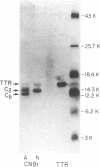
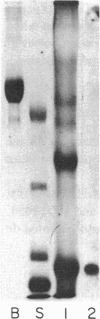
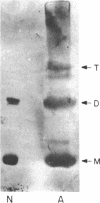
Abstract
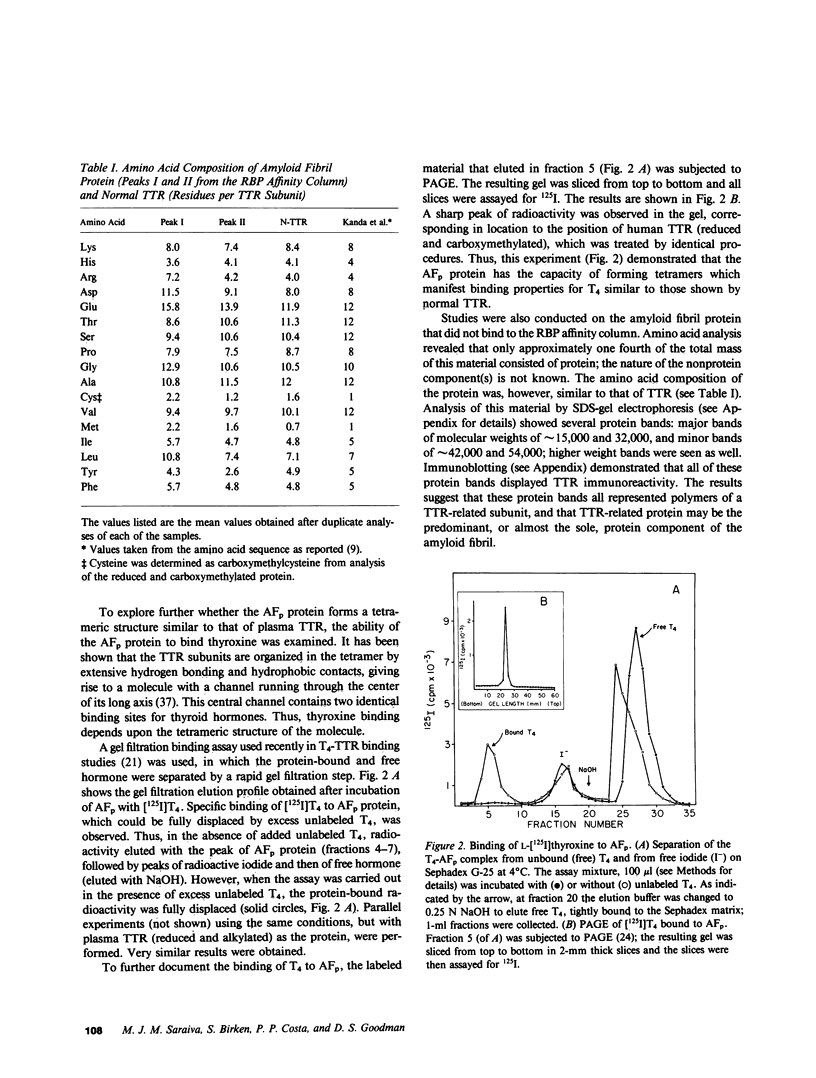
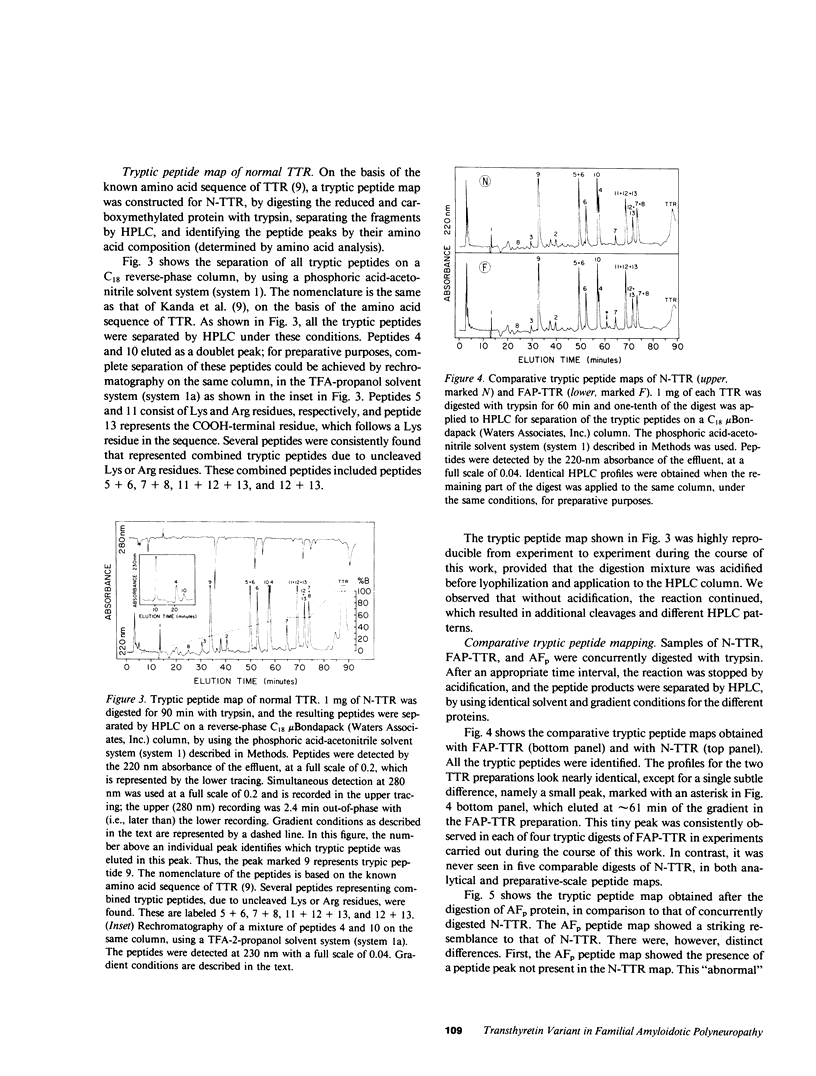
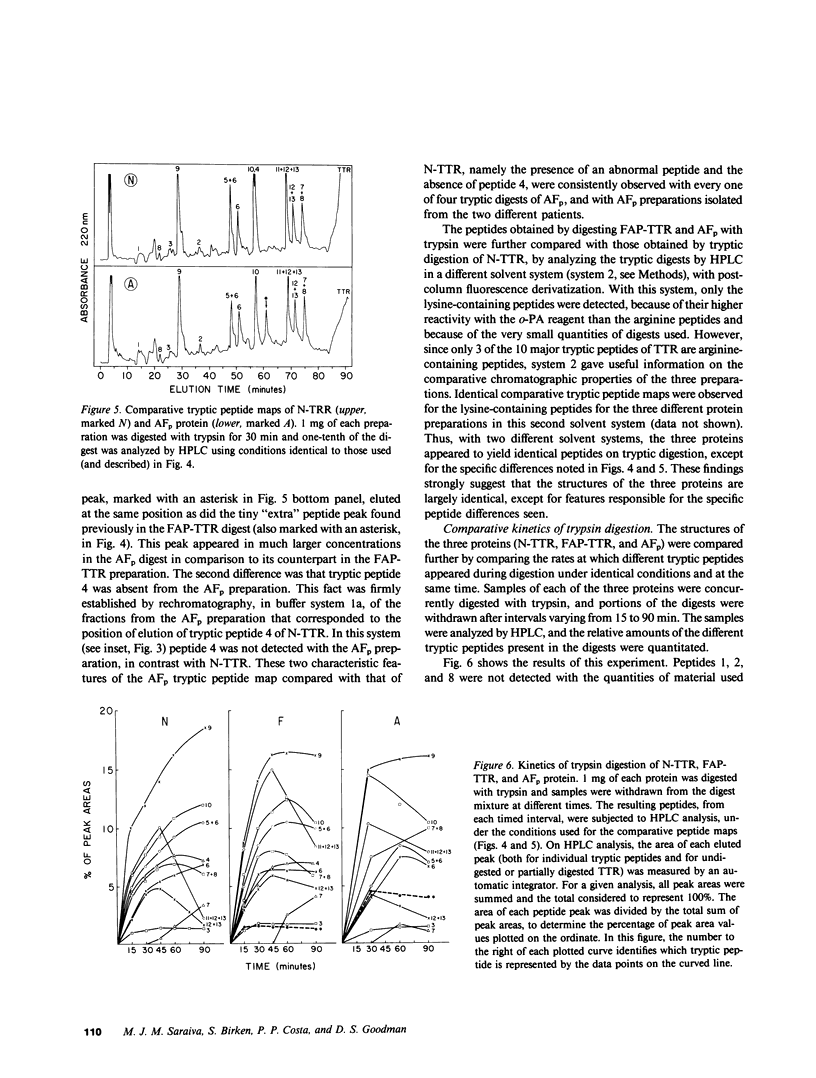
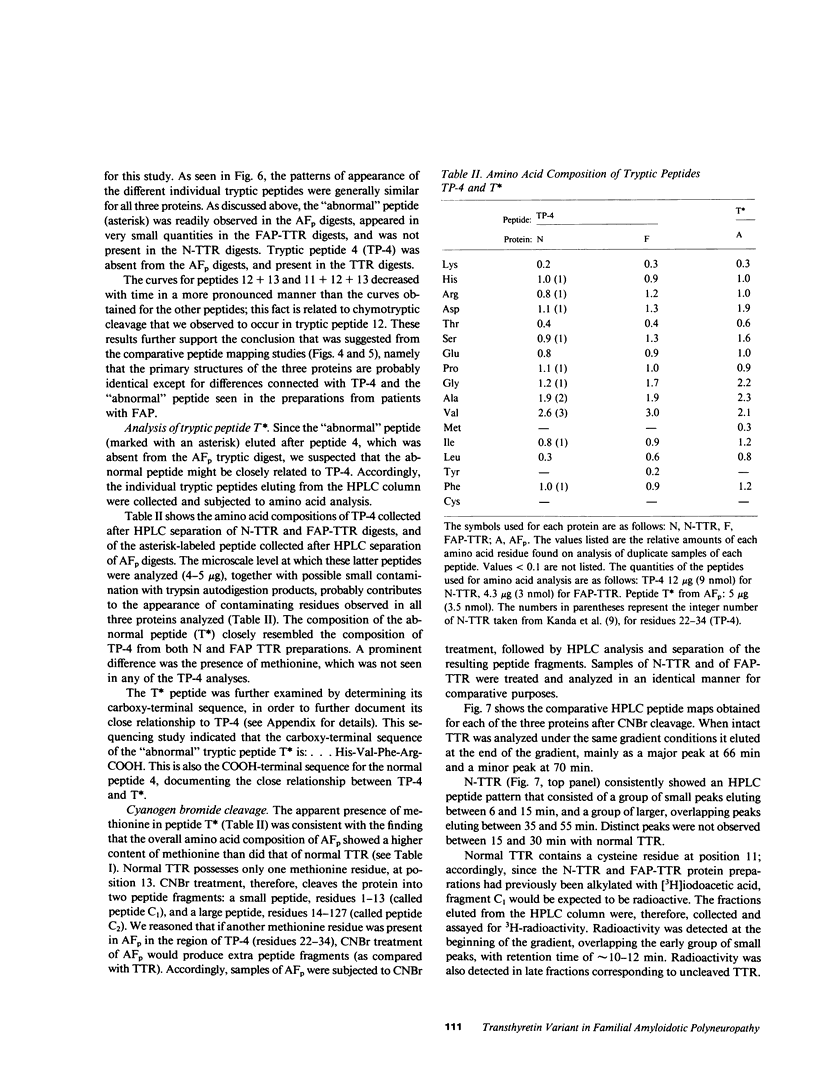
Amyloid fibril protein in patients with familial amyloidotic polyneuropathy is known to be chemically related to transthyretin (TTR), the plasma protein that is usually referred to as prealbumin. A genetically abnormal TTR may be involved in this disease. Studies were conducted on amyloid fibril protein (AFp) isolated from tissues of two Portuguese patients who died with familial amyloidosis, and on TTR isolated from sera of patients with this disease. AFp, purified by affinity chromatography on retinol-binding protein linked to Sepharose, resembled plasma TTR in forming a stable tetrameric structure, and in its binding affinities for both thyroxine and retinol-binding protein. The structural studies included: (a) comparative peptide mappings by reverse-phase high performance liquid chromatography (HPLC) after trypsin digestion; (b) cyanogen bromide cleavage studies; and (c) amino acid microsequence analysis of selected tryptic and CNBr peptides. On the basis of the known amino acid sequence of TTR, comparative tryptic peptide maps showed the presence of a single aberrant tryptic peptide (peptide 4, residues 22-34) in AFp as compared with TTR. This aberrant peptide contained a methionine residue, not present in normal tryptic peptide 4. CNBr cleavage of AFp produced two extra peptide fragments, which were demonstrated, respectively, by HPLC analysis and by sodium dodecyl sulfate-gel electrophoresis. Sequence analyses indicated the presence of a methionine-for-valine substitution at position 30 in AFp as compared with TTR. Thus, the purified amyloid fibril protein comprised a TTR variant with a methionine-forvaline substitution at position 30. A single nucleotide change in a possible codon for valine 30 could explain the substitution. The variant TTR was also present in the TTR isolated from the pooled sera of amyloidoses patients, together with larger (four- to six-fold) amounts of the normal TTR. Thus, in these patients, the variant TTR was circulating in plasma, along with larger amounts of normal TTR. We suggest that the variant TTR represents the specific biochemical cause of the disease, and that this abnormal form of TTR selectively deposits in tissues as the amyloid characteristic of the disease.
Full text
PDF


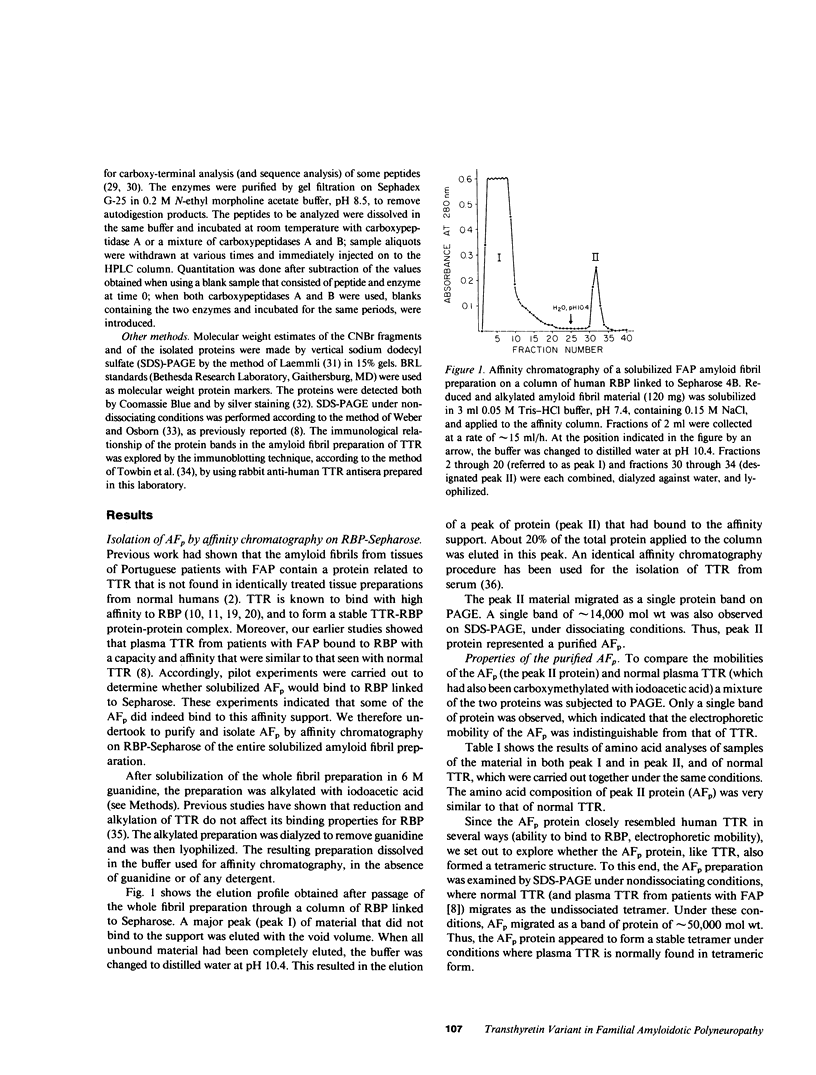
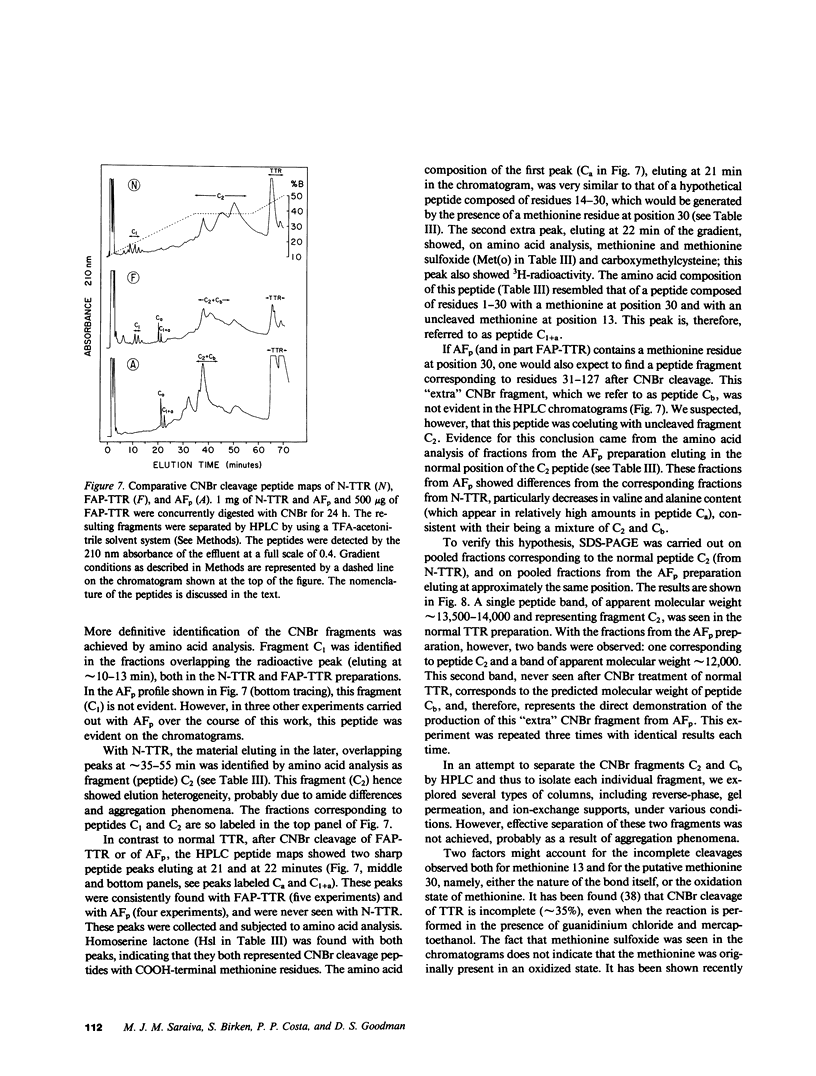
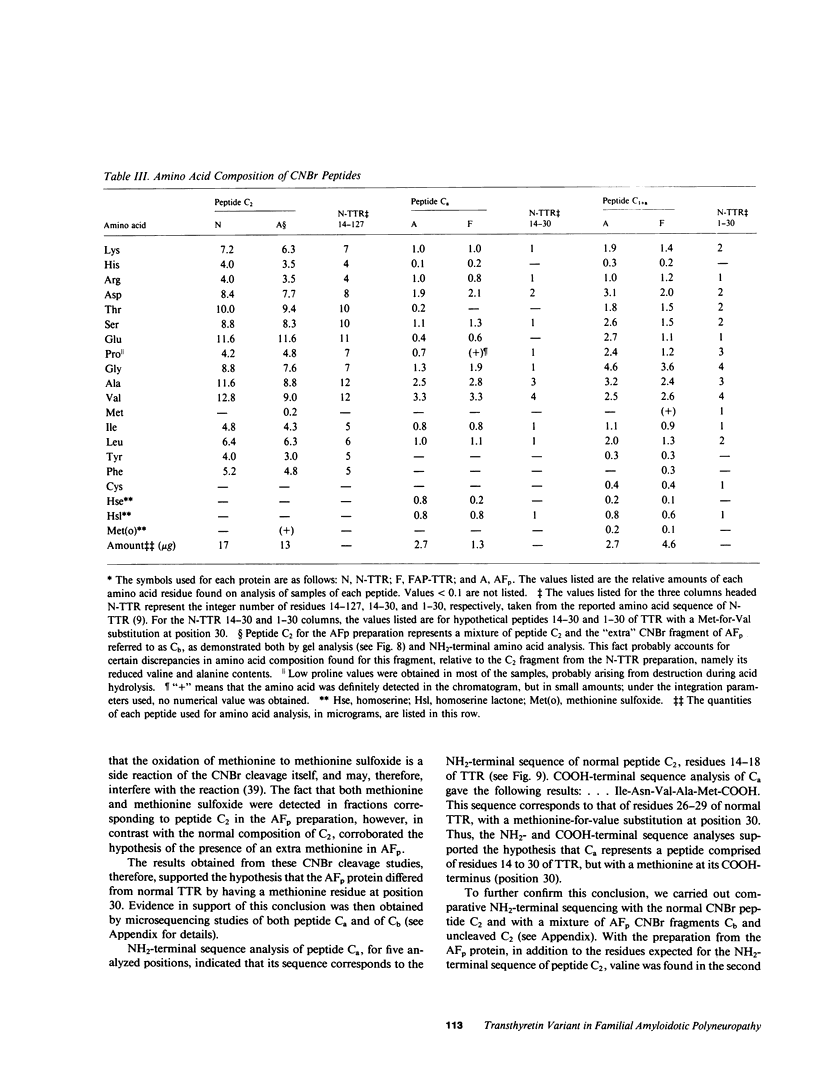
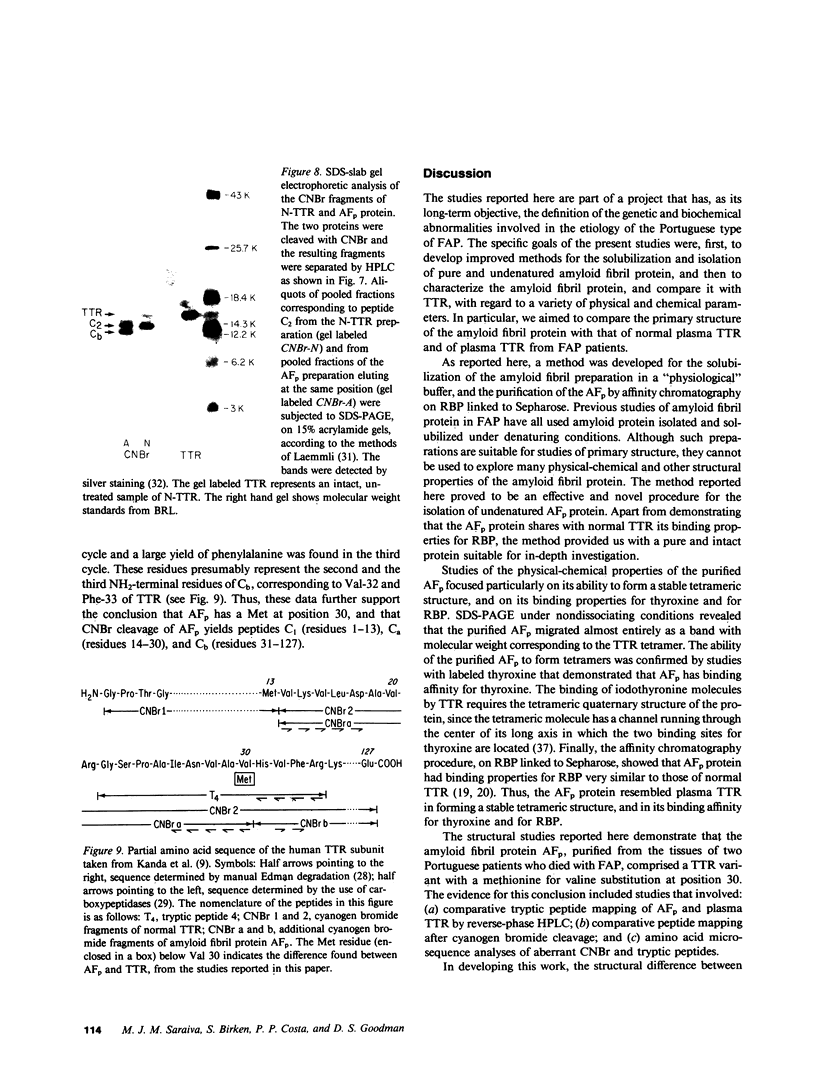


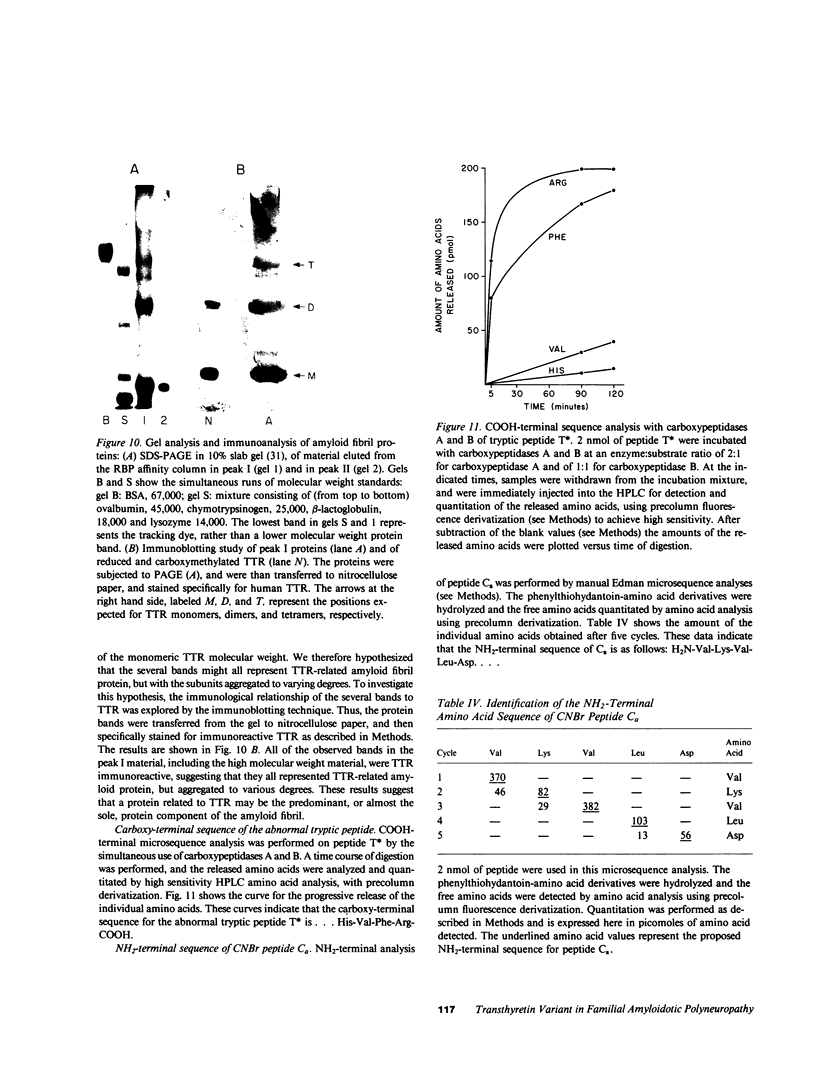

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRADE C. A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain. 1952 Sep;75(3):408–427. doi: 10.1093/brain/75.3.408. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Robin N. I., Refetoff S. Genetic polymorphism of rhesus thyroxine-binding prealbumin: evidence for tetrameric structure in primates. Proc Natl Acad Sci U S A. 1969 Jul;63(3):775–781. doi: 10.1073/pnas.63.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Benson M. D. Partial amino acid sequence homology between an heredofamilial amyloid protein and human plasma prealbumin. J Clin Invest. 1981 Apr;67(4):1035–1041. doi: 10.1172/JCI110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake C. C., Geisow M. J., Oatley S. J., Rérat B., Rérat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. J Mol Biol. 1978 May 25;121(3):339–356. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- Carvalho J., Coimbra A., Andrade C. Peripheral nerve fibre changes in asymptomatic children of patients with familial amyloid polyneuropathy. Brain. 1976 Mar;99(1):1–10. doi: 10.1093/brain/99.1.1. [DOI] [PubMed] [Google Scholar]
- Cheng S. Y., Wilchek M., Cahnmann H. J., Robbins J. Affinity labeling of human serum prealbumin with N-bromoacetyl-L-thyroxine. J Biol Chem. 1977 Sep 10;252(17):6076–6081. [PubMed] [Google Scholar]
- Costa P. P., Figueira A. S., Bravo F. R. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4499–4503. doi: 10.1073/pnas.75.9.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakas M. C., Engel W. K. Amyloid in hereditary amyloid polyneuropathy is related to prealbumin. Arch Neurol. 1981 Jul;38(7):420–422. doi: 10.1001/archneur.1981.00510070054008. [DOI] [PubMed] [Google Scholar]
- Dwulet F. E., Benson M. D. Polymorphism of human plasma thyroxine binding prealbumin. Biochem Biophys Res Commun. 1983 Jul 29;114(2):657–662. doi: 10.1016/0006-291x(83)90831-8. [DOI] [PubMed] [Google Scholar]
- Eanes E. D., Glenner G. G. X-ray diffraction studies on amyloid filaments. J Histochem Cytochem. 1968 Nov;16(11):673–677. doi: 10.1177/16.11.673. [DOI] [PubMed] [Google Scholar]
- Gawinowicz M. A., Goodman D. S. Retinoid affinity label for the binding site of retinol-binding protein. Biochemistry. 1982 Apr 13;21(8):1899–1905. doi: 10.1021/bi00537a030. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Cuatrecasas P., Isersky C., Bladen H. A., Eanes E. D. Physical and chemical properties of amyloid fibers. II. Isolation of a unique protein constituting the major component from human splenic amyloid fibril concentrates. J Histochem Cytochem. 1969 Dec;17(12):769–780. doi: 10.1177/17.12.769. [DOI] [PubMed] [Google Scholar]
- Hill D. W., Walters F. H., Wilson T. D., Stuart J. D. High performance liquid chromatographic determination of amino acids in the picomole range. Anal Chem. 1979 Jul;51(8):1338–1341. doi: 10.1021/ac50044a055. [DOI] [PubMed] [Google Scholar]
- Joppich-Kuhn R., Corkill J. A., Giese R. W. Oxidation of methionine to methionine sulfoxide as a side reaction of cyanogen bromide cleavage. Anal Biochem. 1982 Jan 1;119(1):73–77. doi: 10.1016/0003-2697(82)90666-2. [DOI] [PubMed] [Google Scholar]
- Kanai M., Raz A., Goodman D. S. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968 Sep;47(9):2025–2044. doi: 10.1172/JCI105889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda Y., Goodman D. S., Canfield R. E., Morgan F. J. The amino acid sequence of human plasma prealbumin. J Biol Chem. 1974 Nov 10;249(21):6796–6805. [PubMed] [Google Scholar]
- Kanda Y., Goodman D. S. Partial amino acid sequence of human plasma retinol-binding protein. Isolation and alignment of the five cyanogen bromide fragments and the amino acid sequences of four of the fragments. J Lipid Res. 1979 Sep;20(7):865–878. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy W. P. Manual Edman sequencing techniques for proteins and peptides at the nanomole level. Methods Enzymol. 1981;79(Pt B):27–31. doi: 10.1016/s0076-6879(81)79010-4. [DOI] [PubMed] [Google Scholar]
- Morgan F. J., Canfield R. E., Goodman D. S. The partial structure of human plasma prealbumin and retinol-binding protein. Biochim Biophys Acta. 1971 Jun 29;236(3):798–801. doi: 10.1016/0005-2795(71)90267-4. [DOI] [PubMed] [Google Scholar]
- Navab M., Mallia A. K., Kanda Y., Goodman D. S. Rat plasma prealbumin. Isolation and partial characterization. J Biol Chem. 1977 Jul 25;252(14):5100–5106. [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Peterson P. A. Studies on the interaction between prealbumin, retinol-binding protein, and vitamin A. J Biol Chem. 1971 Jan 10;246(1):44–49. [PubMed] [Google Scholar]
- Pras M., Franklin E. C., Prelli F., Frangione B. A variant of prealbumin from amyloid fibrils in familial polyneuropathy of Jewish origin. J Exp Med. 1981 Sep 1;154(3):989–993. doi: 10.1084/jem.154.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras M., Prelli F., Franklin E. C., Frangione B. Primary structure of an amyloid prealbumin variant in familial polyneuropathy of Jewish origin. Proc Natl Acad Sci U S A. 1983 Jan;80(2):539–542. doi: 10.1073/pnas.80.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A., Goodman D. S. The interaction of thyroxine with human plasma prealbumin and with the prealbumin-retinol-binding protein complex. J Biol Chem. 1969 Jun 25;244(12):3230–3237. [PubMed] [Google Scholar]
- Raz A., Shiratori T., Goodman D. S. Studies on the protein-protein and protein-ligand interactions involved in retinol transport in plasma. J Biol Chem. 1970 Apr 25;245(8):1903–1912. [PubMed] [Google Scholar]
- Roth M. Fluorescence reaction for amino acids. Anal Chem. 1971 Jun;43(7):880–882. doi: 10.1021/ac60302a020. [DOI] [PubMed] [Google Scholar]
- Saraiva M. J., Costa P. P., Goodman D. S. Studies on plasma transthyretin (prealbumin) in familial amyloidotic polyneuropathy, Portuguese type. J Lab Clin Med. 1983 Oct;102(4):590–603. [PubMed] [Google Scholar]
- Skinner M., Cohen A. S. The prealbumin nature of the amyloid protein in familial amyloid polyneuropathy (FAP)-swedish variety. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1326–1332. doi: 10.1016/0006-291x(81)90764-6. [DOI] [PubMed] [Google Scholar]
- Somack R., Andrea T. A., Jorgensen E. C. Thyroid hormone binding to human serum prealbumin and rat liver nuclear receptor: kinetics, contribution of the hormone phenolic hydroxyl group, and accommodation of hormone side-chain bulk. Biochemistry. 1982 Jan 5;21(1):163–170. doi: 10.1021/bi00530a028. [DOI] [PubMed] [Google Scholar]
- Stein S., Moschera J. High-performance liquid chromatography and picomole-level detection of peptides and proteins. Methods Enzymol. 1981;79(Pt B):7–16. doi: 10.1016/s0076-6879(81)79006-2. [DOI] [PubMed] [Google Scholar]
- Tawara S., Araki S., Toshimori K., Nakagawa H., Ohtaki S. Amyloid fibril protein in type I familial amyloidotic polyneuropathy in Japanese. J Lab Clin Med. 1981 Dec;98(6):811–822. [PubMed] [Google Scholar]
- Termine J. D., Eanes E. D., Ein D., Glenner G. G. Infrared spectroscopy of human amyloid fibrils and immunoglobulin proteins. Biopolymers. 1972;11(5):1103–1113. doi: 10.1002/bip.1972.360110512. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Jaarsveld P., Branch W. T., Robbins J., Morgan F. J., Kanda Y., Canfield R. E. Polymorphism of Rhesus monkey serum prealbumin. Purification and partial structure. J Biol Chem. 1973 Nov 25;248(22):7898–7903. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilson J. M., Kobayashi R., Fox I. H., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. J Biol Chem. 1983 May 25;258(10):6458–6460. [PubMed] [Google Scholar]
- van Jaarsveld P. P., Edelhoch H., Goodman D. S., Robbins J. The interaction of human plasma retinol-binding protein and prealbumin. J Biol Chem. 1973 Jul 10;248(13):4698–4705. [PubMed] [Google Scholar]