Abstract
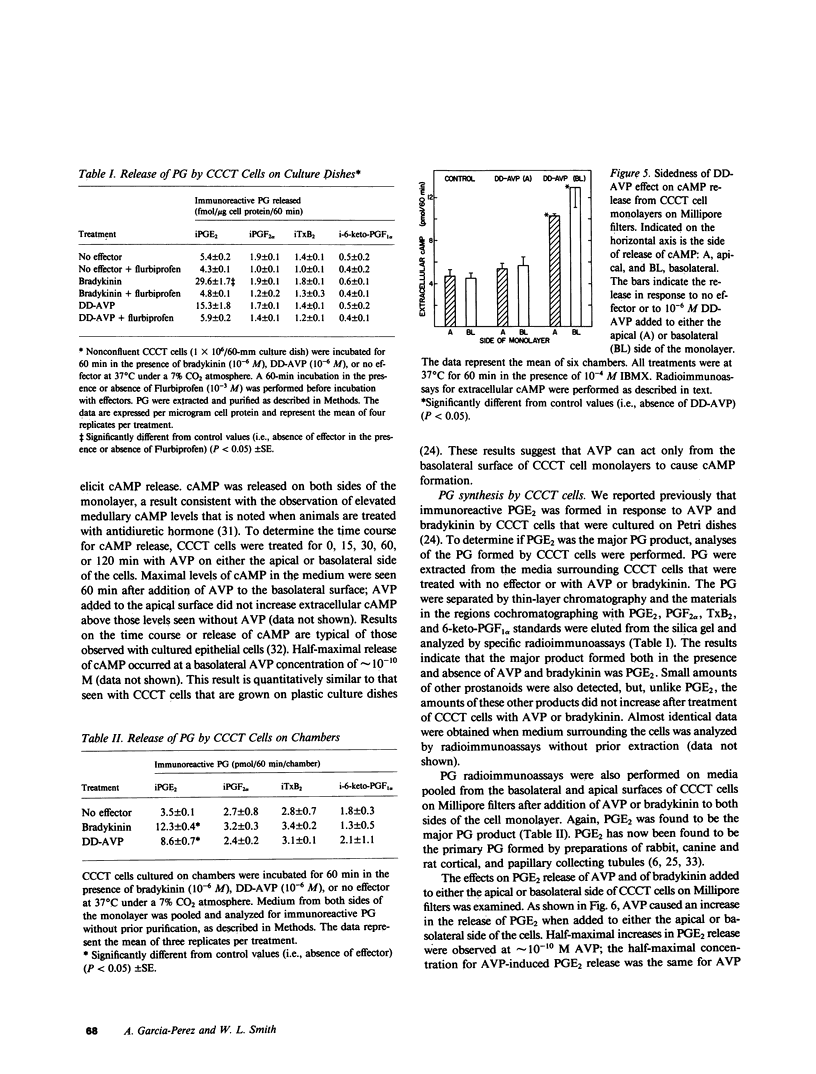
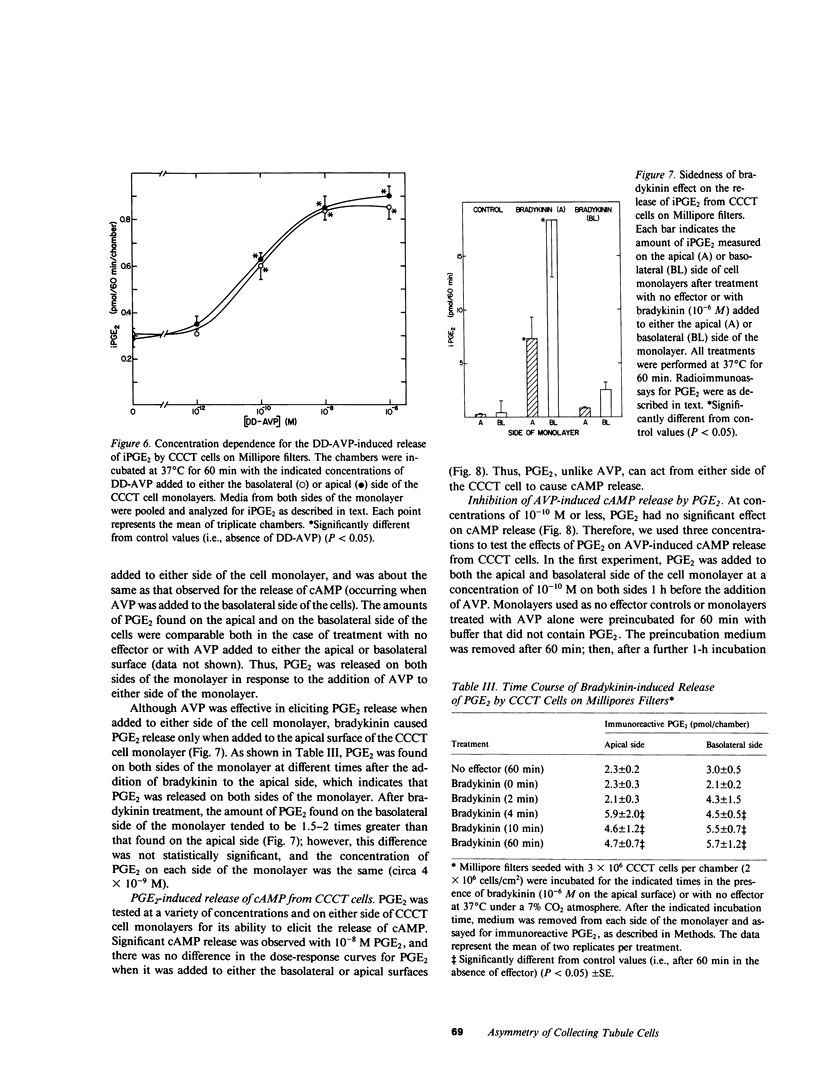
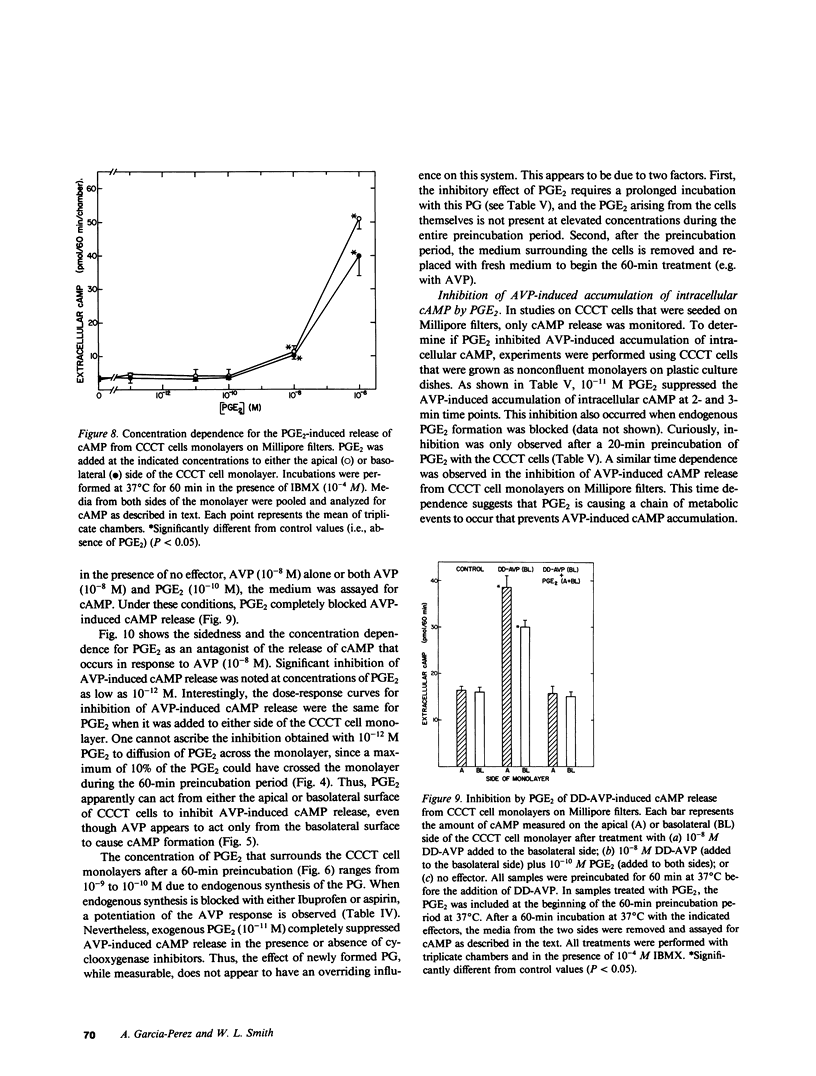
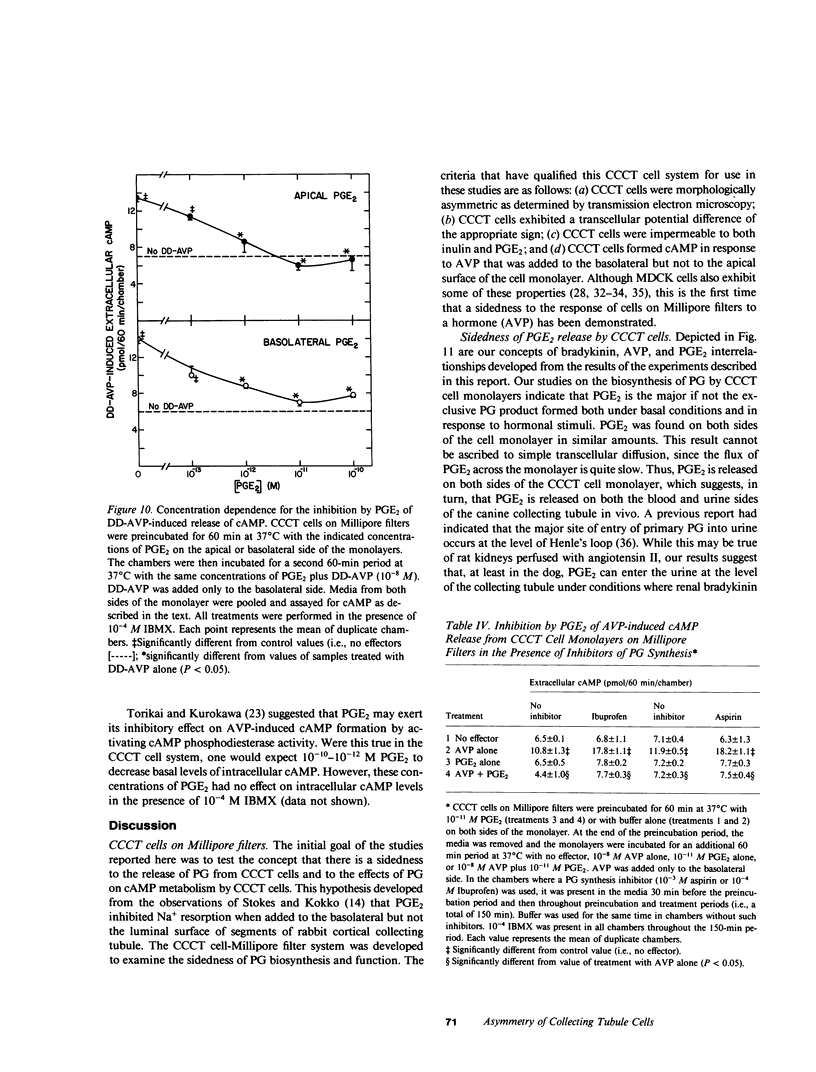
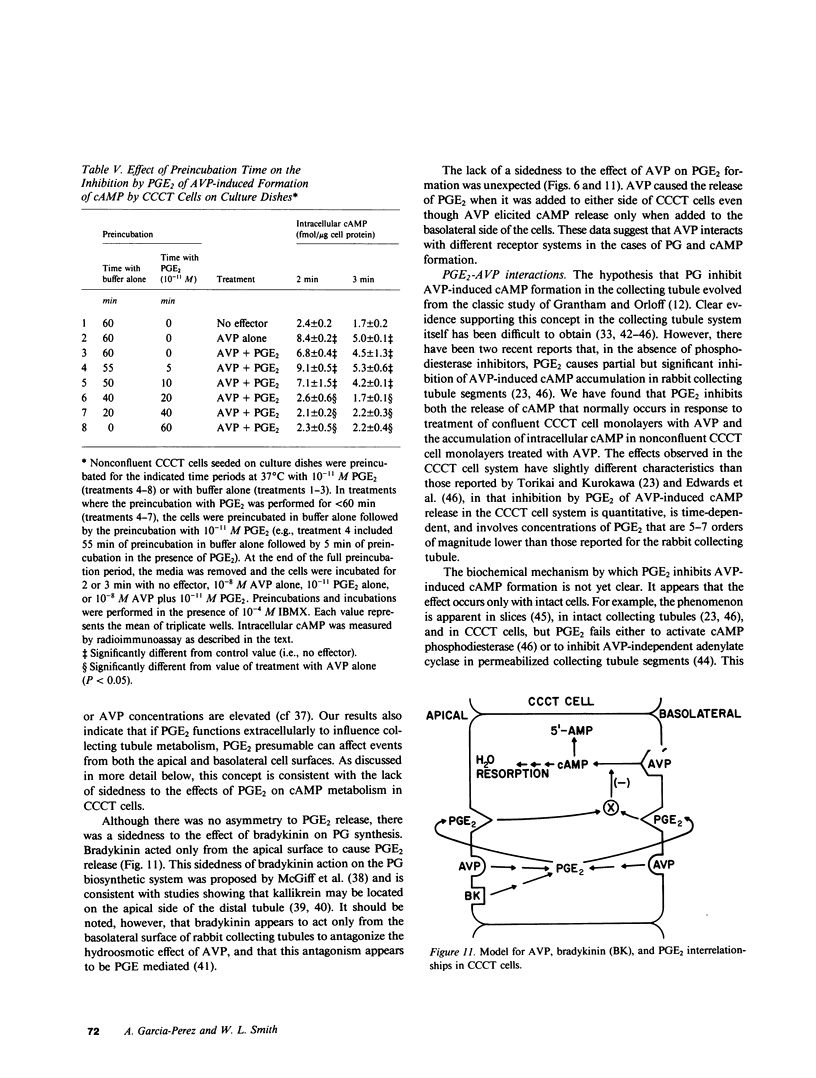
The studies reported here were designed to determine if there is an apical-basolateral asymmetry to the release of prostaglandins by or to the biochemical effects of prostaglandins on the renal collecting tubule. Canine cortical collecting tubule (CCCT) cells were isolated by immunodissection and seeded at supraconfluent densities on Millipore filters. The resulting confluent monolayer of CCCT cells: (a) developed and maintained a transcellular potential difference of 1 mV (apical side negative); (b) exhibited a permeability to inulin that was the same as that obtained with similar monolayers of Madin-Darby canine kidney (MDCK) cells; and (c) released adenosine 3',5'-cyclicmonophosphate (cAMP) in response to arginine vasopressin (AVP) added to the basolateral but not the apical surface of the monolayer. These results indicate that confluent monolayers of CCCT cells on Millipore filters have characteristics of asymmetry that are seen with intact collecting tubules. Moreover, PGE2 added to either side of the CCCT cell monolayer crossed the monolayer at the same slow rate as inulin, which demonstrated the feasibility of examining the sidedness of the effects of and the release of PGE2. Although AVP caused cAMP release only when added to the basolateral side of CCCT cells, AVP caused the release of PGE2 when added to either the apical or basolateral surface. This result implies that there are at least two AVP receptor systems, one coupled to cAMP synthesis and one to PGE2 formation. In contrast to the results observed with AVP, bradykinin caused PGE2 release only when added to the apical surface of CCCT cells, which suggested that urinary but not blood borne kinins elicit PGE2 formation by the canine collecting tubule. PGE2 was released in comparable amounts on each side of the monolayer in response both to AVP and to bradykinin. High concentrations (greater than or equal to 10(-8) M) of PGE2 added to either side of the monolayer caused the release of cAMP. However, at concentrations (10(-10) - 10(-12) M) at which PGE2 had no independent effect on cAMP release, PGE2 inhibited the release of cAMP, which normally occurred in response to AVP. This inhibition occurred with PGE2 added to either the apical or basolateral surface of the CCCT cell monolayer. PGE2 (10(-11) M) also inhibited the AVP-induced accumulation of intracellular cAMP by CCCT cells seeded on culture dishes. This inhibition was only observed when the cells were preincubated with PGE2 for greater than or equal to 20 min. Our results are consistent with the concept that inhibiton by prostaglandins of the hydroosmotic effect of AVP is due to inhibition of AVP-induced cAMP production. This inhibition does not appear to involve a direct physical interaction of PGE2 with the AVP receptor which is coupled to adenylate cyclase, since CCCT cells must be preincubated with PGE2 for 20 min for the inhibition to be observed, and since PGE2 added to the apical surface of CCCT cells inhibits cAMP release in response to AVP acting from the basolateral surface.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler E. M., Flunk L. J., Mullin J. M., Kleinzeller A. Anomalous patterns in cultured cell monolayers. Science. 1982 Aug 27;217(4562):851–853. doi: 10.1126/science.7048529. [DOI] [PubMed] [Google Scholar]
- Anderson R. J., Berl T., McDonald K. D., Schrier R. W. Evidence for an in vivo antagonism between vasopressin and prostaglandin in the mammalian kidney. J Clin Invest. 1975 Aug;56(2):420–426. doi: 10.1172/JCI108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. B., Johnson G. S., Pastan I. Transformation of chick-embryo fibroblasts by wild-type and temperature-sensitive Rous sarcoma virus alters adenylate cyclase activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1055–1059. doi: 10.1073/pnas.70.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck N. P., Kaneko T., Zor U., Field J. B., Davis B. B. Effects of vasopressin and prostaglandin E 1 on the adenyl cyclase-cyclic 3',5'-adenosine monophosphate system of the renal medulla of the rat. J Clin Invest. 1971 Dec;50(12):2461–2465. doi: 10.1172/JCI106746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito L. Z., Baroody R. A. Impermeability of rabbit erythrocytes to prostaglandins. Am J Physiol. 1975 Dec;229(6):1580–1584. doi: 10.1152/ajplegacy.1975.229.6.1580. [DOI] [PubMed] [Google Scholar]
- Bohman S. O. Demonstration of prostaglandin synthesis in collecting duct cells and other cell types of the rabbit renal medulla. Prostaglandins. 1977 Oct;14(4):729–744. doi: 10.1016/0090-6980(77)90201-5. [DOI] [PubMed] [Google Scholar]
- Carretero O. A., Scicli A. G. The renal kallikrein-kinin system. Am J Physiol. 1980 Apr;238(4):F247–F255. doi: 10.1152/ajprenal.1980.238.4.F247. [DOI] [PubMed] [Google Scholar]
- Cereijido M., Robbins E. S., Dolan W. J., Rotunno C. A., Sabatini D. D. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978 Jun;77(3):853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J., Margolius H. S. Studies on rat renal cortical cell kallikrein. II. Identification of kallikrein as an ecto-enzyme. Biochim Biophys Acta. 1979 Oct 11;570(2):330–340. doi: 10.1016/0005-2744(79)90153-0. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Butcher R. W. Desensitization of adenylate cyclase in cultured fibroblasts with prostaglandin E1 and epinephrine. J Biol Chem. 1979 Oct 10;254(19):9373–9378. [PubMed] [Google Scholar]
- Fejes-Tóth G., Magyar A., Walter J. Renal response to vasopressin after inhibition of prostaglandin synthesis. Am J Physiol. 1977 May;232(5):F416–F423. doi: 10.1152/ajprenal.1977.232.5.F416. [DOI] [PubMed] [Google Scholar]
- Frölich J. C., Williams W. M., Sweetman B. J., Smigel M., Carr K., Hollifield J. W., Fleisher S., Nies A. S., Frisk-Holmberg M., Oates J. A. Analysis of renal prostaglandin synthesis by competitive protein binding assay and gas chromatography--mass spectrometry. Adv Prostaglandin Thromboxane Res. 1976;1:65–80. [PubMed] [Google Scholar]
- Garcia-Perez A., Smith W. L. Use of monoclonal antibodies to isolate cortical collecting tubule cells: AVP induces PGE release. Am J Physiol. 1983 Mar;244(3):C211–C220. doi: 10.1152/ajpcell.1983.244.3.C211. [DOI] [PubMed] [Google Scholar]
- Grantham J. J., Orloff J. Effect of prostaglandin E1 on the permeability response of the isolated collecting tubule to vasopressin, adenosine 3',5'-monophosphate, and theophylline. J Clin Invest. 1968 May;47(5):1154–1161. doi: 10.1172/JCI105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier F. C., Allen M. L., Smith W. L. Interrelationships among prostaglandins, vasopressin and cAMP in renal papillary collecting tubule cells in culture. Prostaglandins. 1982 Oct;24(4):547–565. doi: 10.1016/0090-6980(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Grenier F. C., Rollins T. E., Smith W. L. Kinin-induced prostaglandin synthesis by renal papillary collecting tubule cells in culture. Am J Physiol. 1981 Jul;241(1):F94–104. doi: 10.1152/ajprenal.1981.241.1.F94. [DOI] [PubMed] [Google Scholar]
- Handler J. S., Orloff J. Antidiuretic hormone. Annu Rev Physiol. 1981;43:611–624. doi: 10.1146/annurev.ph.43.030181.003143. [DOI] [PubMed] [Google Scholar]
- Hansen H. S. Essential fatty acid supplemented diet increases renal excretion of prostaglandin E2 and water in essential fatty acid deficient rats. Lipids. 1981 Nov;16(11):849–854. doi: 10.1007/BF02535041. [DOI] [PubMed] [Google Scholar]
- Hassid A., Konieczkowski M., Dunn M. J. Prostaglandin synthesis in isolated rat kidney glomeruli. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1155–1159. doi: 10.1073/pnas.76.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt W. F., Lechene C. ADH-PGE2 interactions in cortical collecting tubule. I. Depression of sodium transport. Am J Physiol. 1981 Oct;241(4):F452–F460. doi: 10.1152/ajprenal.1981.241.4.F452. [DOI] [PubMed] [Google Scholar]
- Iino Y., Brenner B. M. Inhibition of Na Transport by prostacyclin (PGI2) in rabbit cortical collecting tubule. Prostaglandins. 1981 Nov;22(5):715–721. doi: 10.1016/0090-6980(81)90210-0. [DOI] [PubMed] [Google Scholar]
- Iino Y., Imai M. Effects of prostaglandins on Na transport in isolated collecting tubules. Pflugers Arch. 1978 Feb 22;373(2):125–132. doi: 10.1007/BF00584850. [DOI] [PubMed] [Google Scholar]
- Kassis S., Fishman P. H. Different mechanisms of desensitization of adenylate cyclase by isoproterenol and prostaglandin E1 in human fibroblasts. Role of regulatory components in desensitization. J Biol Chem. 1982 May 10;257(9):5312–5318. [PubMed] [Google Scholar]
- Kirschenbaum M. A., Lowe A. G., Trizna W., Fine L. G. Regulation of vasopressin action by prostaglandins. Evidence for prostaglandin synthesis in the rabbit cortical collecting tubule. J Clin Invest. 1982 Dec;70(6):1193–1204. doi: 10.1172/JCI110718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum M. A., Serros E. R. Effects of alterations in urine flow rate on prostaglandin E excretion in conscious dogs. Am J Physiol. 1980 Feb;238(2):F107–F111. doi: 10.1152/ajprenal.1980.238.2.F107. [DOI] [PubMed] [Google Scholar]
- Locher R., Vetter W., Block L. H. Interactions between 8-L-arginine vasopressin and prostaglandin E2 in human mononuclear phagocytes. J Clin Invest. 1983 Apr;71(4):884–891. doi: 10.1172/JCI110842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum G. M., Aisenbrey G. A., Dunn M. J., Berl T., Schrier R. W., McDonald K. M. In vivo effect of indomethacin to potentiate the renal medullary cyclic AMP response to vasopressin. J Clin Invest. 1977 Jan;59(1):8–13. doi: 10.1172/JCI108624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumo F., Edelman I. S. Effects of Ca++ and prostaglandin E1 on vasopressin activation of renal adenyl cyclase. J Clin Invest. 1971 Aug;50(8):1613–1620. doi: 10.1172/JCI106649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGiff J. C., Itskovitz H. D., Terragno A., Wong P. Y. Modulation and mediation of the action of the renal kallikrein-kinin system by prostaglandins. Fed Proc. 1976 Feb;35(2):175–180. [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. S. Rapid extraction of arachidonic acid metabolites from biological samples using octadecylsilyl silica. Methods Enzymol. 1982;86:467–477. doi: 10.1016/0076-6879(82)86218-6. [DOI] [PubMed] [Google Scholar]
- Pugliese F., Sato M., Williams S., Aikawa M., Hassid A., Dunn M. Rabbit and rat renal papillary collecting tubule cells in culture: the interactions of arginine vasopressin, prostaglandins, and cyclic AMP. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:517–523. [PubMed] [Google Scholar]
- Rindler M. J., Chuman L. M., Shaffer L., Saier M. H., Jr Retention of differentiated properties in an established dog kidney epithelial cell line (MDCK). J Cell Biol. 1979 Jun;81(3):635–648. doi: 10.1083/jcb.81.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl A. M., Smith J. B., Silver M. J., Nicolaou K. C., Ahern D. Selective binding site for [3H]prostacyclin on platelets. J Clin Invest. 1979 Feb;63(2):215–220. doi: 10.1172/JCI109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. L., Bell T. G. Immunohistochemical localization of the prostaglandin-forming cyclooxygenase in renal cortex. Am J Physiol. 1978 Nov;235(5):F451–F457. doi: 10.1152/ajprenal.1978.235.5.F451. [DOI] [PubMed] [Google Scholar]
- Smith W. L., Wilkin G. P. Immunochemistry of prostaglandin endoperoxide-forming cyclooxygenases: the detection of the cyclooxygenases in rat, rabbit, and guinea pig kidneys by immunofluorescence. Prostaglandins. 1977 May;13(5):873–892. doi: 10.1016/0090-6980(77)90217-9. [DOI] [PubMed] [Google Scholar]
- Sraer J., Foidart J., Chansel D., Mahieu P., Kouznetzova B., Ardaillou R. Prostaglandin synthesis by mesangial and epithelial glomerular cultured cells. FEBS Lett. 1979 Aug 15;104(2):420–424. doi: 10.1016/0014-5793(79)80866-2. [DOI] [PubMed] [Google Scholar]
- Stokes J. B., Kokko J. P. Inhibition of sodium transport by prostaglandin E2 across the isolated, perfused rabbit collecting tubule. J Clin Invest. 1977 Jun;59(6):1099–1104. doi: 10.1172/JCI108733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torikai S., Kurokawa K. Distribution of prostaglandin E2-sensitive adenylate cyclase along the rat nephron. Prostaglandins. 1981 Mar;21(3):427–438. doi: 10.1016/0090-6980(81)90088-5. [DOI] [PubMed] [Google Scholar]
- Torikai S., Kurokawa K. Effect of PGE2 on vasopressin-dependent cell cAMP in isolated single nephron segments. Am J Physiol. 1983 Jul;245(1):F58–F66. doi: 10.1152/ajprenal.1983.245.1.F58. [DOI] [PubMed] [Google Scholar]
- Zusman R. M., Keiser H. R. Prostaglandin biosynthesis by rabbit renomedullary interstitial cells in tissue culture. Stimulation by angiotensin II, bradykinin, and arginine vasopressin. J Clin Invest. 1977 Jul;60(1):215–223. doi: 10.1172/JCI108758. [DOI] [PMC free article] [PubMed] [Google Scholar]