Abstract
Background
Increased levels of delay discounting have been associated with alcoholism and problematic levels of drinking. Attempts to assess the directionality of this relationship by studying individuals with a family history of alcoholism as well as rodent lines selectively bred for high home cage alcohol preference have yielded discordant results. One possible reason for this discordance is that increased levels of delay discounting may only track with specific processes that lead to addiction vulnerability. The current study investigated this possibility by assessing three strains of rats previously identified to exhibit heritable differences in ethanol-seeking and consumption.
Methods
In an adjusting amount delay discounting task, alcohol-preferring P rats who display high levels of both ethanol-seeking and consumption were compared to High Alcohol Drinking (HAD2) rats who only exhibit moderate ethanol-seeking despite high levels of consumption, and Long Evans (LE) rats who display moderate seeking and consumption. Ethanol-seeking and consumption phenotypes were subsequently confirmed in an operant self-administration task with a procedural separation between ethanol-seeking and drinking.
Results
P rats discounted delayed rewards to a greater extent than both HAD2s and LE who did not show differences in discounting. Moreover, the ethanol-seeking and drinking phenotypes were replicated with P rats displaying greater ethanol-seeking compared to both the HAD2s and LE, and both the HAD2s and P rats consuming more ethanol than LEs.
Conclusions
Only the high seeking strain, the P rats, exhibited increased levels of delay discounting. This suggests that this measure of behavioral under-control is specifically associated with alcohol-related appetitive, but not consummatory, processes since the moderate seeking/high drinking line did not show increased levels of impulsivity. This finding supports the hypothesis that delay discounting is specifically associated with only certain processes which are sufficient but not necessary to confer addiction vulnerability, and therefore also supports increased levels of delay discounting as a predisposing risk factor for alcoholism.
Keywords: Appetitive, Consummatory, Rat, Impulsivity, Selected Line
Introduction
Heightened levels of impulsivity have been hypothesized to be a causal factor in addictive disorders (for review: Perry and Carroll 2008). Increased discounting of delayed rewards, a form of cognitive impulsivity, has been repeatedly associated with alcohol use disorders. Greater discounting has been seen in heavy social drinkers and alcoholics (Mitchell et al. 2005; Petry 2001; Vuchinich and Simpson 1998), early compared to late onset alcoholics (Dom et al. 2006), and is associated with greater levels of problematic drinking (Claus et al. 2011; Kollins 2003). A recent meta-analysis of case-control studies (Mackillop et al. 2011) also found a moderate effect size for delay discounting across alcoholism and illicit drug use utilizing only clinical samples, and a small effect size when all samples were included. However, the actual nature of the relationship between increased delay discounting and alcohol use disorders is an open question. Increased discounting could cause or be caused by alcohol use, and both conditions could escalate each other.
One way to examine the directionality of this relationship is to look at individuals with a family history of alcoholism as they are a high risk group for developing alcoholism. However, studies on family history positive (FHP) individuals have been inconclusive. Petry et al. (2002) found FHP females to have steeper discounting and Herting et al. (2010) found that FHP youth trended towards steeper discounting. Acheson et al. (2011) found a modest relationship between the number of affected family members and increased levels of delay discounting that was mediated by anti-social tendencies, whereas Crean et al. (2002) and Bjork et al. (2004) found no relationship between FHP status and delay discounting. Similarly, examinations of delay discounting utilizing rodent lines bidirectionally selected for home cage intake and preference of alcohol has yielded mixed results. In the literature there are two studies showing alcohol preferring lines to have greater discounting (Oberlin & Grahame 2009; Wilhelm & Mitchell 2008) and two null results (Wilhelm et al. 2007; Wilhelm & Mitchell 2012). One possible reason for these disparate results is that the selection phenotype for these lines, high levels of home cage intake and preference of alcohol, can be affected by multiple different factors.
Delay discounting describes choices made between appetitive stimuli. As appetitive processes, such as alcohol seeking, are defined by the motivation to obtain an appetitive stimulus, delay discounting can be said to describe appetitive behavior. Consistent with this idea, recent results have shown increased levels of delay discounting to track with cocaine (Broos et al. 2012) and nicotine (Diergaarde et al. 2008) seeking but not overall levels of intake. The current study attempted to extend these findings to determine whether delay discounting is differentially related to appetitive versus consummatory processes. To this end, alcohol-preferring (P) rats, high alcohol-drinking (HAD2) rats, and Long Evans rats (LE) were compared on a delay discounting task. These three lines were chosen due to a unique combination of alcohol seeking and drinking behavioral phenotypes that they exhibit. Using an operant self-administration paradigm that has a procedural separation between alcohol seeking and drinking, Czachowski and Samson (2002) observed that P rats display both high levels of alcohol seeking and consumption, while HAD2 rats exhibit a similar high level of alcohol consumption (> 1.25 g/kg in less than 20 minutes) but only moderate levels of seeking. The moderate ethanol-seeking was comparable to that of unselected LE (approximately four-fold less than P rats), who also display moderate levels of consumption (Czachowski and Samson 1999). It was therefore hypothesized that delay discounting in the present study would “track with” appetitive behavior, and consequently only the P rats would show increased delay discounting as compared to HAD2 and LE rats.
Materials and Methods
Subjects
The subjects were HAD2s (60th and 62nd generation; Indiana University School of Medicine, Indianapolis, IN), P rats (74th and 75th generation), and Long Evans rats (Harlan, Indianapolis, IN). Animals were male and were aged matched at approximately 50 days. At the start of the study, the weights ranged from 185 to 242g for the P rats, 235 to 280g for the LE, and 112 to 218g for the HAD2s. Animals had ad libitum access to food, were singly housed in plastic shoebox cages, and maintained 12 hour light/dark cycles. Sessions were conducted 5 days/week during the light cycle. Animals were water restricted for the delay discounting receiving access to water 1 hour after each session, for 2 hours during the first week of training and 1 hour thereafter. For the sipper tube paradigm animals were only water restricted for 1 to 2 days during training to initiate lever pressing and from that point forward animals had water ad lib. Animal care procedures were conducted in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (2011) and Institutional Animal Care and Use Committee approval.
Apparatus
All sessions were conducted in modular chambers with electrical inputs and outputs controlled by an IBM compatible PC (Med-Associates, St. Albans, VT; 30×30×24.5cm). All chambers had a stainless steel bar floor, a house light, and were enclosed in sound attenuating boxes with exhaust fans for ventilation and masking external noise. For the delay discounting, chambers were equipped with a nosepoke recess with an internal stimulus light and photocell to record beam breaks centered on the front wall 2cm above the floor. A retractable graduated cylinder tube with a rubber stopper, stainless steel spout with double ball bearings, and a lickometer was located 3cm above the nosepoke recess. On both sides of the nosepoke recess were retractable levers with stimulus lights 4cm above each lever. In the sipper tube appetitive/consummatory paradigm, chambers were equipped with houselight, the two retractable levers and the retractable graduated cylinder tube on the opposite wall.
Delay Discounting
The delay discounting paradigm was an adjusting amount procedure based on Oberlin and Grahame (2009). Subjects responded for 2% sucrose, prepared weight/volume (w/v) in tap water. Training was conducted in 5 stages summarized in Table 1. In stage one, subjects were placed into the chamber with the sipper tube already descended, levers retracted, house light on, and the nosepoke stimulus light off. After 200 “free licks” the sipper tube retracted, the nosepoke's internal stimulus light turned on. Then the subject responded on a fixed ratio (FR) 1 schedule where a nosepoke yielded 20 seconds of access to the sipper tube. When the sipper tube was extended the nosepoke's internal stimulus light was turned off until the tube was retracted. In stage 2 the access to the sipper tube was decreased to 10 seconds and there were no “free licks.” In the third stage, the access to the sipper tube was further reduced to 5 seconds, and a 10 second inter-trial-interval (ITI) was introduced during which there were no programmed consequences for making any response.
Table 1.
Delay discounting training procedure. ITI, Inter-trial Interval; FR, Fixed Ratio; NP, Nosepoke; LP, Lever Press.
| Stage | Procedure | Criterion for Advancement |
|---|---|---|
| 1 | 200 “Free Licks” then FR1NP for 20 seconds of access | 200 licks and 10 Trials |
| 2 | FR1NP for 10 seconds of access | 40 trials in 60 minutes |
| 3 | FR1NP for 5 seconds of access and 10 seconds ITI | 40 trials in 60 minutes |
| 4 | Nosepoke cues levers and lights & 10 second ITI | 40 trials in 60 minutes while responding for 2 seconds of access |
| Day 1: Handshaped to lever press for 10 seconds access | ||
| Day2: FR1LP for 5 seconds access | ||
| Day 3: FR1LP for 2 seconds access | ||
| 5 | Full program: adjusting amount, forced trials, variable length ITI | 20 free choice trials in 60 minutes and an indifference point of 1.5 or greater in 3 of 4 consecutive sessions. |
In stage 4, the levers were extended. To start each trial, the nosepoke light was illuminated. Once the subject nosepoked, the nosepoke's internal stimulus light turned off, and the stimulus lights above both levers turned on signaling the availability of reinforcement for pressing either lever. After pressing a lever, the sipper tube descended into the chamber for 10 seconds, and the stimulus lights turned off. This was followed by a 10 second ITI. Stage 4 was completed over at least 3 sessions. During the first session, subjects were shaped to press both levers after nosepoking for 10 seconds of access to the sipper tube. On subsequent sessions the access time was decreased. The criterion for advancement from stage 4 was the completion of 40 trials in 60 minutes after the access to the sipper tube was decreased to 2 seconds. During the last 3 days of stage 4, lever preference for each subject was assessed.
Stage 5 of training, magnitude discrimination, constituted the first experimental condition. The same pattern of chained responding occurred as in stage 4. However a response on the non-preferred lever yielded a standard reward of 2 seconds of access to the sipper tube, and a response on the preferred lever resulted in the delivery of an adjusting alternative reward. An additional .6 seconds was added to the standard reward to account for the time it takes for the sipper tube to descend. The adjusting alternative reward started at one second of access to the sipper tube, but then changed based upon the subject's choices by .2 seconds ranging between 0 and 2 seconds. Selection of the standard reward caused an increase, and selection of the alternative reward caused a decrease on the next trial. A variable ITI was implemented ensuring 30 seconds always elapsed between when the animal made a selection and when the next trial began preventing subjects from earning several alternative rewards in the time it would take to earn 1 standard reward. Forced trials were introduced to ensure a subject had experience with the changing reward amounts. After any two consecutive choices of the same lever, only the stimulus light above the opposite lever illuminated and had programmed consequences for responding. Across all sessions the locations of the standard and alternative reward were held constant, and sessions were limited to 60 minutes or the completion of 60 free choice trials (whichever occurred first).
To advance from magnitude discrimination, animals had to perform ≥20 free choice trials and have an indifference point, median alternative reward in the last 20 trials, of ≥1.5 seconds in 3 of 4 consecutive sessions. Data from these 3 sessions were used for the zero delay condition. A delay to the standard reward was then implemented. During this delay, only the stimulus light above the standard reward lever remained illuminated until the reward was delivered. Each delay was assessed for 3 sessions each, and delays (2, 4, 8, 12, and 16 seconds) were assessed in ascending order.
Sipper Tube Appetitive/Consummatory paradigm
At least one week elapsed after the delay discounting task, and then animals were trained on a FR 1 schedule for 10 seconds of access to 10% sucrose while water restricted for just one to two days. Over the next three weeks subjects underwent a modified sucrose fading procedure (Samson 1986) in which the concentration of sucrose was decreased as ethanol was introduced. The ethanol concentration was increased to 10% (v/v), and the sucrose was removed. Over these three weeks, the FR requirement increased from 1 to 4, an inactive lever was introduced, and on 2nd to last day the procedural separation between seeking and drinking was implemented. Animals had to complete a single response requirement (RR) of 4 lever presses to receive 20 uninterrupted minutes of access to the sipper tube. Over the course of the next 2-3 weeks the RR was increased to 15 responses, and responding was then maintained for 1 to 3 weeks.
Consummatory variables were measured during 4 days of responding at an RR15 and then averaged across each subject. Total alcohol intake was determined from the change in volume and intake in grams per kilogram was calculated using daily measurements of body weight. Licking behavior was characterized by licks divided into 2 minute time bins. If a subject failed the response requirement on any day, an average of the remaining three days was taken. If the response requirement was not met on 2 of the 4 days, the subject was excluded. After the consummatory measurement, subjects underwent a 12-session extinction curve. During extinction sessions rats received 20 minutes of access to the active and inactive levers with no programmed consequences. Responses were recorded in total responses, responses in 2 minute bins, and cumulative records. The graduated cylinder tube was present but retracted, and filled with 10% ethanol to control for olfactory cues.
Statistical Analysis
For the delay discounting, inclusion of results from a given session was dependent upon the successful completion of 20 free choice trials. A minimum of two sessions completed at each delay was required for each subject following the method of Oberlin and Grahame (2009). Data were averaged across each delay and subjected to a mixed analysis of variance (ANOVA) with delay as the within subjects factor and strain as the between subjects factor. Mean indifference points at each delay were used for non-linear regression to determine the discount function as described by the following equation (Mazur 1987):
In the above equation, V is equal to the subjective value of the standard reward after a given delay (mean indifference point). A is the actual magnitude of the standard reward. D is the delay to the standard reward, and k is the fitted parameter that describes the steepness of the discount function. The k values were examined with a univariate ANOVA with strain as a factor.
For the sipper tube model, intake in g/kg, ml, and number of responses on the first day of extinction were analyzed with a univariate ANOVA. The whole 12 session extinction curve was analyzed with a mixed factorial ANOVA with strain as the between subjects factor and session as the within subjects factor. Responding and licks in 2 minute bins were analyzed with a mixed factorial ANOVA with strain and bin as factors. For both paradigms, significant main effects were followed up by Fisher's LSD tests and interactions were examined with Bonferroni corrected student t tests. Greenouse-Geisser corrections were applied for violations of sphericity and adjusted degrees of freedom are reported.
Results
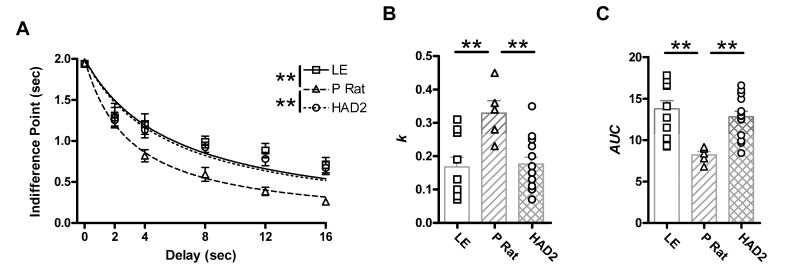
One P rat and 3 LE rats were excluded for “stealing” licks during the inter-trial interval and during the delay to the standard reward (i.e., managing to make contact with the sipper tube even though it was retracted), and one HAD2 was excluded for failing to learn the task. The final n's sizes for the delay discounting were 11 LE, 5 P rats, and 14 HAD2s. Indifference points showed an effect of strain [F(2, 27)=5.5, p=.01], as well as delay [F(3.3, 88.4)=98.1, p<.001], but no interaction [F(6.5, 88.4)=1.6, p=.15]. Post hoc analysis revealed the P rats had lower indifference points than both the HAD2s and LEs who were not different (Fig. 1a). Similarly, analysis of the k values revealed an effect of strain [F(2, 27)=7.2, p=.003]. Follow up tests revealed that the P rats had larger k values, indicative of greater discounting, than both the HAD2s and the LE who were not different (Fig. 1b). Since the adjusting alternative reward was capped at 2 seconds so that animals would not experience large immediate rewards that could disrupt choice behavior, it is possible that the 0 second delay indifference point was underestimated. To insure that this was not unduly influencing the results, we conducted an area under the curve (AUC) analysis excluding the zero second delay indifference point (Myerson et al. 2001). A univariate ANOVA [F(2,27)=7.7, p=.002] and subsequent Fisher's LSD tests revealed that the P rats had less area under the curve than both the HAD2s and LE who were equal, directly paralleling the k value results (Fig. 1c).
Fig 1.
From the delay discounting paradigm: A) Mean indifference points (±SEM) plotted as a function of delay. The hyperbolic curve is defined by the mean k value; B) k values as a function of strain. Bars correspond to mean values (±SEM). C) Area under the curve (AUC) scores as a function of strain. Bars correspond to mean values (±SEM). ** p <.01 on Fishers LSD test.
The number of free choice trials were skewed and subjected to a natural log transformation. The log transformed values showed an effect of delay [F(2.1, 57.6)=6.9, p=.002] and strain [F(2, 27)=4.5, p=.021], but no interaction [F(4.3, 57.6)=1.8, p=.14]. Post hoc analysis revealed that the P rats completed more trials than the HAD2s. Analysis of 2% sucrose intake volume (ml) during the delay discounting showed a main effect of delay [F(3.2, 85.5)=27.3, p<.001]and a main effect of strain [F(2, 27)=17.3, p<.001], but no interaction [F(6.3, 85.5)=1.0, p=.39]. Post hoc analysis revealed all the strains to be different from each other with the greatest intake in the P rats followed by the Long Evans, and then the HAD2s. Similarly analysis of intake in g/kg showed a main effect of delay [F(2.7,72.2)=35.5, <.001] and strain [F(2,27)=3.6, p=.043], but no interaction [F(5.3, 72.2)=1.1, p=.39).However post hoc testing only found a difference between the P rats and the LEs. Both free choice trials and intake are presented in table 2.
Table 2. Delay discounting trial completion and reinforcer intake.
Mean (±SEM) number of completed free choice trials and intake of 2% Sucrose (ml and g/kg) from the delay discounting paradigm.
| Variable | Strain | 0 sec. Delay | 2 sec. Delay | 4 sec. Delay | 8 sec. Delay | 12 sec. Delay | 16 sec. Delay |
|---|---|---|---|---|---|---|---|
| Free | LE | 59.8 (±0.1) | 59.4 (±0.6) | 58.8 (±0.9) | 57.3 (±1.4) | 52.5 (±2.2) | 50.9 (±3.1) |
| Choice | P@@ | 59.9 (±0.1) | 60.0 (±0.0) | 60.0 (±0.0) | 60.0 (±0.0) | 60.0 (±0.0) | 59.9 (±0.1) |
| Trials | HAD%% | 58.2 (±1.3) | 58.8 (±0.7) | 55.4 (±1.4) | 53.8 (±1.7) | 47.6 (±3.1) | 45.9 (±3.2) |
|
| |||||||
| Intake 2% | LE%%@@ | 5.2 (±0.4) | 4.5 (±0.4) | 4.0 (±0.2) | 3.4 (±0.3) | 3.0 (±0.3) | 2.9 (±0.4) |
| Sucrose | P##@@@ | 5.5 (±0.9) | 6.1 (±0.5) | 5.7 (±0.4) | 5.0 (±0.3) | 4.4 (±0.4) | 4.1 (±0.2) |
| (ml) | HAD2##%%% | 3.9 (±0.3) | 3.4 (±0.3) | 3.0 (±0.2) | 2.5 (±0.2) | 1.9 (±0.2) | 2.1 (±0.2) |
|
| |||||||
| Intake 2% | LE% | .41 (±.03) | .33 (±.03) | .28 (±.02) | .23 (±.02) | .19 (±.02) | .18 (±.03) |
| Sucrose | P# | .43 (±.07) | .46 (±.05) | .41 (±.04) | .34 (±.03) | .28 (±.03) | .26 (±.02) |
| (g/kg) | HAD2 | .48 (±.04) | .39 (±.03) | .33 (±.03) | .26 (±.02) | .19 (±.02) | .18 (±.03) |
p<.01 and
p<.001 vs. HAD2 on Fishers LSD.
p<.05,
p<.01, and
p<.001 on Fishers LSD vs. P rat.
p<.05 and
p<.01 vs. Long Evans (LE) on Fishers LSD.
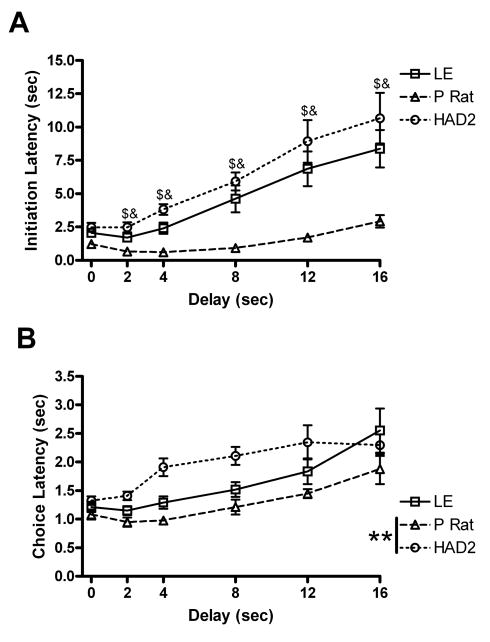
The trial initiation latencies were also skewed and were subjected to a log transformation. The log transformed trial initiation latencies revealed an effect of delay [F(2.9, 77.7)=63.7, p<.001], strain [F(2, 27)=28.9, p<.001], and an interaction [F(5.8, 77.7)=2.7, p=.02]. Post hoc analysis revealed that the P rats had shorter latencies than both the LEs and the HAD2s at all delays except the 0 second delay (Fig. 2a). The choice latencies showed an effect of strain [F(2, 27)=4.6, p=.02], delay [F(2.6, 71.5)=17.3, p<.001], but no interaction [F(5.3, 71.5)=1.7, p=.13]. Post hoc analysis revealed that the P rats had shorter choice latencies than the HAD2s and but not the LEs (Fig. 2b).
Fig 2.
Latency variables from the delay discounting paradigm. A) Mean trial initiation latencies (±SEM) as a function of delay; B) Mean choice latencies (±SEM) as a function of delay. $ and & indicate differences between the P rats vs. LE and HAD2s respectively after Bonferroni correction for multiple comparison. ** p<.01 on Fishers LSD test.
Sipper Tube
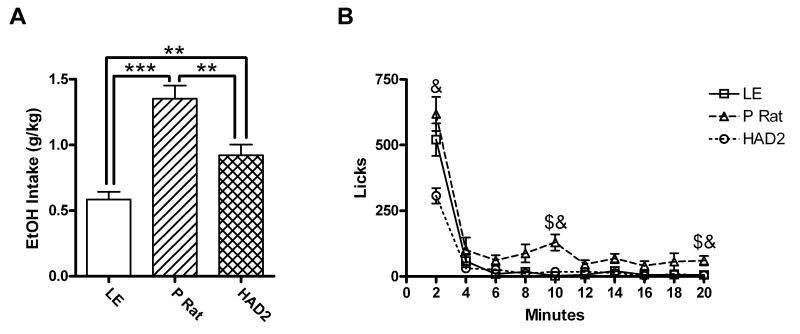
For the sipper tube model, 3 LE and 3 HAD2s were excluded for failing to train to and maintain responding at a response requirement of 15 (final n's: 11 LE, 6 P, and 12 HAD2s). Ethanol intake showed a main effect of strain when measured in volume (ml) [F(2, 26)=44.95, p<.001] and in g/kg [F(2, 26)=18.8, p<.001]. Post hoc analysis revealed that in both cases P rats drank more than both the HAD2s and LE, and the HAD2s drank more ethanol in g/kg than the LE (Fig. 3a) Furthermore when licks were analyzed in two minute bins, there was an effect of strain [F(2, 26)=21.13, p<.001], bin [F(2.1, 54.8)=144.4, p<.001] and a bin by strain interaction [F(4.2, 54.8)=5.5, p<.001]. Follow up analysis revealed that all the strains' licking behavior showed an initial large bout, but only the P rats came back to the sipper tube for additional bouts in addition to licking more than the other two strains (Fig 3b).
Fig. 3.
Consummatory variables from the sipper tube paradigm. A) Mean 10% ethanol intake in grams per kilogram (±SEM) as a function of strain; B) Mean licks (±SEM) per 2 minute bin over the 20 minutes of sipper tube access. ** and *** indicate p<.01 and p<.001 on Fishers LSD test. $ and & indicate differences between the P rats versus the LE and HAD2s respectively after Bonferroni correction for multiple comparison.
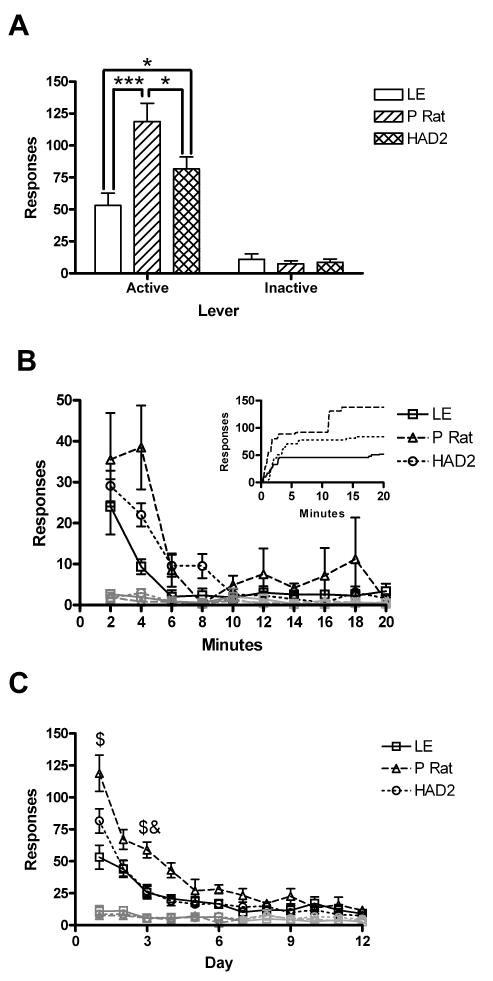
On the first day of extinction, total responding on the active lever showed an effect of strain [F(2,26)=7.96, p=.002], but responding on the inactive lever did not [F(2,26)=.25, p=.78). Follow up analysis showed that the P rats responded more on the active lever than both the HAD2s and the LE, and the HAD2s responded more than the LE (Fig 4a). When responding was broken down into 2 minute bins, both active [F(3.2, 83.2)=24.3, p<.001] and inactive responding [F(2.9, 75.2)=2.8, p=.049] showed a main effect of bin such that responding was greater at the start of the session, but neither showed a bin by strain interaction [F(6.4, 83.2)=2.0, p=.067; F(5.8, 75.2)=.50, p=.8; Fig. 4b]. Throughout the entire extinction curve, active responding showed an effect of day [F(4.5, 117.5)=68.3, p<.001], strain [F(2, 26)=8.2, p=.002 ], and an interaction[F(9.0, 117.5)=4.0, p<.001]. The inactive lever revealed an effect of day [F(4.3, 111.7)=3.9, p=.01] but not strain [F(2, 26)=.18, p=.83 ] and no interaction [F(8.6, 111.7)=.79, p=62]. Follow up analysis showed that the P rats continued to respond more later into the extinction curve than the other two strains (Fig. 4c).
Fig. 4.
Appetitive variables from the sipper tube paradigm. A) Mean responses (±SEM) on the first day of extinction as a function of strain; B) Mean responses (±SEM) per 2 minute bin on the first day of extinction. Representative cumulative records are graphed in the inset. C) Mean responses (±SEM) during the entire 12 session extinction curve. Responding on the active lever is in black and on the inactive lever is in the lighter shade. * p<.05 Fishers LSD; *** p<.001 on Fishers LSD; $ and & indicate differences between the P rats versus the LE and HAD2s respectively after Bonferroni correction for multiple comparison. Table 1 Delay Discounting Training
Bivariate Analysis
A trend towards a positive relationship between k values and responding on the first day of extinction was observed [r(24)=.38, p=.055], while the correlation between k values and intake in g/kg was not significant [r(24)=.27, p=.19]. For AUC scores, a significant correlation was observed with responding on the first day of extinction [r(24)=-.42] such that responding tended to increase with lower AUC scores, while intake in g/kg only trended towards significance [r(24)-.38, p=.058].
Discussion
Overall, these findings demonstrate that increased delay discounting “tracks with” alcohol seeking versus alcohol drinking. Specifically, P rats show greater impulsivity with larger k values and lower indifference points than the HAD2 and LE rats. This parallels the pattern of alcohol seeking in these strains as characterized previously using both across-session breakpoint and single, probe extinction sessions (Czachowski and Samson 1999; 2002) and in the present experiment with the P rats responding more on the first day of extinction (a single, probe extinction session). In addition, P rats showed a resistance to extinction over days compared to the other two lines, suggesting that they may also be more perseverative and/or that their greater intake of ethanol during reinforced trials increased this measure of appetitive responding. The elevated ethanol intake of the P rats in the sipper tube model compared to the HAD2s can be explained by their drinking pattern. All three strains have a large initial bout, but the P rats are the only strain to initiate a second bout inside of the 20 minute access period. Coming back for a second bout suggests that the P rats are reengaging in focal search behavior and indicates that they have a smaller inter-bout interval than the other two lines. The initiation of a second bout and the shorter inter-bout interval could be considered an appetitive behavior, as opposed to a consummatory behavior, and is consistent with the overall findings of high appetitive drive in addition to greater impulsivity in the P rat.
The P rats also had shorter trial initiation latencies than the other two strains in the delay discounting task. This is also consistent with the P rats exhibiting increased appetitive drive. However, they did not have decreased choice latencies compared to the LEs. This likely reflects the fact that the trial initiation latency measure is more reflective of appetite drive as compared to choice latency. While choice latencies may also be affected by appetitive drive, they are also subject to the influence of other factors such as the characteristics of the choice that the subject is making. Moreover, a general lack of variability in choice latencies (mean values ranged from approximately 1 to 2.25 seconds as compared to 0.5 to 10.5 seconds for trial initiation latencies) would contribute to this as well. This lower variability of choice latency also may be due to subjects initiating a trial and then responding on a choice lever “all in one motion.” Conversely another possibility is that the P rats are more attentive in addition to possessing increased appetitive drive allowing them to initiate trials faster. This would contrast with the P rats being considered more “impulsive.” However impulsivity is not a unitary construct, but it is rather a constellation of related but distinct attributes that all pertain to a lack of behavioral control (Cyders and Coskunpinar 2011; Dick et al 2010; Evenden 1999). Consequently even if the P rats are more attentive, this possibility would not conflict with them having increased cognitive impulsivity in the form of delay discounting.
The fact that the P rats obtained higher intakes of 2% sucrose than the other two lines despite lower indifference points during the delay discounting could be concerning because it raises the possibility that they were obtaining more reward per unit of access time to the sipper tube. If this were the case they would effectively have a larger delayed reward than the other two lines rendering a possible alternative explanation for their escalated delay discounting. However, larger magnitude rewards tend to be discounted less steeply (Baker et al 2003), the opposite of what was seen in the P rats. Furthermore, magnitude effects are a point of divergence between the animal and human literature. In both pigeons and rats, changing either the amount or the quality of the reinforcer has not been shown to affect discounting behavior (Calvert et al. 2010; Green et al. 2004; Richards et al. 1997). Another potential problem of the higher intake of the P rats is the possibility of greater satiation earlier in the session, but the faster choice and trial initiation latencies of the P rats as well as the trial completion data rule out this possibility. Moreover, examination of intake in g/kg showed the P rats and the HAD2s, which differ in delay discounting, do not differ on intake further ruling out magnitude effects as well as heightened satiation as a possible alternative explanation.
The current findings are consistent with those of Broos et al. (2012) showing that steeper discounting rats had greater context-induced reinstatement, but not different levels of baseline cocaine self-administration. Similarly, Diergaarde et al. (2008) found greater delay discounting was associated with higher levels of responding for and intake of nicotine at high FRs but not low FRs. Furthermore, Koffarnus and Woods (2011) found levels of increased delay discounting to predict more inelastic demand for cocaine, but that they were unrelated to the intensity of cocaine demand in rats. Contrasting with the present results, Anker et al. (2009) did not show delay discounting to be associated with responding on a within session progressive ratio schedule for cocaine even though they did show that steeper discounters had a greater increase in intake when switching from short to long access conditions. However, their design did not utilize a procedural separation between drug seeking and taking, so there is no way to separately examine appetitive and consummatory responding in their subjects. Using a similar design, Perry et al. (2005) did find that greater delay discounters displayed greater self-administration of cocaine and that steeper discounters were also more likely to acquire cocaine self-administration and had faster acquisition.
Initial levels of delay discounting for a natural reward being positively associated with later alcohol seeking supports the hypothesis that heightened impulsivity is a predisposing characteristic for alcohol use disorders. Moreover, increased delay discounting being associated with increased alcohol seeking but not intake provides a possible explanation for discordant results from prior selected line studies using delay discounting (Oberlin & Grahame 2009 and Wilhelm & Mitchell 2008 versus Wilhelm et al. 2007 and Wilhelm & Mitchell 2012). Increased alcohol seeking behavior is not necessarily recruited during selection for intake of “free” alcohol. Consequently, lines in which delay discounting is not a correlated response to selection may not have recruited genes underlying increased appetitive drive. Wilhelm and Mitchell's (2012) finding are consistent with this explanation since one of the selected lines they studied, the Alko Alcohol rats, has been shown to not defend its intake of alcohol when responding for dipper presentations of alcohol by increasing their responding when the response requirement increased similar to HAD rats and unlike P rats in the same study (Files et al. 1998).
The differences between the P and HAD2 rats in delay discounting also support their validity as animal models. Alcoholism is an extremely heterogeneous disorder. The findings of Moss et al. (2008) are a case in point. They showed that DSM-IV alcohol dependence criteria endorsement in the National Epidemiological Survey on Alcohol and Related Conditions conformed to 6 unique clusters using a latent class analysis. Multiple different attempts have been made to describe this inter-individual variability seen in alcoholism ranging from Cloningers and colleagues' (1981) type I/II alcoholics and Babor et al.'s (1992) type A/B alcoholics to systems based purely on motivation for drinking (Dow and Kelley 2013). The high ethanol-seeking and steep discounting of the P rats contrasting with the HAD2s who have reduced seeking and no differences in delay discounting compared to unselected LE parallel this heterogeneity of “alcohol behavioral phenotypes.”
In summary, the current findings extend those of prior studies by demonstrating that increased levels of delay discounting are specifically associated with alcohol seeking and increased appetitive drive as opposed to alcohol consumption. This supports the hypothesis that heightened impulsivity is a predisposing characteristic for alcohol use disorders as delay discounting was assessed prior to any alcohol exposure. This study is unable to address if alcohol exposure could further exacerbate impulsive decision making, nor does this study rule out a third common factor that could underlie the relationship between increased delay discounting and alcohol seeking. Future research should attempt to dissect this relationship further in addition to continuing to characterize differences in behavioral phenotypes of alcohol use. In particular, the comprehensive phenotype of the P rat from the present study and others suggests that, in addition to increased appetitive drive for both sucrose and ethanol (Czachowski and Samson 2002), they show excessive perseverative behavior which could be assessed using paradigms to measure behavioral flexibility.
Acknowledgments
This work was supported by P60AA007611 from NIAAA. The authors wish to thank Dr. Brandon Oberlin for his programming advice and assistance setting up the initial delay discounting paradigm and Katie Anne Havard for her assistance with data collection.
This work was supported by P60AA007611 from the National Institute on Alcohol Abuse and Alcoholism.
Contributor Information
Steven Wesley Beckwith, Department of Psychology, Indiana University Purdue University Indianapolis, Indianapolis, Indiana
Cristine Lynn Czachowski, Department of Psychology, Indiana University Purdue University Indianapolis, Indianapolis, Indiana
References
- Acheson A, Vincent AS, Sorocco KH, Lovallo WR. Greater Discounting of Delayed Rewards in Young Adults with Family Histories of Alcohol and Drug Use Disorders: Studies from the Oklahoma Family Health Patterns Project. Alcohol Clin Exp Res. 2011;35(9):1607–1613. doi: 10.1111/j.1530-0277.2011.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93(3):343–348. doi: 10.1016/j.pbb.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B. Types of Alcoholics, I Evidence for an Empirically Derived Typology Based on Indicators of Vulnerability and Severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay Discounting in Current and Never-Before Cigarette Smokers: Similarities and Differences Across Commodity, Sign, and Magnitude. J Abnorm Psychol. 2003;112(3):382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type1-/type 2-like traits. Alcohol. 2004;34:133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Broos N, Diergaarde L, Schoffelmeer ANM, Pattij T, De Vries TJ. Trait Impulsive Choice Predicts Resistance to Extinction and Propensity to Relapse to Cocaine Seeking: A Bidirectional Investigation. Neuropsychopharmacology. 2012;37(6):1377–1386. doi: 10.1038/npp.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert AL, Green L, Myerson J. Delay Discounting of Qualitatively Different Reinforcers in Rats. J Exp Anal Behav. 2010;93:171–184. doi: 10.1901/jeab.2010.93-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Kiehl KA, Hutchison KE. Neural and Behavioral Mechanisms of Impulsive Choice in Alcohol Use Disorder. Alcohol Clin Exp Res. 2011;35(7):1209–1219. doi: 10.1111/j.1530-0277.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of Alcohol Abuse Cross-Fostering Analysis of Adopted Men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Crean J, Richards JB, de Wit H. Effect of tryptophan depletion on impulsive behavior in men with or without a family history of alcoholism. Behav Brain Res. 2002;136:349–357. doi: 10.1016/s0166-4328(02)00132-8. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A. Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clin Psychol Rev. 2011;31:965–982. doi: 10.1016/j.cpr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Breakpoint Determination and Ethanol Self-Administration Using an Across-Session Progressive Ratio Procedure in the Rat. Alcohol Clin Exp Res. 1999;23(10):1580–1586. [PubMed] [Google Scholar]
- Czachowski CL, Samson HH. Ethanol- and Sucrose-Reinforced Appetitive and Consummatory Responding in HAD1, HAD2, and P Rats. Alcohol Clin Exp Res. 2002;26(11):1653–1661. doi: 10.1097/01.ALC.0000036284.74513.A5. [DOI] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O'Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poorvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, De Vries TJ. Impulsive Choice and Impulsive Action Predict Vulnerability to Distinct Stages of Nicotine Seeking in Rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Dom G, D'Haene P, Sabbe B. Impulsivity in early- and late-onset alcoholics: differences in self-report measures and a discounting task. Addiction. 2006;101:50–59. doi: 10.1111/j.1360-0443.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- Dow SJ, Kelly JF. Listening to Youth: Adolescents' Reasons for Substance Use as a Unique Predictor of Treatment Response and Outcome. Psychol Addict Behav. 2013;27(4):1122–1131. doi: 10.1037/a0031065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Files FJ, Samson HH, Denning CE, Marvin S. Comparison of Alcohol-Preferring and Nonpreferring Selectively Bred Rat Lines. II. Operant Self-Administration in a Continuous-Access Situation. Alcohol Clin Exp Res. 1998;22(9):2147–2158. [PubMed] [Google Scholar]
- Green L, Myerson J, Holt DD, Slevin JR, Estle SJ. Discounting of Delayed Food Rewards and Rats: Is there a Magnitude Effect. 2004;81(1):39–50. doi: 10.1901/jeab.2004.81-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Schwartz D, Mitchell SH, Nagel BJ. Delay Discounting Behavior and White Matter Microstructure Abnormalities in Youth With a Family History of Alcoholism. Alcohol Clin Exp Res. 2010;34(9):1590–1602. doi: 10.1111/j.1530-0277.2010.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Woods Individual differences in discount rate are associated with demand for self-administered cocaine, but not sucrose. Addict Biol. 2011;18:8–18. doi: 10.1111/j.1369-1600.2011.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollins SH. Delay discounting is associated with substance use in college students. Addict Behav. 2003;28:1167–1173. doi: 10.1016/s0306-4603(02)00220-4. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 2011;216(3):305–321. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur J. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. The Effect of Delay and of Intervening Events on Reinforcement Value. Vol. 5. Lawrence Erlbaum; Hillsdale,NJ: 1987. pp. 55–73. Quantitative analysis of behavior series. [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, Boettiger CA. Impulsive Responding in Alcoholics. Alcohol Clin Exp Res. 2005;29(12):2158–2169. doi: 10.1097/01.alc.0000191755.63639.4a. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi H. DSM-IV Criteria Endorsement Patterns in Alcohol Dependence: Relationship to Severity. Alcohol Clin Exp Res. 2008;32(2):306–313. doi: 10.1111/j.1530-0277.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. J Exp Anal Behav. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Eighth. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- Oberlin BG, Grahame NJ. High-Alcohol Preferring Mice Are More Impulsive than Low-Alcohol Preferring Mice as Measured in the Delay Discounting Task. Alcohol Clin Exp Res. 2009;33(7):1294–1303. doi: 10.1111/j.1530-0277.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berl) 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of Gender and Family History of Alcohol Dependence on a Behavioral Task of Impulsivity in Healthy Subjects. J Stud Alcohol. 2002;63(1):83–90. [PubMed] [Google Scholar]
- Richards JB, Mitchell SH, de Wit H, Seiden LS. Determination of Discount Functions in Rats with an Adjusting- Amount Procedure. J Exp Anal Behav. 1997;67:353–366. doi: 10.1901/jeab.1997.67-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH. Initiation of Ethanol Reinforcement using a Sucrose Substitution Procedure in Food- and Water-Sated Rats. Alcohol Clin Exp Res. 1986;10(4):436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic Temporal Discounting in Social Drinkers and Problem Drinkers. Exp Clin Psychopharmacol. 1998;6(3):292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Acute Ethanol Does Not Always Affect Delay Discounting in Rats Selected to Prefer or Avoid Ethanol. Alcohol Alcohol. 2012;47(5):518–524. doi: 10.1093/alcalc/ags059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse Lines Selected for Alcohol Consumption Differ on Certain Measures of Impulsivity. Alcohol Clin Exp Res. 2007;31(11):1839–1845. doi: 10.1111/j.1530-0277.2007.00508.x. [DOI] [PubMed] [Google Scholar]