT cells by David et al. (25, 38, 39) but contrast those of Wongpiyabovorn et al. (40) in HPKs. The latter group reported that only IL-4 combined with IFN-γ, but not IL-4 or IL-13 alone, upregulated IL-13Rα2 mRNA. Additionally, they observed that IFN-γ and IL-13 could upregulate the mRNA expression of IL-13Rα1 in HPKs. Potential reasons for the disparate results could be the investigators’ use of conventional PCR and measurements taken at only one time point versus our use of quantitative real-time PCR and multiple time points. Furthermore, the increase in IL-13Rα2 mRNA in our experiments correlated with induction of IL-13Rα2 protein in keratinocytes. These other studies did not examine IL-13Rα2 protein levels.
Recent studies indicate that IL-13Rα2 may have a dual role in the pathogenesis of Th2 inflammation. First, IL-13Rα2 serves as a decoy receptor through its high IL-13–binding affinity, short cytoplasmic tail, and lack of a definitive signaling motif, as well as its existence as a soluble receptor. This inhibitory role is supported by in vitro studies demonstrating that IL-13Rα2 overexpression leads to decreased IL-13 signaling and in vivo studies using IL-13Rα2 gene-targeted mice (19–21). Because IL-13 can induce IL-13Rα2, the receptor functions as part of a self-regulatory feedback loop designed to attenuate IL-13 responses. Second, IL-13Rα2 may contribute to inflammation through TGF-β, as demonstrated recently by Fichtner-Feigl et al. (22) in murine models of oxalazone-induced colitis and bleomycin-induced lung fibrosis. These opposing roles of IL-13Rα2 may hinge on tissue specificity, because the mechanisms by which IL-13 mediates its own effects were shown to differ between tissues. For example, IL-13–mediated lung fibrosis is TGF-β dependent (41), whereas IL-13–mediated liver fibrosis following Shistosoma mansoni infection is TGF-β independent (42).
Further evidence for the complex role of IL-13Rα2 in regulating Th2 inflammatory responses comes from studies demonstrating that the membrane-bound receptor can inhibit IL-4 signaling in glioblastoma cells and primary human fibroblasts (43, 44). The relative distribution of IL-13Rα2 within the subcellular compartments of various tissues may also be a factor in determining whether the cumulative effect of the receptor is proinflammatory or anti-inflammatory. Alternative splicing of IL-13Rα2 in mice leads to distinct membrane-bound and soluble IL-13Rα2, and we demonstrated differential regulation of these two transcripts in vivo in an experimental allergic asthma model (45). These alternatively spliced variants of IL-13Rα2 may have distinct biologic functions. In conclusion, our research demonstrates that IL-4 and IL-13 can induce total IL-13Rα2 in skin cells, and this receptor modulates IL-13 signaling pathways in keratinocytes. Although further studies are warranted to delineate the role of this receptor in cutaneous Th2 inflammation, our data suggest that IL-13Rα2 may be a useful biomarker of AD and likely has a key role in other allergic disorders.
Acknowledgments
This work was supported by a National Research Service Award Institutional Research Training grant (to M.R.W.) and National Institutes of Health Grant R01 AI58157 (to G.K.K.H.).
We thank Dr. Keith F. Stringer and Betsy DiPasquale for assistance with the CD3 staining.
Abbreviations used in this paper
- AD
atopic dermatitis
- ASP
Aspergillus
- HPK
human primary keratinocyte
- HPRT
hypoxanthine phosphoribosyltransferase
- KO
knockout
- MBP
major basic protein
- siRNA
small interfering RNA
- TEWL
trans-epidermal water loss
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 4.Wills-Karp M. IL-12/IL-13 axis in allergic asthma. J Allergy Clin Immunol. 2001;107:9–18. doi: 10.1067/mai.2001.112265. [DOI] [PubMed] [Google Scholar]
- 5.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. quiz 691. [DOI] [PubMed] [Google Scholar]
- 6.Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, Leung DY. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–231. doi: 10.1016/s0091-6749(96)70246-4. [DOI] [PubMed] [Google Scholar]
- 7.Lange J, Ngoumou G, Berkenheide S, Moseler M, Mattes J, Kuehr J, Kopp MV. High interleukin-13 production by phytohaemagglutinin- and Der p 1-stimulated cord blood mononuclear cells is associated with the subsequent development of atopic dermatitis at the age of 3 years. Clin Exp Allergy. 2003;33:1537–1543. doi: 10.1046/j.1365-2222.2003.01789.x. [DOI] [PubMed] [Google Scholar]
- 8.Ohshima Y, Yasutomi M, Omata N, Yamada A, Fujisawa K, Kasuga K, Hiraoka M, Mayumi M. Dysregulation of IL-13 production by cord blood CD4+ T cells is associated with the subsequent development of atopic disease in infants. Pediatr Res. 2002;51:195–200. doi: 10.1203/00006450-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 9.La Grutta S, Richiusa P, Pizzolanti G, Mattina A, Pajno GB, Citarrella R, Passalacqua G, Giordano C. CD4(+)IL-13(+) cells in peripheral blood well correlates with the severity of atopic dermatitis in children. Allergy. 2005;60:391–395. doi: 10.1111/j.1398-9995.2005.00733.x. [DOI] [PubMed] [Google Scholar]
- 10.McKenzie AN, Li X, Largaespada DA, Sato A, Kaneda A, Zurawski SM, Doyle EL, Milatovich A, Francke U, Copeland NG, et al. Structural comparison and chromosomal localization of the human and mouse IL-13 genes. J Immunol. 1993;150:5436–5444. [PubMed] [Google Scholar]
- 11.Tazawa T, Sugiura H, Sugiura Y, Uehara M. Relative importance of IL-4 and IL-13 in lesional skin of atopic dermatitis. Arch Dermatol Res. 2004;295:459–464. doi: 10.1007/s00403-004-0455-6. [DOI] [PubMed] [Google Scholar]
- 12.Zheng T, Oh MH, Oh SY, Schroeder JT, Glick AB, Zhu Z. Transgenic expression of interleukin-13 in the skin induces a pruritic dermatitis and skin remodeling. J Invest Dermatol. 2009;129:742–751. doi: 10.1038/jid.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aman MJ, Tayebi N, Obiri NI, Puri RK, Modi WS, Leonard WJ. cDNA cloning and characterization of the human interleukin 13 receptor alpha chain. J Biol Chem. 1996;271:29265–29270. doi: 10.1074/jbc.271.46.29265. [DOI] [PubMed] [Google Scholar]
- 14.Caput D, Laurent P, Kaghad M, Lelias JM, Lefort S, Vita N, Ferrara P. Cloning and characterization of a specific interleukin (IL)-13 binding protein structurally related to the IL-5 receptor alpha chain. J Biol Chem. 1996;271:16921–16926. doi: 10.1074/jbc.271.28.16921. [DOI] [PubMed] [Google Scholar]
- 15.Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci USA. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miloux B, Laurent P, Bonnin O, Lupker J, Caput D, Vita N, Ferrara P. Cloning of the human IL-13R alpha1 chain and reconstitution with the IL4R alpha of a functional IL-4/IL-13 receptor complex. FEBS Lett. 1997;401:163–166. doi: 10.1016/s0014-5793(96)01462-7. [DOI] [PubMed] [Google Scholar]
- 17.Guo J, Apiou F, Mellerin MP, Lebeau B, Jacques Y, Minvielle S. Chromosome mapping and expression of the human interleukin-13 receptor. Genomics. 1997;42:141–145. doi: 10.1006/geno.1997.4628. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JG, Hilton DJ, Willson TA, McFarlane C, Roberts BA, Moritz RL, Simpson RJ, Alexander WS, Metcalf D, Nicola NA. Identification, purification, and characterization of a soluble interleukin (IL)-13-binding protein. Evidence that it is distinct from the cloned Il-13 receptor and Il-4 receptor alpha-chains. J Biol Chem. 1997;272:9474–9480. doi: 10.1074/jbc.272.14.9474. [DOI] [PubMed] [Google Scholar]
- 19.Wood N, Whitters MJ, Jacobson BA, Witek J, Sypek JP, Kasaian M, Eppihimer MJ, Unger M, Tanaka T, Goldman SJ, et al. Enhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med. 2003;197:703–709. doi: 10.1084/jem.20020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiaramonte MG, Mentink-Kane M, Jacobson BA, Cheever AW, Whitters MJ, Goad ME, Wong A, Collins M, Donaldson DD, Grusby MJ, Wynn TA. Regulation and function of the interleukin 13 receptor alpha 2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197:687–701. doi: 10.1084/jem.20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daines MO, Hershey GK. A novel mechanism by which interferon-gamma can regulate interleukin (IL)-13 responses. Evidence for intracellular stores of IL-13 receptor alpha -2 and their rapid mobilization by interferon-gamma. J Biol Chem. 2002;277:10387–10393. doi: 10.1074/jbc.M108109200. [DOI] [PubMed] [Google Scholar]
- 22.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 23.Esche C, de Benedetto A, Beck LA. Keratinocytes in atopic dermatitis: inflammatory signals. Curr Allergy Asthma Rep. 2004;4:276–284. doi: 10.1007/s11882-004-0071-8. [DOI] [PubMed] [Google Scholar]
- 24.Akei HS, Brandt EB, Mishra A, Strait RT, Finkelman FD, Warrier MR, Hershey GK, Blanchard C, Rothenberg ME. Epicutaneous aeroallergen exposure induces systemic TH2 immunity that predisposes to allergic nasal responses. J Allergy Clin Immunol. 2006;118:62–69. doi: 10.1016/j.jaci.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 25.David M, Ford D, Bertoglio J, Maizel AL, Pierre J. Induction of the IL-13 receptor alpha2-chain by IL-4 and IL-13 in human keratinocytes: involvement of STAT6, ERK and p38 MAPK pathways. Oncogene. 2001;20:6660–6668. doi: 10.1038/sj.onc.1204629. [DOI] [PubMed] [Google Scholar]
- 26.Lü ZR, Park D, Lee KA, Ryu JW, Bhak J, Shi L, Lee DY, Park YD, Zou F, Yang JM. Profiling the dysregulated genes of keratinocytes in atopic dermatitis patients: cDNA microarray and interactomic analyses. J Dermatol Sci. 2009;54:126–129. doi: 10.1016/j.jdermsci.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Khodoun M, Lewis CC, Lewis C, Yang JQ, Orekov T, Potter C, Wynn T, Mentink-Kane M, Hershey GK, Wills-Karp M, Finkelman FD. Differences in expression, affinity, and function of soluble (s)IL-4Ralpha and sIL-13Ralpha2 suggest opposite effects on allergic responses [Erratum in 2008 J. Immunol 180: 664] J Immunol. 2007;179:6429–6438. doi: 10.4049/jimmunol.179.10.6429. [DOI] [PubMed] [Google Scholar]
- 28.Brandt EB, Rothenberg ME. Eosinophil levels in mice are significantly higher in small blood vessels than in large blood vessels. J Allergy Clin Immunol. 2001;108:142–143. doi: 10.1067/mai.2001.116121. [DOI] [PubMed] [Google Scholar]
- 29.Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, Brenneise IE, Horton MA, Haczku A, Gelfand EW, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–2156. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews RP, Rosa LR, Daines MO, Khurana Hershey GK. Reconstitution of a functional human type II IL-4/IL-13 receptor in mouse B cells: demonstration of species specificity. J Immunol. 2001;166:1716–1722. doi: 10.4049/jimmunol.166.3.1716. [DOI] [PubMed] [Google Scholar]
- 31.Daines MO, Chen W, Tabata Y, Walker BA, Gibson AM, Masino JA, Warrier MR, Daines CL, Wenzel SE, Hershey GK. Allergen-dependent solubilization of IL-13 receptor alpha2 reveals a novel mechanism to regulate allergy. J Allergy Clin Immunol. 2007;119:375–383. doi: 10.1016/j.jaci.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daines MO, Tabata Y, Walker BA, Chen W, Warrier MR, Basu S, Hershey GK. Level of expression of IL-13R alpha 2 impacts receptor distribution and IL-13 signaling. J Immunol. 2006;176:7495–7501. doi: 10.4049/jimmunol.176.12.7495. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Sivaprasad U, Tabata Y, Gibson AM, Stier MT, Finkelman FD, Hershey GK. IL-13R alpha 2 membrane and soluble isoforms differ in humans and mice. J Immunol. 2009;183:7870–7876. doi: 10.4049/jimmunol.0901028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta J, Grube E, Ericksen MB, Stevenson MD, Lucky AW, Sheth AP, Assa’ad AH, Khurana Hershey GK. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725–730. doi: 10.1016/j.jaci.2007.12.1161. [DOI] [PubMed] [Google Scholar]
- 35.Purwar R, Werfel T, Wittmann M. Regulation of IL-13 receptors in human keratinocytes. J Invest Dermatol. 2007;127:1271–1274. doi: 10.1038/sj.jid.5700687. [DOI] [PubMed] [Google Scholar]
- 36.Ellis CN, Drake LA, Prendergast MM, Abramovits W, Boguniewicz M, Daniel CR, Lebwohl M, Stevens SR, Whitaker-Worth DL, Cheng JW, Tong KB. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46:361–370. doi: 10.1067/mjd.2002.120528. [DOI] [PubMed] [Google Scholar]
- 37.Zuo L, Fulkerson PC, Finkelman FD, Mingler M, Fischetti CA, Blanchard C, Rothenberg ME. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13R alpha 2-inhibited pathway. J Immunol. 2010;185:660–669. doi: 10.4049/jimmunol.1000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.David MD, Bertoglio J, Pierre J. Functional characterization of IL-13 receptor alpha2 gene promoter: a critical role of the transcription factor STAT6 for regulated expression. Oncogene. 2003;22:3386–3394. doi: 10.1038/sj.onc.1206352. [DOI] [PubMed] [Google Scholar]
- 39.David M, Bertoglio J, Pierre J. TNF-alpha potentiates IL-4/IL-13-induced IL-13Ralpha2 expression. Ann N Y Acad Sci. 2002;973:207–209. doi: 10.1111/j.1749-6632.2002.tb04633.x. [DOI] [PubMed] [Google Scholar]
- 40.Wongpiyabovorn J, Suto H, Ushio H, Izuhara K, Mitsuishi K, Ikeda S, Nakao A, Okumura K, Ogawa H. Up-regulation of interleukin-13 receptor alpha1 on human keratinocytes in the skin of psoriasis and atopic dermatitis. J Dermatol Sci. 2003;33:31–40. doi: 10.1016/s0923-1811(03)00148-8. [DOI] [PubMed] [Google Scholar]
- 41.Lee CG, Homer RJ, Zhu Z, Lanone S, Wang X, Koteliansky V, Shipley JM, Gotwals P, Noble P, Chen Q, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1) J Exp Med. 2001;194:809–821. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaviratne M, Hesse M, Leusink M, Cheever AW, Davies SJ, McKerrow JH, Wakefield LM, Letterio JJ, Wynn TA. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-beta independent. J Immunol. 2004;173:4020–4029. doi: 10.4049/jimmunol.173.6.4020. [DOI] [PubMed] [Google Scholar]
- 43.Rahaman SO, Sharma P, Harbor PC, Aman MJ, Vogelbaum MA, Haque SJ. IL-13R(alpha)2, a decoy receptor for IL-13 acts as an inhibitor of IL-4-dependent signal transduction in glioblastoma cells. Cancer Res. 2002;62:1103–1109. [PubMed] [Google Scholar]
- 44.Andrews AL, Nasir T, Bucchieri F, Holloway JW, Holgate ST, Davies DE. IL-13 receptor alpha 2: a regulator of IL-13 and IL-4 signal transduction in primary human fibroblasts. J Allergy Clin Immunol. 2006;118:858–865. doi: 10.1016/j.jaci.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 45.Tabata Y, Chen W, Warrier MR, Gibson AM, Daines MO, Hershey GK. Allergy-driven alternative splicing of IL-13 receptor alpha2 yields distinct membrane and soluble forms. J Immunol. 2006;177:7905–7912. doi: 10.4049/jimmunol.177.11.7905. [DOI] [PubMed] [Google Scholar]