Abstract
Background
Recent changes in the legality of cannabis have prompted evaluation of whether blood levels of Δ9-tetrahydrocannabinol (THC) or its metabolites could be used to substantiate impairment, particularly related to behavioral tasks such as driving. However, because marked tolerance develops to behavioral effects of THC, the applicability of a particular threshold of blood THC as an index of impairment in people with different patterns of use remains unclear. Studies relevant to this issue are difficult to accomplish in humans, as prior drug exposure is difficult to control.
Methods
Here, effects of THC to decrease rectal temperature and operant response rate compared to levels of THC and its metabolites were studied in blood in two groups of monkeys: one received intermittent treatment with THC (0.1 mg/kg i.v.) and another received chronic THC (1 mg/kg/12 h s.c.) for several years.
Results
In monkeys with intermittent THC exposure, a single dose of THC (3.2 mg/kg s.c.) decreased rectal temperature and response rate. The same dose did not affect response rate or rectal temperature in chronically exposed monkeys, indicative of greater tolerance. In both groups, blood levels of THC peaked 20–60 min post-injection and had a similar half life of elimination, indicating no tolerance to the pharmacokinetics of THC. Notably, in both groups, the behavioral effects of THC were not apparent when blood levels were maximal (20-min post-administration).
Conclusion
These data indicate that thresholds for blood levels of THC do not provide a consistent index of behavioral impairment across individuals with different patterns of THC exposure.
Keywords: marijuana, 11-OH-THC, THC-COOH, rhesus, legal limit, driving under the influence, DUI
1. INTRODUCTION
Recent changes in the legal status of cannabis have led to the development of laws establishing legal limits for Δ9-tetrahydrocannabinol (THC) in the blood when driving. However, marked tolerance to the psychomotor effects of THC develop with repeated or chronic use, which may result in overly conservative legal limits for medical or recreational users (e.g., Hruba et al., 2012; Ramaekers et al., 2009). The relationship between the behavioral effects of THC and blood levels of THC and its major metabolites has not been widely evaluated, especially following repeated or chronic exposure.
Cannabis is a widely-used illicit drug and THC is the major active component responsible for its psychoactive effects (Lile et al., 2009; Wiley, 1999). Recently, the medical use of cannabis was approved in several states, and recreational use was approved in Colorado and Washington (Hartman, 2013; Seamon, 2006). This legalization of cannabis has raised the issue of whether blood levels of THC can be used to determine impairment for driving or other tasks (e.g., Grotenhermen et al., 2007; Hartman, 2013; Hollister et al., 1981). To this end, Colorado recently enacted a law stipulating a threshold of 5 ng/ml of THC in blood for driving under the influence, based on a recently published report (Hartman and Huestis, 2013; Walker et al., 2013). Another recent study indicated that blood levels below 10 ng/ml were not associated with increased accident risk, and recommended a threshold of 7–10 ng/ml for driving under the influence (Grotenhermen et al., 2007). However, the relevance of either of these limits among regular or chronic cannabis users is unclear because repeated or chronic exposure to THC results in marked tolerance to the psychomotor effects of THC (e.g., Hollister et al., 1981) and THC levels remain relatively constant in the blood of chronic users for over a week after cannabis use ceases (Bergamaschi et al., 2013),
Tolerance to THC has been shown to develop to both the behavioral and the physiological effects in humans, monkeys, and mice (Hruba et al., 2012; Hunt and Jones, 1980; Miczek, 1979; Singh et al., 2011). Clinical studies also show that chronic cannabis users are less sensitive to the impairing effects of THC on cognitive and psychomotor tasks, compared to occasional users (D’Souza et al., 2008; Ramaekers et al., 2009). Specifically, smoked cannabis (500 µg/kg) produced significant impairment of tracking performance, divided attention, and inhibitory control in occasional (≤1x/week) cannabis users, but only modestly affected inhibitory control in heavy (>4 days/week) users (Ramaekers et al., 2009). Similarly, in a sample of heavy cannabis users (>4 days/week for more than 2 years), smoked cannabis (700 µg/kg) produced no impairment on tasks measuring tracking errors or reaction time, and produced statistically significant, but modest effects on a divided attention task (Schwope et al., 2012). Levels of THC and its major metabolites were measured in that study, and remained above the legal threshold of 5 ng/ml for 2–4 hours after cannabis consumption. However, in this study, no cannabis use restrictions were enforced prior to study admission, daily patterns and the last incidence of cannabis use varied widely among participants, and it is unclear what levels participants were at intake (THC blood levels were first assessed 15–20 hours after intake, prior to experimental THC administration).
The goal of this study was to compare behavioral (operant response rates) and physiological (rectal temperature) effects with the pharmacokinetics of THC in two groups of monkeys with different levels of exposure to THC. One group received a relatively small dose of THC intermittently (0.1 mg/kg i.v. every 3 or 4 days) and the second group received a relatively large dose of THC every day (1 mg/kg s.c. every 12 h) for several years. We examined the effects of single doses of THC (1 mg/kg and 3.2 mg/kg s.c.) on operant response rates and rectal temperature in these two groups of monkeys while simultaneously collecting blood to measure time-matched levels of THC and its metabolites.
2. MATERIALS AND METHODS
2.1 Subjects
Two groups of adult rhesus monkeys (Macaca mulatta) were used in our experiment: one group (intermittent) included two male and two female adult monkeys (n=4 total) that were intermittently exposed to THC as a training drug (0.1 mg/kg i.v. every 3 or 4 days). The other group (chronic) included two female and three male adult monkeys (n=5 total) that were exposed to THC daily (1 mg/kg THC s.c., every 12 h). Monkeys were housed separately on a 14-h light/10-h dark schedule and fed enough food to maintain healthy weights (range, 8.4–12.5 kg for intermittent THC group and 5.9- 9.8 kg for daily THC group) with a diet consisting of primate chow (mean: 201g, range: 186 – 227g; High Protein Monkey Diet; Harlan Teklad, Madison, WI), fresh fruit, and peanuts; water was provided freely in the home cage. Monkeys were trained to discriminate THC (intermittent) or rimonabant (chronic) from vehicle and had previous acute exposures to other cannabinoids in previous studies (Ginsburg et al., 2012; Hruba et al., 2012). Monkeys were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 2011).
2.2 Apparatus
Monkeys were seated in chairs (model R001; Primate Products, Miami, FL) that provided restraint at the neck area and shoulders. Chairs were placed in ventilated, sound-attenuating chambers equipped with two levers; a light was positioned above each lever. Feet were secured in shoes containing brass electrodes to which a brief electric stimulus (3 mA, 250 ms) could be delivered from an a.c. generator. The chambers were connected to a computer with an interface (MED Associates, St. Albans, VT); experimental events were controlled and recorded with Med-PC software (MED Associates).
2.3 Response rate
Response rate was assessed in monkeys trained to respond on a lever to terminate a light stimulus associated with shock (stimulus shock termination) by dividing the number of responses made while the light was on by the time the light was on during the cycle. Response rates were assessed during 5-min cycles (preceded by a 10-min timeout in the test chamber) 10, 30, 60, 120, 240, 360, 480, 600, 720, and 1440 min after THC (3.2 mg/kg) or vehicle treatment. Response rate was expressed as a percentage of vehicle control for each monkey.
2.4 Temperature
Rectal temperature was assessed by inserting a digital thermometer (Physitemp, Clifton, New Jersey, USA) 2 cm into the rectum (procedure room temperature averaged 22.6–22.7°C) immediately after each operant cycle. Temperature was expressed as a change from time-matched vehicle control.
2.5 Collection and quantification of THC, 11-OH-THC and THC-COOH
Saphenous venous blood was collected in EDTA tubes of 3 ml immediately after rectal temperature was assessed at each time point. After blood collection the tubes were put in ice in containers and stored at a temperature of at least −80°C until analysis.
2.6 Measurement of THC and metabolites using HPLC-tandem MS
THC, and its metabolites 11-OH-THC and THC-COOH along with the internal standard deuterated (−)-Δ9-THC-D3 were obtained from Cerilliant Corp. (Round Rock, Texas). HPLC grade methanol was purchased from Fisher (Fair Lawn, NJ). All other reagents were purchased from Sigma Chemical Company (St. Louis, MO). Milli-Q water was used for preparation of all solutions. THC metabolites and THC internal standard super stock solutions were prepared in methanol at a concentration of 1 mg/ml and stored in aliquots at −80°C. A working stock solution prepared each day from the super stock solutions at a concentration of 10 µg/ml was used to spike the calibrators.
THC and metabolites were quantified in monkey blood using HPLC with tandem mass spec detection. Briefly, 500 µl of blood for spiked calibrators and unknown samples were homogenized with 2 ml of isopropanol by vortexing vigorously with 10 µL of 10 µg/mL of deuterated THC (internal standard). Three ml of hexane were then added to each sample while vortexing for 30 sec. The samples were then centrifuged for 10 min at 13K g, and the supernatants transferred to glass tubes and dried under a nitrogen stream. The residue was dissolved in 100 µL of mobile phase A (10 mM ammonium formate and 0.1% formic acid dissolved in 90% HPLC grade methanol) and 50 µl were injected into the LC/MS/MS. The ratio of the peak area of THC and metabolites to that of the internal standard (response ratio) were compared against a linear regression of calibrator response ratios at THC and metabolite concentrations of 0, 1.56, 3.13, 6.25, 12.5, 25.0, 50.0 and 100 ng/ml of blood to quantify THC and metabolites. The concentration of THC and metabolites in blood was expressed as ng THC/ml blood.
The HPLC system consisted of a Shimadzu SCL-10A Controller, LC-10AD pump with a FCV- 10AL mixing chamber (quarternary gradient), SIL-10AD autosampler, and an AB Sciex API 3200 tandem mass spectrometer with turbo ion spray. The analytical column was a Grace Alltima C18 (4.6 × 150 mm, 5 µ) purchased from Alltech (Deerfield, IL) and was maintained at 40°C during the chromatographic runs using a Shimadzu CTO-10A column oven. The flow rate of the mobile phase was 0.6 ml/min. THC was eluted with a step gradient. The column was equilibrated with 100% mobile phase A. At 6.1 minutes after injection, the system was switched to 100% mobile phase B (10 mM ammonium formate and 0.1% formic acid dissolved in HPLC grade methanol ). At 15.1 min, the system was switched back to 100% mobile phase A in preparation for the next injection. LC-ESIMS/ MS analysis using selected reaction monitoring was used to detect and quantify the major fragments of THC and its metabolites. Analyst 1.1 software (Applied Biosystems) was used to identify the precursor ions and to quantify the following fragments: 315.2 →193.2 Da (THC), 345.2→193.2 Da (THC-COOH), 331→193.2 Da (11-OH-THC), and 318.24→196.2 Da (deuterated- THC).
2.7 Drugs
THC (500 mg/ml in absolute ethanol; The Research Technology Branch, National Institute on Drug Abuse, Rockville, MD), and rimonabant base (The Research Technology Branch, National Institute on Drug Abuse.) were dissolved in a mixture of 1 part absolute ethanol, 1 part Emulphor-620 (Rhodia Inc.), and 18 parts physiologic saline and were administered subcutaneously in a volume from 0.1 ml/kg to 1.0 ml/kg. Doses were expressed as the weight of the forms listed above in milligrams per kilogram of body weight.
2.8 Data Analyses
Temperature and response rate data were each analyzed with mixed-effects ANOVA with time as a within-subject factor and prior THC exposure (intermittent or chronic) as a between-subject factor. One-sample t-tests were conducted to determine points that differed significantly from 100% (control temperature or response rate) after correction for multiple comparisons using the method of Benjamini and Hochberg (1995). ANOVA was performed using the Anova package of the R statistical language (R Development Core Team, 2012).
Descriptive statistics were calculated for the plasma concentrations of THC, 11-OH-THC, and THC-COOH at each time point for peak blood concentration (Cmax), time to peak blood concentration (Tmax), apparent terminal half-life (t1/2), and area under the curve from t = 0 to 24 ng-min/mL (AUC0→24) with Prism (Prism version 5.0 for Windows; GraphPad Software Inc., San Diego, CA). The 95% confidence levels for each statistic were determined and values for the chronic group that fell outside of the confidence interval for the intermittent group were considered to differ significantly.
The relationship between response rate or temperature and plasma levels of THC was compared using hysteresis plots. For each time point, temperature or response rate was plotted as a function of mean THC levels in both groups. Mean temperature and response rate values for the chronic group that fell outside of the 95% confidence limits for the Intermittent group were considered significantly different. Points for which the confidence limits did not include 0°C or 100% control response rate (no change from control values) were considered to indicate a significant effect of THC.
3. RESULTS
3.1 Temperature
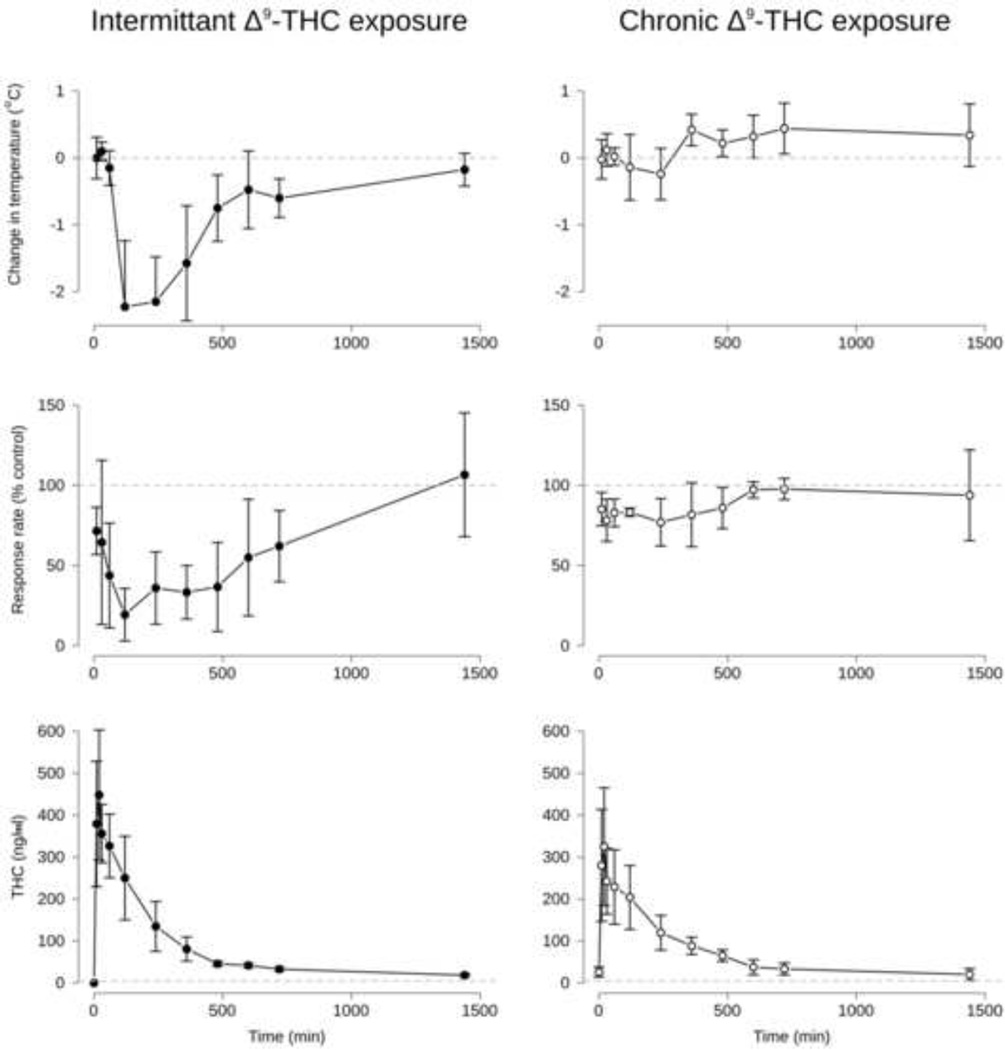
Control rectal temperatures in degrees C in individual monkeys were 36.5, 36.7, 37.0, and 37.8 for the intermittent group and 36.0, 36.1, 36.1, 36.5, and 36.6 for the chronic group. Following administration of 3.2 mg/kg THC, rectal temperature was maximally reduced by 2.2° C and 0.2° C at 120-min and 240-min for the intermittent and chronic groups, respectively. An ANOVA showed a significant interaction between group intermittent vs. chronic) and time on change in temperature (F[9, 63] = 13.2, p<0.001. Main effects of group (F[1, 7] = 20.9, p<0.005) and time (F[9, 63] = 13.6, p<0.0001) were also present. Results are shown in Figure 1 (top panel).
Figure 1.
Time course of changes in rectal temperature (top panels), response rate suppression (middle panels), and blood levels of THC (bottom panels). Points represent the mean and error bars represent the 95% confidence intervals for each point. Points where the confidence intervals do not include 0° C or 100% control rate (horizontal dashed lines for temperature and response rate, respectively) reflect significant effects of 3.2 mg/kg THC. Note that only at time = 0 (prior to s.c. THC administration) in the Intermittent group (bottom left panel) were blood levels of THC below 5 ng/ml (horizontal dashed line in the bottom panels).
3.2 Response Rate
Control rates of responding for individual monkeys were 0.71, 0.89, 1.30, and 2.16 responses per s for the intermittent group and 1.79, 1.98, 2.07, 2.44, and 2.76 responses per s for the chronic group. Following administration of 3.2 mg/kg THC, response rates were maximally reduced to 19.3% and 77% of control at 120-min and 240-min for the intermittent and chronic groups, respectively. An ANOVA showed a significant interaction between group (intermittent vs. chronic) and Time on response rate (F[9, 63] = 2.4, p<0.05. Main effects of group (F[1, 7] = 18.2, p<0.005) and Time (F[9, 63] = 3.6, p<0.005) were also present. Results are shown in Figure 1 (middle panel).
3.3 Blood levels of Δ9-THC, 11-OH-THC and THC-COOH
Prior to THC administration, monkeys in the intermittent group had no detectable levels of THC, 11-OH-THC, or THC-COOH in their blood. However, monkeys in the chronic group had THC levels of 26.16 [14.4 – 38.0] ng/ml, 11-OH-THC levels of 8.12 [4.8 – 11.4] ng/ml, and THC-COOH levels of 13.4 [17.4 – 19.4] ng/ml (mean [95% confidence interval], respectively).
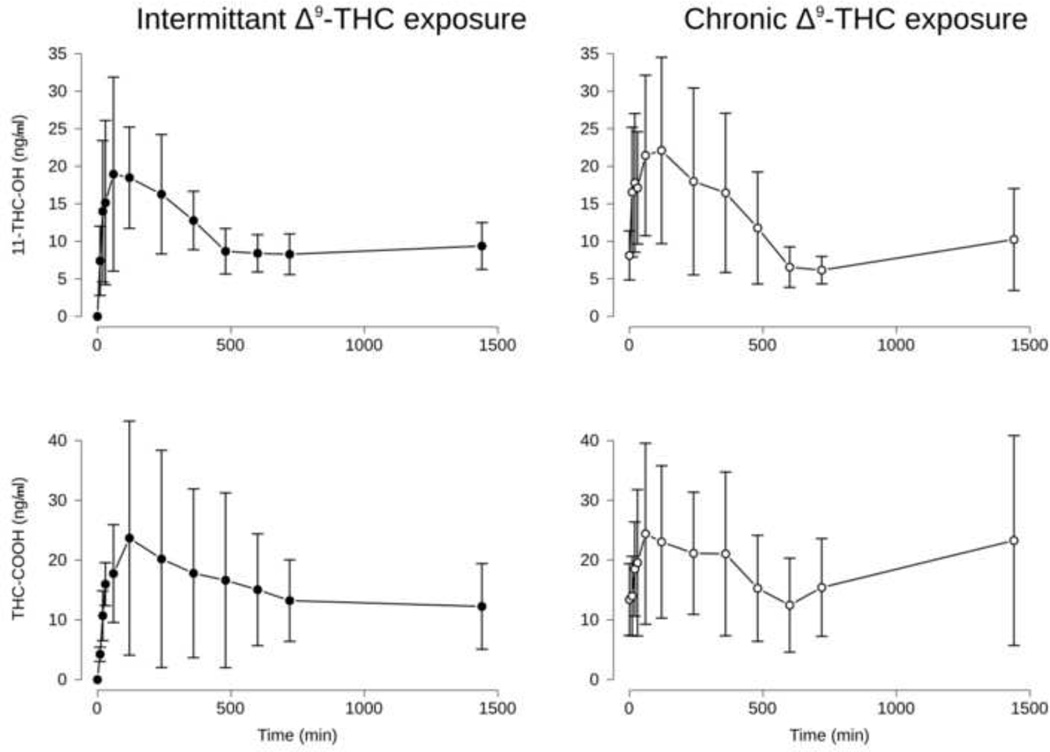
Blood levels of THC after administration of 3.2 mg/kg are shown in Figure 1 (bottom), blood levels of 11-OH-THC and THC-COOH after the same treatment are shown in Figure 2. Mean maximal blood levels [95% confidence interval] for THC were 457.0 [317.7 – 596.3] and 355.2 [206.5 – 504.0] for the intermittent and chronic groups, respectively, which occurred 20-min after injection for both groups. Mean maximal blood levels for 11-OH-THC were 24.6 [15.1 – 34.1] and 26.1 [13.8 – 39.9] ng/ml. Peak 11-OH-THC blood levels occurred at 60-min for the intermittent group and 120-min after injection for the chronic group. Mean maximal blood levels for THC-COOH were 26.3 [9.0 – 43.6] and 29.8 [12.2 – 47.4] ng/ml. Peak THC-COOH blood levels occurred at 120-min for the intermittent group and 60-min after injection for the chronic group. No significant differences in the maximum blood levels of THC, 11-OH-THC, or THC-COOH were found between the chronic and intermittent groups. An ANOVA was consistent with these results, revealing a main effect of Time, but not group on THC, 11-OH-THC, and THC-COOH blood levels (F[11, 76] = 36.0, 6.4, and 3.4, respectively).
Figure 2.
Time course of metabolites of THC, 11-OH-THC (top panels) and THC-COOH (bottom panels). Points represent the mean and error bars represent the 95% confidence intervals for each point. Note that metabolites were present in every sample except for time = 0 (prior to s.c. THC administration) in the Intermittent group.
For the intermittent and chronic groups, respective AUC0→24 h (mean [95% confidence interval]) for THC was 109603 [94674 – 124533] and 98408 [84683 – 112134] ; 11-OH-THC was 15418 [13065 – 17771] and 16287 [12373 – 20201]; and THC-COOH was and 21724 [15812 – 27635] and 27202 [18137 – 36267]. No significant differences were present between the intermittent and chronic groups for THC or its metabolites.
The half-life for THC (mean [95% confidence interval]) was calculated as 120.6 [83.26 – 218.7] and 161.7 [98.8 – 445.4] -min for the intermittent and chronic groups, respectively. There was no significant difference in these half-lives. THC plateaued at 23.3 and 21.1 ng/ml in the intermittent and chronic groups, respectively, and these values did not significantly differ.
3.4 Hysteresis
As shown in Figure 3, THC had significantly greater effects on rectal temperature (left panel) and response rate (right panel) in the intermittent group versus the chronic group across a wide range of blood THC levels. THC levels were not significantly different at any time point. For both measures, effects in the intermittent group were outside of the 95% confidence limits of the chronic group when blood levels for each group were between 50–250 ng/ml. Interestingly, although blood THC levels were highest at 30-min post-injection, maximal effects on temperature and response rate did not occur until 120-min after injection in the intermittent group, after blood THC levels had substantially decreased. By the last 2–3 time points, effects on temperature and response rate were not different from control (0° C temperature change or 100% control response rate) although blood THC levels remained above 5 ng/ml.
Figure 3.
Hysteresis plots of mean blood levels of THC versus mean rectal temperature (left) and response rate (right). Each point represents a sample time and the direction of time is indicated by the flow of arrows. Shaded areas represent the 95% confidence intervals for THC levels (x-direction) and for each measure (y-direction). Points for the chronic group that fall outside of the 95% confidence interval for the intermittent group are significantly different. Similarly, portions of the curve where the confidence region does not include 0° C or 100% control rate (horizontal dashed lines) indicate significant effects of THC on each measure, respectively. Note that at no point after administration (up to 24-hours later) did blood THC levels fall below 5 ng/ml, indicated by the vertical dashed lines.
4. DISCUSSION
In this study, a history of greater THC exposure resulted in greater tolerance to the hypothermic and response rate decreasing effects of THC, as expected. However, no differences in the pharmacokinetics of THC were observed between the groups. Hypothermic and response rate decreasing effects of 3.2 mg/kg THC were significantly greater in the group intermittently exposed to THC versus chronic exposure, and these effects did not occur at the time of peak THC blood levels in either group. No differences in blood levels of 11-OH-THC or THC-COOH over time were present between the groups. While THC (or its metabolites) was not detected in the blood of intermittently exposed monkeys prior to single dose THC administration, monkeys in the chronic exposure group had substantial levels of THC and metabolites in their blood. Together, these data indicate that determining a threshold for THC or metabolites in the blood that reflects physiological or behavioral effects is difficult, and further complicated by differential tolerance that follows from different patterns of exposure.
Tolerance to the hypothermic effects of THC was apparent between the two groups. This is consistent with tolerance we and others have described in mice and rats (Fan et al., 1994; Singh et al., 2011; Uran et al., 1980). Thus, this study extends the generality of this phenomenon to monkeys. Though no change in rectal temperature was observed in the chronic group, the ability of 3.2 mg/kg THC to significantly reduce rectal temperature in the intermittent group demonstrates that this was an effective dose. The magnitude of the hypothermic effect in this group is consistent with the hypothermic effect reported by others in essentially drug-naive rhesus monkeys following similar i.p. doses of THC (Matsuzaki et al., 1987). However, in the intermittent group, the hypothermic effects of 3.2 mg/kg THC subsided even though blood levels of THC remained elevated compared with pre treatment levels. This may reflect acute tolerance to the hypothermic effects of THC, similar to earlier observations in rats (Lomax, 1971).
The mechanism of the hypothermic effect of THC remains poorly understood, but appears to be mediated by CB1 receptors (McMahon et al., 2005). Tolerance to hypothermic effects of THC has been reported in rodents (Järbe, 1978; McKinney et al., 2008; Singh et al., 2011). Taffe (2012a) reports that THC (0.3 mg/kg i.m.) produces approximately a 1 degree Celsius decrease in body temperature for up to 8 h in rhesus monkeys; thus the time course and magnitude of the effect was similar to the present results. However, the monkeys in the study reported by Taffe (2012a) likely had a history of less THC exposure than the monkeys in the present study. Together with those prior results, the present study demonstrates that tolerance to the hypothermic effects of THC occurs in monkeys.
Tolerance to the response rate-decreasing effects of THC was also apparent between the two groups. This is consistent with the marked behavioral tolerance to rate-suppressing effects of THC reported by us and others in pigeons, mice, rats, and monkeys (Lamb et al., 2000; McMahon, 2011; McMillan et al., 1980; Singh et al., 2011). Only modest effects on response rate were observed in the chronic group, and the significant and substantial effect of 3.2 mg/kg THC on response rate in the intermittent group demonstrates that this was an effective dose. Also similar to the hypothermic effect, rate-decreasing effects of THC had subsided even though blood levels of THC remained elevated above pre-treatment levels. This is similar to acute tolerance to ethanol effects that we and others previously reported (Ginsburg et al., 2008; Hiltunen and Järbe, 1992).
THC impairs performance in a variety of learning- and memory-based operant conditioning tasks in rhesus monkeys (Schulze et al., 1988; Winsauer et al., 1999; Taffe, 2012b). The effects of THC on cognitive performance generally overlap with the doses of THC that produce hypothermia and disruptions in fixed ratio responding in rhesus monkeys (McMahon et al., 2005), although direct comparisons across studies are complicated by differences in THC treatment history, route of administration, and pretreatment time. Here, a relatively large dose of THC was needed to decrease response rate and rectal temperature. Although the intermittent and chronic treatment groups differed markedly in THC treatment history, both groups had a more extensive history of THC treatment than monkeys used in a previous study (e.g., McMahon et al., 2005). The monkeys used in that prior study appeared to be less tolerant than monkeys used in this study inasmuch as doses of THC smaller than 3.2 mg/kg decreased rectal temperature and response rate.
No tolerance to the pharmacokinetics of THC was observed between the two groups. Others have reported similarly that tolerance to THC pharmacokinetics does not occur, despite the development of tolerance to THC (Dewey et al., 1973; Hunt and Jones, 1980; Martin et al., 1976). For example,Slikker et al. (1991) reports that marijuana (inhalation of a combusted 0.8 g cigarette containing 2.6% THC) produced 59 ng/ml of THC and 5.5 ng/ml of 11-nor-9-carboxy-THC at 45 min in plasma. The ratio of THC/11-THC-OH is similar to that reported in our study. Moreover, this level of plasma THC and 11-THC-OH remained the same after one year of daily exposure to this inhalation regimen, consistent with our results. Thus, differences in the hypothermic and rate-suppressing effects of THC between intermittent and chronically exposed monkeys do not appear to be explained by differences in the pharmacokinetics or metabolism of THC. Further, this suggests that blood levels of THC should be similar following acute THC exposure in tolerant and non-tolerant individuals, despite potentially great differences between the individuals in impairment due to the exposure.
A relationship between blood levels of THC metabolites and hypothermic or rate-suppressing effects of THC was not apparent. Blood levels of 11-OH-THC and THC-COOH increased, then decreased as a function of time after treatment similarly in both groups, yet as noted above, monkeys in the chronic group never showed hypothermia or rate-suppression. THC is oxidized to its active metabolite 11-OH-THC and then to 11-nor-9 carboxytetrahydrocannabinol (THC-COOH). The time course of this conversion has been detailed elsewhere (Huestis, 2005). The present results do not indicate that either metabolite is any better suited to reflect impairment from cannabis use than THC is.
Different physiological and behavior effects were observed between the groups despite similar blood levels of THC and its metabolites. These results are consistent with those of others showing that blood levels of THC do not necessarily reflect physiological or behavioral effects of the drug, depending on the history or prior exposure (Grotenhermen et al., 2007; Hollister et al., 1981). Such results indicate that using blood levels as a measure of impairment may be limited among frequent cannabis users.
While consistent with previous reports, this study has limitations, which should be considered. We only report on the effects of a single dose of THC. More importantly, the measures used may not reflect driving impairment. Thermoregulation is a closely controlled physiological function, which is necessarily resistant to disruption. Similarly, response rates under stimulus-shock termination procedures are typically quite high and resistant to disruption. Thus, both of these measures are more likely to reveal tolerance than other less well-defended outcomes. By extension, driving while intoxicated can have serious consequences and thus, tolerance is also more likely for this relatively well-defended endpoint.
Although we only report the time course of effects following 3.2 mg/kg of THC, a dose of 1.0 mg/kg of THC was without effect on rectal temperature and had modest effects on response rate over the same time period in the intermittent group (Table 1). Effects on rate for the group following 1.0 mg/kg were not significantly different from control responding at any time point. The hypothermic and rate-decreasing effects of THC are therefore dose-dependent and 3.2 mg/kg is the minimally effective dose in the intermittent group.
Table 1.
Maximal effects of THC for each subject
| Group | Subject | Age (years) |
3.2 mg/kg THC | 1.0 mg/kg THC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| THC (ng/ml) |
THC-OH (ng/ml) |
THC- COOH (ng/ml) |
Temperature (°C) |
Response Rate (% Control) |
THC (ng/ml) |
THC-OH (ng/ml) |
THC- COOH (ng/ml) |
Temperature (°C) |
Response Rate (% Control) |
|||
| Chronic | L | 13 | 137 | 6.2 | 4.4 | −0.4 | 44.1 | 147 | 18.6 | 15.9 | n.d. | n.d. |
| S | 12 | 513 | 28.2 | 53.5 | 0.1 | 80.8 | 100 | 11.6 | 23.5 | n.d. | n.d. | |
| B | 7 | 322 | 22.5 | 43.2 | −0.9 | 63.2 | 148 | 7.3 | 25.2 | n.d. | n.d. | |
| A | 14 | 308 | 44.7 | 30.8 | −0.2 | 76.2 | 173.3 | 19.3 | 20 | n.d. | n.d. | |
| T | 13 | 463 | 25.2 | 30.3 | −0.3 | 58 | 137 | 15.4 | 22.8 | n.d. | n.d. | |
| Intermittent | E | 12 | 594 | 15.8 | 25.6 | −3.4 | 0 | 227 | 9.7 | 17.6 | 0.5 | 85.8 |
| X | 6 | 526 | 23.2 | 18 | −1.7 | 33.8 | 59.4 | 3.9 | 3 | 0.1 | 32.3 | |
| J | 12 | 265 | 38.4 | 10.6 | −2.1 | 25.5 | 44 | 4.8 | 3.3 | −0.8 | 51.3 | |
| D | 6 | 443 | 21.1 | 51.1 | −2.7 | 9.3 | 127 | 9.2 | 15.9 | 0 | 21.4 | |
n.d.: data were not collected
Metabolite data represent peak plasma levels observed
Temperature data represent the larget decrease in temperature observed
Rate data represent the lowest rate observed (expressed as percent of control rate)
Age at the time studies were conducted
The pharmacokinetics of THC we observed were similar to those seen in humans. The pharmacokinetics of THC in human plasma in laboratory experiments is dose-dependent and depends on the route of administration (smoking, oral, oromucosal spray), with smoking resulting in the highest plasma levels of THC. Stott et al (2013) administered THC by oromucosal spray at doses of ~0.1 to 0.3 mg/kg which resulted in peak plasma THC levels of 0.5 to 6.0 ng/mL and mean Tmax values of 1.5 h. Oral THC administration (~0.25 mg/kg) resulted in peak plasma levels of 0.52 to 10.1 ng/ml with Tmax values between 3 and 4 h (Sachse-Seeboth et al, 2009). A higher orally administered dose (~0.50 to 2 mg/kg orally either as single or multiple doses) resulted in higher peak plasma levels, ranging from 4.1 to 51.9 ng.ml with Tmax values of ~3 h (Karschner et al 2012). Huestis and Cone (2004) reported that smoking THC (~0.25 to 0.50 mg/kg) produced peak plasma levels of 100 to 230 ng/ml with Tmax of about 0.5 h. Hunault et al (2008), and Toennes et al (2011) reported that smoked doses (~0.25 to 1.0 mg/kg) resulted in peak plasma levels of 38 to 462 ng/ml with Tmax values <1 h. Thus, oromucosal or oral administration results in lower peak plasma levels and greater Tmax values for plasma THC than smoking does. These results suggest that plasma levels of THC in this study with rhesus monkeys best match those of humans who smoked cannabis during controlled, human laboratory studies, particularly when the THC content of the cannabis is relatively high, which appears to be increasingly prevalent (Cascini et al., 2012).
The measures we assessed include an involuntary physiological measure and a voluntary behavior. Similar patterns of results were seen on both measures. In particular, response rate represents an acquired behavior that is fairly sensitive to many psychoactive drugs, and requires attention and coordination. However, the tolerance we observed, especially to rate-decreasing effects of THC may not parallel tolerance to the far more demanding task of driving. Similar arguments can be made for other difficult tasks performed by humans during a typical day. Others have reported substantial tolerance to the effects of THC on simulated driving tasks or other psychomotor tasks, which would seem consistent with our results (Bosker et al., 2012; Downey et al., 2013; Schwope et al., 2012). In these studies, blood levels of THC or its metabolites were not determined across groups with known patterns of prior THC exposure. Two recent studies have suggested thresholds of 5 ng/ml or 7–10 ng/ml (Grotenhermen et al., 2007; Hartman and Huestis, 2013). Hartman and Huestis (2013) note that impairment on complex tasks is still evident in frequent cannabis users and suggest a threshold of 5 ng/ml. Grotenhermen et al., (2007) however express concern that such a low threshold may result in misclassification of previous or low level cannabis use and note that blood levels of THC often decline below 5ng/ml within 10 hours of consumption, even in frequent users Thus, these authors proposed a 7–10 ng/ml limit to avoid misclassification of previous cannabis use. However, these authors do not account for the development of tolerance that may result in frequent cannabis users and whether 7–10 ng/ml is appropriate in this instance. While our study does not directly address whether 5 ng/ml THC represents a blood level that generally reflects driving impairment, it adds to the growing body of literature that suggests that this threshold may be too low in many cases.
The relationship between blood levels of THC and behavioral impairment differ from the relationship for ethanol. In a review provided to the U.S. Department of Transportation, Moskowitz and Fiorentino (2000) found that driving impairment is apparent with any amount of ethanol detectable in the blood. Further, at a blood level of 0.080 g/dl, 94% of the studies reviewed reported impairment, and virtually all subjects exhibited impairment on some critical driving measure. Thus, the current standard for alcohol impairment while driving represents a blood level of alcohol that is correlated with impairment despite varying levels of tolerance across the subjects. This contrasts with the primary finding of the present study, that the profound tolerance that can develop to THC results in a lack of correspondence between blood levels of THC and impairment.
Together with previous reports, this study indicates that among frequent and even intermittent cannabis users, 5 ng/ml THC may not reflect impaired behavior. Further, the lack of impairment we observed at early time points after THC administration (when blood levels were highest) indicates that blood levels of THC may not be an appropriate instantaneous measure of impairment at any threshold. These results suggest that caution should be used in applying thresholds of blood levels of THC or metabolites as substantiating evidence of driving or other task impairment due to cannabis use, especially among those who frequently use the drug for recreational or medical purposes.
Acknowledgements
This work was supported by PHS grant DA19222. The NIDA had no further role in the study design; collection, interpretation, or analysis of the data; the writing of the report; or in the decision to submit this work for publication. The authors wish to thank Greg Friesenhahn for his excellent technical assistance.
Role of funding source: Nothing declared
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures:
Contributors: Author Ginsburg wrote the manuscript. Author Hruba designed and conducted the study. Ginsburg and Hruba contributed equally to the manuscript. Author Zaki collected and compiled data. Author Javors developed the analytical procedure to detect blood levels of each chemical. Work was performed under a project developed and administered by Author McMahon.
Conflict of Interest: No author has any conflicts of interest to declare.
REFERENCES
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Methodol. 1995;57:289–300. [Google Scholar]
- Bergamaschi MM, Karschner EL, Goodwin RS, Scheidweiler KB, Hirvonen J, Queiroz RHC, Heustis MA. Impact of prolonged cannabinoid excretion in chronic daily cannabis smokers’ blood on per se drugged driving laws. Clin. Chem. 2013;59:519–526. doi: 10.1373/clinchem.2012.195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosker WM, Kuypers KPC, Theunissen EL, Surinx A, Blankespoor RJ, Skopp G, Jeffery WK, Walls HC, van Leeuwen CJ, Ramaekers JG. Medicinal Δ(9) -tetrahydrocannabinol (dronabinol) impairs on-the-road driving performance of occasional and heavy cannabis users but is not detected in Standard Field Sobriety Tests. Addiction. 2012;107:1837–1844. doi: 10.1111/j.1360-0443.2012.03928.x. [DOI] [PubMed] [Google Scholar]
- Cascini F, Aiello C, Di Tanna G. Increasing delta-9-tetrahydrocannabinol (Δ-9-THC) content in herbal cannabis over time: systematic review and meta-analysis. Curr. Drug Abuse Rev. 2012;5:32–40. doi: 10.2174/1874473711205010032. [DOI] [PubMed] [Google Scholar]
- Dewey WL, McMillan DE, Harris LS, Turk RF. Distribution of radioactivity in brain of tolerant and nontolerant pigeons treated with 3 H-9 -tetrahydrocannabinol. Biochem. Pharmacol. 1973;22:399–405. doi: 10.1016/0006-2952(73)90420-6. [DOI] [PubMed] [Google Scholar]
- Downey LA, King R, Papafotiou K, Swann P, Ogden E, Boorman M, Stough C. The effects of cannabis and alcohol on simulated driving: Influences of dose and experience. Accid. Anal. Prev. 2013;50:879–886. doi: 10.1016/j.aap.2012.07.016. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between delta 9-tetrahydrocannabinol, CP 55,940 and WIN 55,212JPharmacol. Exp. Ther. 1994;271:1383–1390. [PubMed] [Google Scholar]
- Ginsburg BC, Martinez G, Friesenhahn G, Javors M, Lamb RJ. Acute tolerance to ratedecreasing effects of single doses of ethanol. Physiol. Behav. 2008;94:374–383. doi: 10.1016/j.physbeh.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, McMahon LR. JWH-018 and JWH-073: Δ9-tetrahydrocannabinol-like discriminative stimulus effects in monkeys J. Pharmacol. Exp. Ther. 2012;340:37–45. doi: 10.1124/jpet.111.187757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhermen F, Leson G, Berghaus G, Drummer OH, Krüger H-P, Longo M, Moskowitz H, Perrine B, Ramaekers JG, Smiley A, Tunbridge R. Developing limits for driving under cannabis. Addiction. 2007;102:1910–1917. doi: 10.1111/j.1360-0443.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- Hartman HL. Legalized marijuana and the workplace: preparing for the trend. Employee Relat. Law J. 2013;38:72–75. [Google Scholar]
- Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin. Chem. 2013;59:478–492. doi: 10.1373/clinchem.2012.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiltunen AJ, Järbe TU. Acute and chronic ethanol tolerance: operant behaviour in naive and ethanol tolerant rats. Psychopharmacology (Berl.) 1992;107:511–516. doi: 10.1007/BF02245264. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Gillespie HK, Ohlsson A, Lindgren JE, Wahlen A, Agurell S. Do plasma concentrations of delta 9-tetrahydrocannabinol reflect the degree of intoxication? J. Clin. Pharmacol. 1981;21:171S–177S. doi: 10.1002/j.1552-4604.1981.tb02593.x. [DOI] [PubMed] [Google Scholar]
- Hruba L, Ginsburg BC, McMahon LR. Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Δ9-tetrahydrocannabinol treatment in rhesus monkeys. J. Pharmacol. Exp. Ther. 2012;342:843–849. doi: 10.1124/jpet.112.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA. Pharmacokinetics and metabolism of the plant cannabinoids, delta9-tetrahydrocannabinol, cannabidiol and cannabinol. Handb. Exp. Pharmacol. 2005:657–690. doi: 10.1007/3-540-26573-2_23. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Cone EJ. Relationship of Delta 9-tetrahydrocannabinol concentrations in oral fluid and plasma after controlled administration of smoked cannabis. J. Anal. Toxicol. 2004;28:394–399. doi: 10.1093/jat/28.6.394. [DOI] [PubMed] [Google Scholar]
- Hunault CC, Mensinga TT, de Vries I, Kelholt-Dijkman HH, Hoek J, Kruidenier M, Leenders ME, Meulenbelt J. Delta-9-tetrahydrocannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg THC. Psychopharmacology (Berl.) 2008;20:171–181. doi: 10.1007/s00213-008-1260-2. [DOI] [PubMed] [Google Scholar]
- Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J. Pharmacol. Exp. Ther. 1980;215:35–44. [PubMed] [Google Scholar]
- Järbe TU. delta9-Tetrahydrocannabinol: tolerance after noncontingent exposure in rats. Arch. Int. Pharmacodyn. Ther. 1978;231:49–56. [PubMed] [Google Scholar]
- Karschner EL, Schwope DM, Schwilke EW, Goodwin RS, Kelly DL, Gorelick DA, Huestis MA. Predictive model accuracy in estimating last Δ9-tetrahydrocannabinol (THC) intake from plasma and whole blood cannabinoid concentrations in chronic, daily cannabis smokers administered subchronic oral THC. Drug Alcohol Depend. 2012;125:313–319. doi: 10.1016/j.drugalcdep.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Järbe TU, Makriyannis A, Lin S, Goutopoulos A. Effects of Delta 9-tetrahydrocannabinol, (R)-methanandamide, SR 141716,and d-amphetamine before and during daily Delta 9-tetrahydrocannabinol dosing. Eur. J. Pharmacol. 2000;398:251–258. doi: 10.1016/s0014-2999(00)00318-6. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Delta9-tetrahydrocannabinol, triazolam, hydromorphone, and methylphenidate in humans discriminating Delta9-tetrahydrocannabinol. Psychopharmacology (Berl.) 2009;203:241–250. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax P. Acute tolerance to the hypothermic effect of marihuana in the rat. Res. Commun. Chem. Pathol. Pharmacol. 1971;2:159–167. [PubMed] [Google Scholar]
- Maccarrone M, Wenger T. Effects of cannabinoids on hypothalamic and reproductive function. Handb. Exp. Pharmacol. 2005:555–571. doi: 10.1007/3-540-26573-2_18. [DOI] [PubMed] [Google Scholar]
- Martin BR, Dewey WL, Harris LS, Beckner JS. 3H-delta9-tetrahydrocannabinol tissue and subcellular distribution in the central nervous system and tissue distribution in peripheral organs of tolerant and nontolerant dogs. J. Pharmacol. Exp. Ther. 1976;196:128–144. [PubMed] [Google Scholar]
- Matsuzaki M, Casella GA, Ratner M. delta 9-Tetrahydrocannabinol: EEG changes, bradycardia and hypothermia in the rhesus monkey. Brain Res. Bull. 1987;19:223–229. doi: 10.1016/0361-9230(87)90087-6. [DOI] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, Sim-Selley LJ. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, Amin MR, France CP. SR 141716A differentially attenuates the behavioral effects of delta9-THC in rhesus monkeys. Behav. Pharmacol. 2005;16:363–372. doi: 10.1097/00008877-200509000-00008. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Chronic Δ9-tetrahydrocannabinol treatment in rhesus monkeys: differential tolerance and cross-tolerance among cannabinoids. Br. J. Pharmacol. 2011;162:1060–1073. doi: 10.1111/j.1476-5381.2010.01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DE, Leander JD, Dudley KH. Effects of drugs on behavior in pigeons tolerant to delta 9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 1980;212:85–90. [PubMed] [Google Scholar]
- Miczek KA. Chronic delta9-tetrahydrocannabinol in rats: effect on social interactions, mouse killing, motor activity, consummatory behavior, and body temperature. Psychopharmacology (Berl.) 1979;60:137–146. doi: 10.1007/BF00432284. [DOI] [PubMed] [Google Scholar]
- Moskowitz H, Fiorentino D. A Review of the Literature on the Effects of Low Doses of Alcohol on Driving-Related Skills. Washington, DC: National Highway Traffic Safety Administration; 2000. [Google Scholar]
- Pertwee R. In vivo interactions between psychotropic cannabinoids and other drugs involving central and peripheral neurochemical mediators. In: Murphy L, Bartke A, editors. Marijuana/cannabinoids, Neurobiology and Neurophysiology. Boca Raton (FL): CRC Press; 1992. p. 165. [Google Scholar]
- Ramaekers JG, Kauert G, Theunissen EL, Toennes SW, Moeller MR. Neurocognitive performance during acute THC intoxication in heavy and occasional cannabis users J. Psychopharmacol. Oxford. 2009;23:266–277. doi: 10.1177/0269881108092393. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing R. Vienna, Austria: Foundation for Statistical Computing; 2012. [Google Scholar]
- Sachse-Seeboth C, Pfeil J, Sehrt D, Meineke I, Tzvetkov M, Bruns E, Poser W, Vormfelde SV, Brockmöller J. Interindividual variation in the pharmacokinetics of Delta9-tetrahydrocannabinol as related to genetic polymorphisms in CYP2C9. Clin. Pharmacol. Ther. 2009;85:273–276. doi: 10.1038/clpt.2008.213. [DOI] [PubMed] [Google Scholar]
- Schwope DM, Bosker WM, Ramaekers JG, Gorelick DA, Huestis MA. Psychomotor performance, subjective and physiological effects and whole blood Δ9-tetrahydrocannabinol concentrations in heavy, chronic cannabis smokers following acute smoked cannabis. J. Anal. Toxicol. 2012;36:405–412. doi: 10.1093/jat/bks044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze GE, McMillan DE, Bailey JR, Scallet A, Ali SF, Slikker W, Jr, Paule MG. Acute effects of delta-9-tetrahydrocannabinol in rhesus monkeys as measured by performance in a battery of complex operant tests. J. Pharmacol. Exp. Ther. 1988;245:178–186. [PubMed] [Google Scholar]
- Seamon MJ. The legal status of medical marijuana. Ann. Pharmacother. 2006;40:2211–2215. doi: 10.1345/aph.1H399. [DOI] [PubMed] [Google Scholar]
- Singh H, Schulze DR, McMahon LR. Tolerance and cross-tolerance to cannabinoids in mice: schedule-controlled responding and hypothermia. Psychopharmacology (Berl.) 2011;215:665–675. doi: 10.1007/s00213-010-2162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slikker W, Jr, Paule MG, Ali SF, Scallet AC, Bailey JR. Chronic marijuana smoke exposure in the rhesus monkeyIPlasma cannabinoid and blood carboxyhemoglobin concentrations and clinical chemistry parameters. Fundam. Appl. Toxicol. 1991;17:321–334. doi: 10.1016/0272-0590(91)90222-p. [DOI] [PubMed] [Google Scholar]
- Stott CG, White L, Wright S, Wilbraham D, Guy GW. A phase I study to assess the effect of food on the single dose bioavailability of the THC/CBD oromucosal spray. Eur. J. Clin. Pharmacol. 2013;69:825–834. doi: 10.1007/s00228-012-1393-4. [DOI] [PubMed] [Google Scholar]
- Taffe MA. Δ9-Tetrahydrocannabinol attenuates MDMA-induced hyperthermia in rhesus monkeys. Neuroscience. 2012a;201:125–133. doi: 10.1016/j.neuroscience.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA. Δ9-Tetrahydrocannabinol impairs visuo-spatial associative learning and spatial working memory in rhesus macaques. J. Psychopharmacol. 2012b;26:1299–1306. doi: 10.1177/0269881112443743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toennes SW, Schneider K, Kauert GF, Wunder C, Moeller MR, Theunissen EL, Ramaekers JG. Influence of ethanol on cannabinoid pharmacokinetic parameters in chronic users. Anal. Bioanal. Chem. 2011;400:145–152. doi: 10.1007/s00216-010-4449-2. [DOI] [PubMed] [Google Scholar]
- Uran B, Tulunay FC, Ayhan IH, Ulkü E, Kaymakçalan S. Correlation between the dose and development of acute tolerance to the hypothermic effect of THC. Pharmacology. 1980;21:391–395. doi: 10.1159/000137458. [DOI] [PubMed] [Google Scholar]
- Walker S, Fields R, King S. A bill for an act concerning penalties for persons who drive while under the influence of alcohol or drugs and in connection therewith making an appropriation. House Bill 114, Colorado State Assembly. 2013 [Google Scholar]
- Wiley JL. Cannabis: discrimination of “internal bliss”? Pharmacol. Biochem. Behav. 1999;64:257–260. doi: 10.1016/s0091-3057(99)00059-3. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Lambert P, Moerschbaecher JM. Cannabinoid ligands and their effects on learning and performance in rhesus monkeys. Behav. Pharmacol. 1999;10:497–511. doi: 10.1097/00008877-199909000-00008. [DOI] [PubMed] [Google Scholar]