Abstract
In mice the chemokine Cxcl12 and its receptor Cxcr4 participate in maintenance of the spermatogonial population during postnatal development. More complexity arises since Cxcl12 also binds to the non-classical/atypical chemokine receptor Cxcr7. We explored the expression pattern of Cxcl12, Cxcr4 and Cxcr7 during postnatal development in mouse testes and investigated the response of Cxcl12, Cxcr4, Cxcr7 and SSC-niche associated factors to busulfan-induced germ cell depletion and subsequent recovery by RNA expression analysis and localization of the proteins. In neonatal testes transcript levels of Cxcl12, Cxcr4 and Cxcr7 were relatively low and protein expression of Cxcr7 was restricted to gonocytes and spermatogonia. During development, RNA expression of Cxcl12 remained stable but that of Cxcr4 and Cxcr7 increased. Cxcr7 was expressed in germ cells located at the basement membrane of the seminiferous tubules. In adult testes, transcript levels of Cxcl12 were highest while the localization of Cxcr7 did not change. Following germ cell depletion, a significantly increased expression of Cxcl12 and a decreased expression of Cxcr7 were observed. Germ cells repopulating the seminiferous tubules were immunopositive for Cxcr7. We conclude that Cxcr7 expression to be restricted to premeiotic germ cells throughout postnatal testicular development and during testicular recovery. Hence, the spermatogonial population may not only be simply controlled by interaction of Cxcl12 with Cxcr4 but may also involve Cxcr7 as an important player.
Introduction
In mammalian testes, the unipotent spermatogonial stem cells (SSCs) reside within specialized microenvironments called ‘niches’, essential for the regulation of stem cell self-renewal and differentiation. The Sertoli cells, forming the blood-testis barrier (BTB), and the basal lamina are the structural components of this SSC niche [1]–[3]. Sertoli, peritubular as well as Leydig cells provide extrinsic factors that are essential for migration, stem cell retention, and for the regulation of SSC functions [4]–[9]. Amongst such factors are the colony stimulating factor-1 (Csf-1) produced by Leydig cells as well as the glial cell-line-derived neurotrophic factor (Gdnf), which is secreted by Sertoli cells from birth through adulthood. Gdnf regulates the proliferation and survival of undifferentiated spermatogonia [9]–[12].
Interestingly, recent studies have revealed that the chemokine (C-X-C motif) ligand 12 (Cxcl12) is involved in the postnatal maintenance of the SSC pool in mouse testes [13]–[15]. While the chemokine is a product of the Sertoli cells only, the C–X-C chemokine receptor type 4 (Cxcr4) is expressed by spermatogonia, Sertoli and interstitial cells [14]–[16]. The functional role of the Cxcl12/Cxcr4 interaction within the adult mouse testis has been investigated in vitro and it was suggested that the ligand/receptor pair is involved in SSC propagation but prevents SSC differentiation [15]. In addition, it has been experimentally shown by germ cell transplantation experiments, that in congenitally infertile W/Wv mouse testes an increased expression of Cxcl12 by Sertoli cells leads to an elevated/enhanced colonization of seminiferous tubules by Cxcr4 positive SSCs. Based on these studies it was concluded that the Cxcr4 mediated action of Cxcl12 also plays a role for the homing and colonization process of SSCs into their niches [14], [15]. The stimulation of colonization and migration of stem cells into their niches via Cxcr4 is also known to play a role during hematopoiesis [17], [18]. Furthermore, the interaction between Cxcl12 and Cxcr4 is also required for the migration as well as for the maintenance of primordial germ cells (PGCs) in mice [19], [20].
Moreover, the C–X-C chemokine receptor type 7 (Cxcr7) was identified in zebrafish as an alternative receptor for Cxcl12 which created more complexity to this rather simple model regarding the regulation of PGC migration [21]–[24]. In contrast to Cxcr4, Cxcr7 belongs to the group of atypical chemokine receptors [25]–[28] and the interaction with Cxcl12 rather results in an internalization of the chemokine without inducing downstream signaling. Consequently, the action of Cxcr7 rather results in the generation of a Cxcl12 gradient thereby facilitating the directed migration of PGCs in zebrafish [21], [22], [29]. So far, transcripts of Cxcr7 were detected in testes from adult rats and humans [30], [31]. More specifically, Eva et al. 1993 reported previously a cloning and sequencing analysis of Cxcr7 (also called Rdc1) in a rat forebrain library and demonstrated mRNA expression of Cxcr7 in lung, kidney and testes of adult rats by northern blot analysis [30]. Twenty years later, McIver et al. 2013 investigated normal and pathological human testes and found that CXCR7 transcripts in seminomas and non-seminomas to be significantly lower compared to the matched control tissue [31]. However, neither the localization nor the function of this receptor during fetal and postnatal mammalian germ cell development has been investigated. Moreover, to date the capacity of the Cxcl12/Cxcr4/Cxcr7 axis to support the re-establishment of spermatogenesis following an induced germ cell loss, remains largely unknown.
In the present study, we investigated the expression pattern of Cxcr7 during postnatal germ cell development in mouse testes. Moreover, we evaluated the response of several niche-associated factors, including the chemokine Cxcl12 as well its receptors Cxcr4 and Cxcr7 to experimental spermatogonial depletion and subsequent repopulation.
Materials and Methods
Ethics statement
NMRI mice at the age of 1, 7, 14, 21 days post-partum (dpp) and adult animals (>37 dpp; between 6 and 21 weeks of age) were purchased from the Central Animal Facility of the Medical Faculty (Muenster, Germany). Mice were maintained under a twelve hours light/dark regime, with pelleted food and water available ad libitum. All experimental protocols in this paper were approved by the regional/state authority “Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen” (State Agency for Nature, Environment and Consumer Protection North Rhine-Westphalia) (animal license No. 87.5104.2010.A244, LANUV NRW, Germany).
All procedures on mice were performed in accordance with the granted procedures and 3R guidelines optimizing animal welfare. The busulfan treatment of mice was performed as outlined below in the animal protocol to minimize suffering. Mice were sacrificed by cervical dislocation under KX anaesthesia (ketamine/xylazine solution in saline; i.p.; Sigma Aldrich, K-113, Steinheim, Germany).
Tissue collection for characterization of Cxcl12 and its receptors Cxcr4/Cxcr7 during testicular development
Mice aged 1 (n = 3), 7, 14, 21 dpp (n = 4 per age group) and adult animals (n = 3; between 19 and 21 weeks of age) were sacrificed. One testis from each animal was weighted (testis weights increased gradually from 1.3±0.5 mg on day 1 to 111.8±15.2 mg on day>37 (Fig. 1A)) and snap-frozen for RNA expression analysis, whereas the other testis was fixed for 6 hrs at room temperature (RT) in 4% paraformaldehyde (PFA) for immunohistochemical analysis.
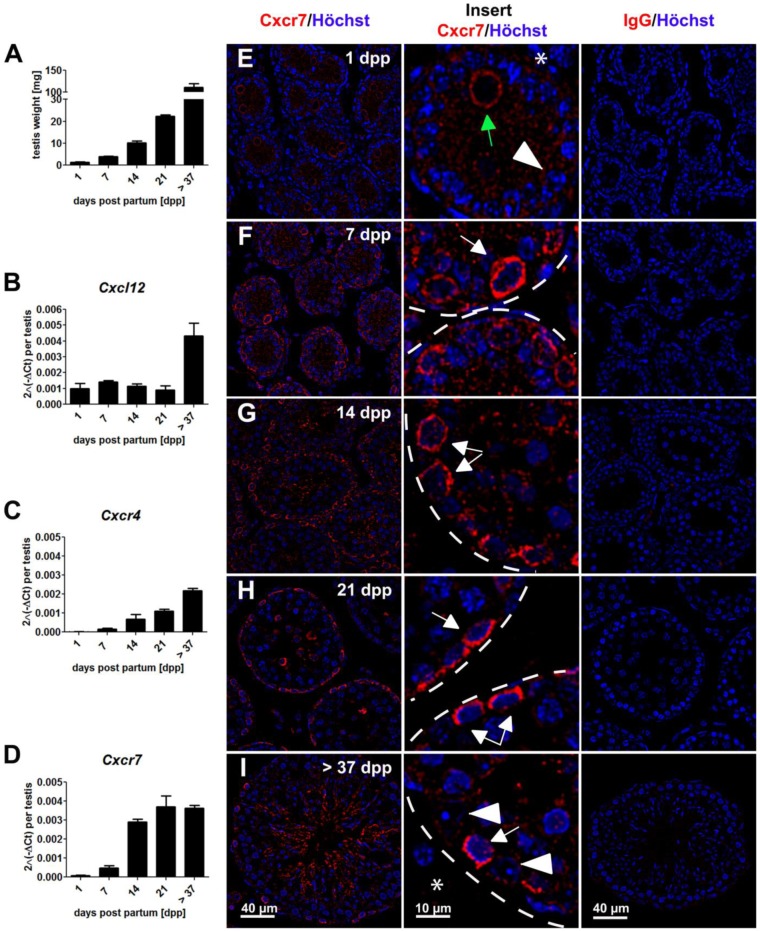
Figure 1. Expression pattern of Cxcr7 during testicular germ cell development.
Changes in testicular weight (A) as well as changes in transcript levels of Cxcl12 (B), Cxcr4 (C) and Cxcr7 (D) during postnatal development in mouse testes ((1dpp and <37dpp (n = 3); 7dpp, 14 dpp, 21 dpp (n = 4)), 2 (−ΔCt) per testis, relative to luciferase). Results are shown as mean ± SD. Representative images showing immunofluorescence stainings for Cxcr7 (red) and Hoechst (blue) on days 1, 7, 14, 21,>37 of postnatal testicular development (first column, E–I). Within the inserts (second column) expression of Cxcr7 was observed in gonocytes (green arrow), spermatogonia (white arrows) and interstitial cells (asterisks). Sertoli cells are indicated with white arrowheads. Results of the respective negative controls using nonspecific IgG antibodies are shown in the right column (IgG/Hoechst). Scale bars represent 10 µm and 40 µm, respectively.
Gonadotoxic treatment
Butane-1,4-diyl dimethanesulfonate (busulfan) was used as a cytotoxic agent to induce germ cell loss in the testes of adult mice [32], [33]. Busulfan powder (Sigma Aldrich, B2635, Steinheim, Germany) was dissolved in dimethylsulfoxid (DMSO, Sigma Aldrich, D5879, Steinheim, Germany). Adult male NMRI mice (6 weeks old) were either given a single injection (i.p.) of DMSO (vehicle control group, n = 50) or of busulfan at a concentration of 38 mg/kg (treated group, n = 50). On days 1, 3, 7, 21 and 28 post treatments, ten animals per time point from both groups were killed as described above and evaluated. In addition, to control for potential DMSO effects, eight mice received a single injection (i.p.) of 0.9% saline (sham control group) and were killed 24 hrs later. Sham-treated NMRI mice had a mean testis weight of 96±4.5 mg. DMSO treatment alone had no apparent effect on the testicular weight, however, a significant reduction was detected on days 21 (57.0±13.6 mg) and 28 (41.6±3.6 mg) after busulfan treatment (Fig. S1).
Tissue collection following gonadotoxic treatment
Following treatment, mice were sacrificed and one testis from each animal was weighed and snap-frozen for RNA expression analysis, whereas the other testis was divided into two parts, one of which was fixed in Bouin's solution and the other in 4% PFA for periodic acid Schiff's (PAS) staining and immunohistochemical analysis. While busulfan treatment was effective in the majority of animals, two mice showed no decrease in testicular weight and histological analysis revealed normal spermatogenesis. Therefore, these animals were excluded from the study.
RNA isolation and relative gene expression analysis
Per testis sample, 20 ng luciferase mRNA (Promega, L4561, Mannheim, Germany) were added [32], before RNA extraction was performed using Ultraspec (Biotecx, Houston, USA) and genomic DNA was removed by DNase treatment (DNA-free Kit, Ambion, Darmstadt, Germany). Using 1 µg of total RNA, cDNA was generated employing SuperScript II Reverse Transcriptase (Invitrogen, Darmstadt, Germany) and random hexamer primers (Promega, Mannheim, Germany).
Quantitative real-time PCR (qRT-PCR) analysis was performed using SYBR Green technology. For relative quantification of SSC marker genes and niche-associated factors, the following genes were selected based on the current literature (Table S1) Lin28a (undifferentiated spermatogonia), Ddx4 (general germ cell marker), Cxcl12, Gdnf, Amh (Sertoli cells), Erm, Itgb1, Cxcr4 (Sertoli cells and spermatogonia) and Cxcr7 (to be determined). Specific primers were designed using Primer Express 3.0 Software. Optimal primer concentrations, primer specificity and PCR efficiency were evaluated following the Power SYBR Green PCR User Guide (Applied Biosystems, Darmstadt, Germany). Primer sequences, localization of proteins within the mouse testis and the respective references are summarized in Table S1. For qRT-PCR analyses, cDNA was diluted 1∶10 and 2 µl were used for each 20 µl PCR reaction with Power SYBR Green Mastermix (Applied Biosystems, Darmstadt, Germany). The PCR programme consisted of initial steps of activation and denaturation which were run once for 10 min at 95°C, followed by 40 cycles of denaturation (15 sec at 95°C), annealing and elongation (1 min at 60°C). qRT-PCRs were run on the StepOnePlus (Applied Biosystems, Darmstadt, Germany) and were subsequently analyzed using the StepOne software 2.2 (Applied Biosystems, Darmstadt, Germany).
To calculate relative expression levels of Cxcr7, Cxcr4 and Cxcl12 during postnatal germ cell development in mice, the 2 (-ΔCT) method was applied [34]. Rather than using an internal reference gene, luciferase was employed as an external standard, facilitating the calculation of expression levels per testis, as previously described [32]. Therefore, this approach allowed for a direct comparison of expression levels between tissues of different weights and cellular composition.
Following gonadotoxic treatments, the 2 (-ΔΔCT) method was applied [34] using luciferase as external standard and sham control animals (n = 8) as calibrator. As Leydig and Sertoli cell numbers are not affected by busulfan and DMSO treatment, no further corrections regarding the transcript levels per testis were required [32].
Histological analyses and repopulation index
Testes fixed in Bouin's solution were transferred into 70% ethanol the next day, whereas PFA fixed tissues were placed into 30%, 50% and 70% ethanol (30 min each), respectively. Routine paraffin-embedding was performed and testes were sectioned at 3 µm using a Leica SM2000R microtome (Leica, Wetzlar, Germany). For histological evaluation, sections were stained using PAS staining followed by haematoxylin counterstaining [35].
Following gonadotoxic treatment and germ cell depletion, the repopulation index (RI) was determined on day 28 after busulfan treatment (RI28d, n = 4). In each testicular section, 25 seminiferous tubules were evaluated for the presence of germ cells and based on these results the percentage of seminiferous tubules showing germ cells that had reached the spermatogonial stage or later was determined [36].
Immunohistochemical stainings on testicular tissue sections
Paraffin was removed from sections using Pro Taqs Clear (Pro Taqs Clear, Cat. No. 4003011, Quartett Immunodiagnostika & Biotechnologie, Berlin, Germany) and sections were subsequently rehydrated in a decreasing ethanol series. After rinsing with distilled water and Tris-buffered saline (TBS), sections were placed into citrate buffer (pH 6) and were heated in the microwave for 12 min. After cooling to RT, sections were washed in TBS and non-specific peroxidases were blocked with 3% (v/v) H2O2 for 15 min at RT. In order to block non-specific binding sites, sections were incubated with 25% chicken serum in TBS containing 0.5% (w/v) bovine serum albumin (BSA) for 30 min at RT. Subsequently, primary antibodies against LIN28a (rabbit polyclonal anti-LIN28a, A177 Cell Signaling, dilution of 1∶50, Darmstadt, Germany) and DDX4 (rabbit polyclonal anti-DDX4, ab-13840 Abcam, dilution of 1∶200, Wiesbaden, Germany) were applied and sections were incubated in a humid chamber at 4°C overnight. Incubation with the corresponding immunoglobulin G (IgG) fractions served as negative controls. After three washes in TBS, primary antibodies were detected using secondary antibodies labeled with biotin and tertiary antibodies with a streptavidin-conjugated horseradish peroxidase (S5512, Sigma-Aldrich, Steinheim, Germany). Finally, staining was visualized using 3,3′-diaminobenzidine as chromogen (D4168, Sigma-Aldrich, Steinheim, Germany), and hematoxylin as counterstain.
Immunofluorescence stainings on testicular tissue sections
Sections were dewaxed and rehydrated as described above. After rinsing with distilled water and phosphate-buffered saline (PBS), sections were incubated with 25% goat serum in TBS containing 5% (w/v) bovine serum albumin (BSA) for 30 min at RT. Subsequently, primary antibodies against CXCR7 (rabbit polyclonal anti-CXCR7, Abcam, dilution of 1∶400, Cambridge, UK), CXCR4 (rat monoclonal anti-CXCR4, R&D System, dilution of 1∶20, Wiesbaden, Germany), LIN28a (rabbit polyclonal anti-LIN28a, A177 Cell Signaling, dilution of 1∶50, Darmstadt, Germany) and SALL4 (mouse monoclonal anti-SALL4, Abcam, dilution of 1∶150, Cambridge, UK) were applied and sections were incubated in a humid chamber at 4°C for 60 min. The specificity of the anti-CXCR7 antibody was confirmed immunohistochemically using peptide competition (Fig. S2A–D). For this, the human GPCR RDC1 peptide was applied in a ratio of 1∶1 with the anti-CXCR7 antibody to testicular tissue sections (14 dpp, incubation 1 hr at RT, Abcam, Cambridge, UK). Incubation with corresponding immunoglobulin G (IgG) fractions was used as negative control. Following two PBS washing steps the appropriate Alexa fluor 488-linked or 546-linked secondary antibodies, diluted in TBS/5% BSA, were applied for 45 min at RT in the dark. Cells were counter-stained with Hoechst for visualization of nuclei (33258, Sigma-Aldrich, Steinheim, Germany).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, USA). For the gonadotoxic treatment study, significant differences were determined using the Mann-Whitney test and significance levels are indicated as *, P = 0.01 to 0.05; **, P = 0.001 to 0.01 or ***, P<0.001.
Results
Cxcl12, Cxcr7 and Cxcr4 expression during testicular germ cell development in mice
Transcript levels of the chemokine Cxcl12 per testis remained constant from birth until day 21 and increased only in adult animals (4-fold, Fig. 1B). mRNA levels of the chemokine receptors Cxcr4 (Fig. 1C) and Cxcr7 (Fig. 1D) were low at birth and increased on day 21 pp and in adult animals (>37 days dpp), respectively.
Immunofluorescent staining revealed that gonocytes were positive for Cxcr7 on day 1 pp (Fig. 1E, Insert). On days 7 (Fig. 1F), 14 pp (Fig. 1G) and 21 pp (Fig. 1H), germ cells located at the basement membrane were positive for the receptor Cxcr7. Additionally, on days 14 and 21 pp differentiated germ cells were also Cxcr7-positive. Finally, in testes at 37 dpp (Fig. 1I), some interstitial cells and germ cells at the basement membrane as well as spermatids stained positive for Cxcr7. The cellular distribution pattern of Cxcr7 during testicular germ cell development in mouse testes is summarized in Table S2. Double immunohistochemical stainings showed that Cxcr7 and Sall4 co-localize and are expressed in spermatogonia on days 7 (Fig. S2E–G), 14 (Fig. S2I–K) and>37 pp (Fig. S2M–O).
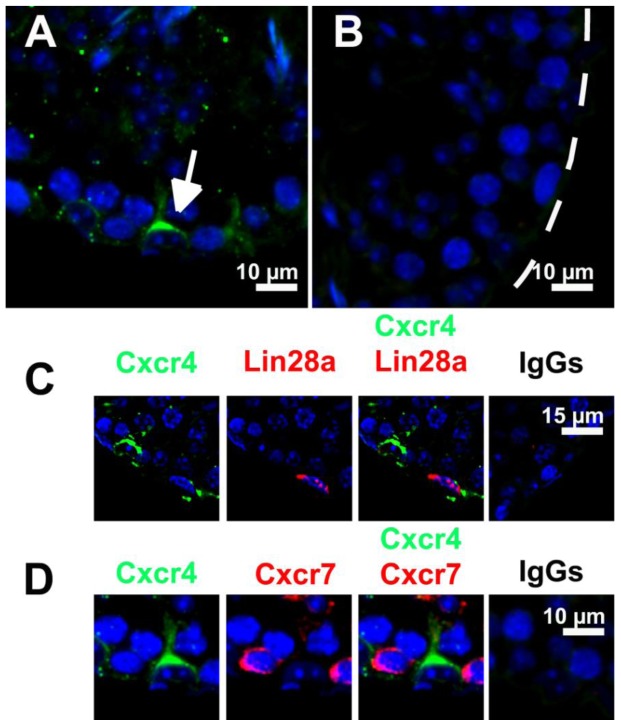
In testes of adult mice, the chemokine receptor Cxcr4 was detected in testicular cells located at the basement membrane (Fig. 2). Some interstitial cells were also Cxcr4-positive (data not shown). Neither Lin28a-positive (Fig. 2C) nor Cxcr7-positive (Fig. 2D) spermatogonia co-expressed Cxcr4.
Figure 2. Cxcr4 and Cxcr7 expression in adult mouse testes.
Representative images show immunofluorescence staining for Cxcr4 (green, A) in adult mouse testes. Cxcr4 is expressed by testicular cells at the basement membrane (arrow) of seminiferous tubules (indicated by dotted lines). Co-stainings revealed no Cxcr4 expression in Lin28a-positive (C) and Cxcr7-positive (D) spermatogonia. Incubation with corresponding IgG antibodies was used as negative control (B, C and D right column). All sections were counterstained with Hoechst (blue). Scale bars represent 10 and 15 µm, respectively.
Histological analysis after busulfan treatment
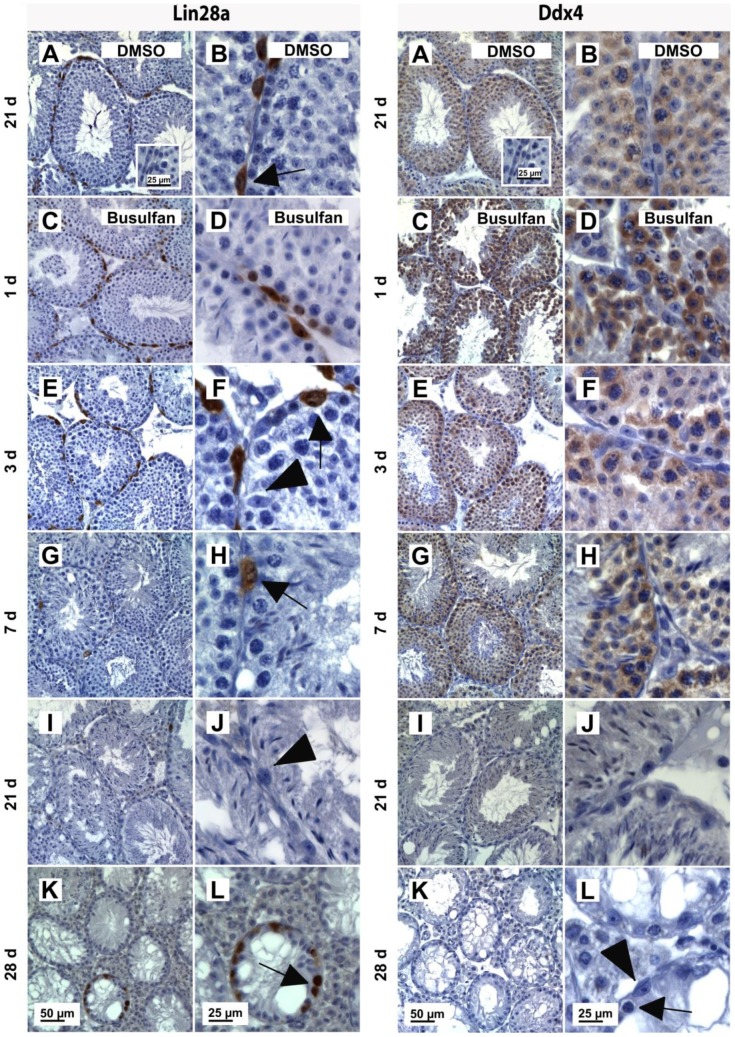
Histological analysis of PAS stained testicular tissue (Fig. S3) revealed that the injection of DMSO alone had no apparent effect on the somatic or the germ cell population (Fig. S3A–E). In contrast, busulfan treatment resulted in a progressive germ cell loss (Fig. S3F–O). Immunohistochemical stainings furthermore showed that Lin28a positive undifferentiated spermatogonia were located at the basal membrane of the seminiferous tubules until day 3 after treatment (Fig. 3; left column; C, E; Inserts: D, F). On days 7 and 21 however, the majority of tubules were depleted of Lin28a positive cells (Fig. 3; left column; G and I; Inserts: H and J). Finally, on day 28 after treatment, Lin28a expression was observed in several seminiferous tubules showing repopulation of spermatogonia (Fig. 3; left column; K; Insert: L). Immunohistochemical analyses for Ddx4 showed positive spermatocytes and round spermatids in the testes of busulfan treated mice on days 1, 3 and 7 (Fig. 3; right column; C, E, G; Inserts: D, F and H). 21 days after busulfan treatment, Ddx4 expression was still present in some seminiferous tubules containing residual round spermatids (Fig. 3; right column; I; Insert: J) and after 28 days, very few Ddx4 positive cells were detected (Fig. 3; right column; K; Insert: L). Subsequent quantification of those seminiferous tubules containing spermatogonia 28 days after busulfan treatment revealed a repopulation index of RI28d = 0.2±0.2%.
Figure 3. Expression of Lin28a and Ddx4 in adult mouse testes following busulfan treatment.
Representative images showing immunohistochemical stainings for Lin28a (left column) and Ddx4 (right column) on testis sections of DMSO (A, B) and busulfan treated (C–L) adult mice. For Lin28a and Ddx4 stainings on days 1 (C), 3 (E), 7 (G), 21 (I) and 28 (K) after busulfan treatment higher magnifications are shown for each time point (D, F, H, J, L). Germ cells are indicated by arrows and Sertoli cells by arrow heads. As negative control, stainings with nonspecific IgGs were performed (A; Insert). Scale bars represent 25 µm and 50 µm.
Relative expression of germ cell- and SSC niche-associated factors following cytotoxic treatment
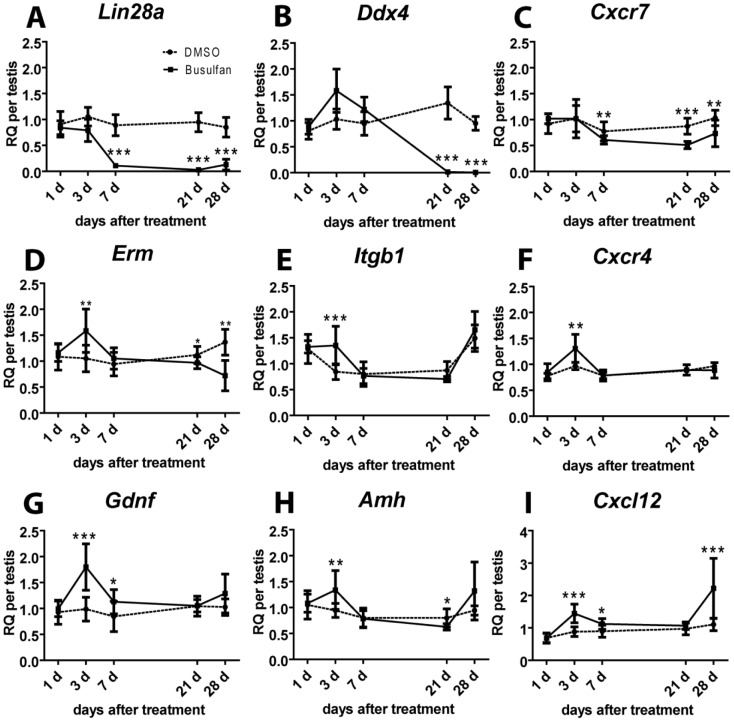
The progressive germ cell loss is reflected at the transcript level. qRT-PCR analyses showed significantly reduced transcript levels of the spermatogonial marker gene Lin28a on days 7, 21 and 28 (Fig. 4A) and the general germ cell marker gene Ddx4 (Fig. 4B) after 21 and 28 days post busulfan treatment compared to DMSO controls. In line with Lin28a and compared to DMSO controls, the transcript levels of Cxcr7 were also significantly lower on days 7 (1.3-fold), 21 (1.7-fold) and 28 (1.4-fold, Fig. 4C) after busulfan treatment. In contrast to Cxcr7 and compared to DMSO controls, transcript levels of Cxcr4 (1.4-fold, Fig. 4F) and of other niche factors (Itgb1∶1.6-fold, Fig. 4E; Gdnf: 1.8-fold, Fig. 4G; Amh: 1.4-fold, Fig. 4H) were significantly higher on day 3. Finally, the mRNA levels of the chemokine Cxcl12 were significantly higher on days 3 (1.6-fold) and 28 (2.0-fold, Fig. 4I) compared to the DMSO controls.
Figure 4. Gene expression patterns of Lin28a, Ddx4 and of SSC niche-associated factors in adult mouse testes after cytotoxic treatments.
Changes in transcript levels of Lin28a (A), Ddx4 (B), Cxcr7 (C), Erm (D), Itgb1 (E), Cxcr4 (F), Gdnf (G), Amh (H) and Cxcl12 (I) on days 1, 3, 7, 21 and 28 after DMSO (•) and busulfan (▪, 38 mg/kg) treatment (n = 10 per time point and treatment). Results were calculated using luciferase as external standard and values from the sham-treated control group (n = 8) as calibrator and are presented as fold change in gene expression per testis (RQ per testis). Significant differences between the DMSO and the busulfan groups are marked with asterisks (*, P = 0.01 to 0.05; **, P = 0.001 to 0.01 or ***, P<0.001).
Cxcr7 and Cxcr4 expression in seminiferous tubules 28 days after gonadotoxic treatment
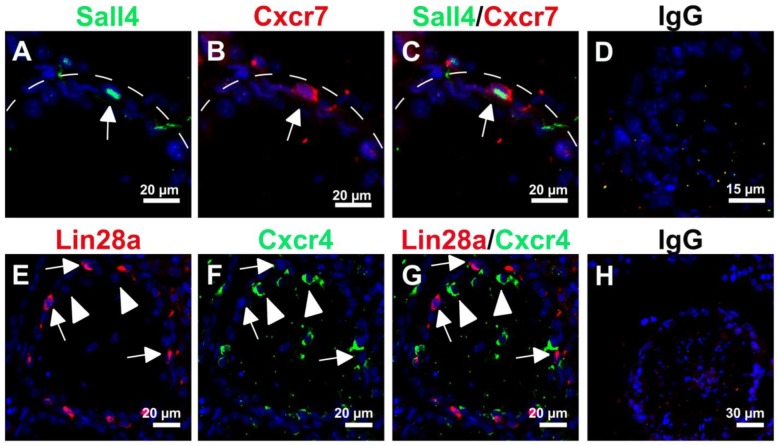
28 days after busulfan treatment, Cxcr7-positive as well as Cxcr4-positive cells were observed in some seminiferous tubules. Cxcr7 expression was observed in Sall4-positive spermatogonia (Fig. 5A–D). Lin28a-positive spermatogonia repopulating the seminiferous tubules 28 days post busulfan exposure were negative for Cxcr4 as shown by co-localization (Fig. 5E–H).
Figure 5. Cxcr7 and Cxcr4 expression in germ cell-depleted mouse testes.
Representative immunofluorescence images of testicular tissue section 28 days after busulfan treatment of adult mice. Cxcr7 expression (B, red, arrow) is restricted to Sall4-positive spermatogonia (A, green, arrow) but Cxcr4 expression (F, green, arrowheads) is not solely observed in Lin28-positive spermatogonia (E, red, arrows). The respective merged images are shown in (C) and (G). Hoechst (blue) was used as nuclear counterstain. Incubation with corresponding IgG antibodies was used as negative control and a representative image is shown in (D, H). Scale bars represent 15, 20 and 30 µm.
Discussion
In the present study, we describe the expression pattern of Cxcr7 during postnatal testicular development and demonstrate that spermatogonial depletion and repopulation has effects on the Cxcl12/Cxcr4/Cxcr7 axis in adult mouse testes.
So far studies in mammalian testes have focused on the tissue distribution and interaction of Cxcl12 and Cxcr4 in embryonic, neonatal and adult mice [14], [15], [19], [20], [37]. In line with our results, Pellegrino et al. (2012) have recently demonstrated mRNA expression of Cxcl12 during various phases of testicular development in mice. In addition, stainings revealed protein expression in the cytoplasm of Sertoli cells in 3 dpp, 6 dpp and adult mice [13], [15]. Our finding that Cxcr4 transcript expression can be detected throughout testicular development is in general agreement with previous studies. In neonatal mice, gonocytes as well as undifferentiated spermatogonia express Cxcr4, and its expression has also been described in adult mouse testes [13]–[15].
So far, results regarding the localization of the Cxcr4 protein in the adult mouse testis are controversial [14]–[16]. Whereas Yoon et al. 2009 localized Cxcr4 in spermatogonia, Sertoli and interstitial cells [16], Kanatsu-Shinohara et al. 2012 and Yang et al. 2013 localized Cxcr4 only in undifferentiated spermatogonia [14], [16] using co-staining against Plzf or Id4 for characterization of spermatogonia. Our stainings exclusively showed Cxcr4 expression in testicular cells residing along the basement membrane. However, in contrast to Kanatsu-Shinohara et al. 2012 and Yang et al. 2013, co-expression analysis with the spermatogonial marker Lin28a and Cxcr7 did not provide evidence for co-expression of Cxcr4 in undifferentiated spermatogonia [14], [15], [38], [39]. Therefore, the unequivocal identification of Cxcr4-positive cells in adult NMRI mice remains to be elucidated. Compared to Kanatsu-Shinohara et al. 2012 and Yang et al. 2013, possible reasons for the different results include the use of a different mouse strain and a different fixation agent.
Unexpectedly, we found that the atypical chemokine receptor Cxcr7 is expressed by Sall4-positive spermatogonia throughout testicular development [40]. As this receptor is expressed by somatic cells during PGC migration and acts as a scavenger for Cxcl12 [21]–[24], the germ cell-specific expression was unexpected. However, recent studies suggest, that CXCR7 has diverse cell type-specific functions. Using human vascular smooth muscle cells, it has been demonstrated that CXCR7 can act as an endogenous ß-arrestin-biased signaling receptor to induce cell migration [41]. In addition, CXCR7 may also serve as a co-receptor for CXCR4 and therefore enhance the classical CXCL12-mediated G-protein signaling, including cell migration [42]–[44]. Whether these mechanisms play a role in Cxcr7 expressing germ cells during the postnatal development in mouse testes, however, remains to be elucidated.
In the second part of our study, the effect of busulfan treatment and subsequent germ cell loss on SSC-niche associated factors, including the Cxcl12/Cxcr4/Cxcr7 axis was investigated. Interestingly, previous studies already investigated the impact of busulfan administration of the somatic environment in adult mouse testes and showed that the numbers of Leydig cells and Sertoli cell were not decreased and therefore changes in transcript levels per testis are therefore a reflection of changes per Sertoli cell or Leydig cell, respectively [32]. Regarding germ cells, our histological analyses and immunohistochemical stainings revealed that the seminiferous tubules were sporadically repopulated with Lin28a-positive spermatogonia 28 days after busulfan treatment, supporting previous findings demonstrating spermatogonial repopulation to be initiated as early as day 15 after busulfan administration (dose-dependent) [45], [46]. Furthermore, we demonstrated that Ddx4-positive spermatocytes and spermatids were absent from the tubules between days 7 and 21 after treatment. Performing qPCR analyses for Lin28a and Ddx4, we demonstrated that the loss of the respective testicular cell type is also reflected at the RNA level. In line with our results, O'Shaughnessy et al. (2008) showed that transcript levels of Stra8 (spermatogonia associated gene) and of Spo11 (spermatocytes associated gene) decreased on day 5 and day 15 after busulfan administration, respectively [32]. These findings revealed that RNA expression analysis for specific marker genes is a valid tool to evaluate changes regarding the composition of testicular cell types. Interestingly, despite the fact that busulfan treatment had not yet resulted in a loss of all spermatogonia on day 3 after treatment, we found that expression levels of the ligand Cxcl12 and its receptor Cxcr4 as well as of the growth factors Amh, Gdnf and the transcription factor Erm were significantly increased compared to the vehicle control (DMSO). Consistent with our findings, previous studies have shown a similar increase of Gdnf transcripts during the loss of spermatogonia following gonadotoxic treatments [47], [48]. Zhoni et al., (2011) and Ventelä et al. (2012) reported a 2-fold increase of Gdnf transcript levels 5 days after busulfan treatment and 24 hours after X-irradiation, respectively. Both studies suggested that Sertoli cells respond rapidly and temporarily to the loss of spermatogonia and support the survival of remaining germ cells and stimulate their proliferation [48], [49]. Moreover, our data reveal significantly increased expression levels of the chemokine Cxcl12 at the time of the early spermatogenic repopulation (on day 28 after busulfan treatment). These results are in line with previous reports, showing that most changes in transcript levels of 26 Sertoli cell-specifc genes were associated with the loss of the last spermatids and the early time of spermatogenic repopulation [32]. The expression pattern of Cxcr4, which was not detected on Lin28a-positive or Cxcr7-positive spermatogonia, was reflected at the RNA level following busulfan treatment, as transcript levels of Cxcr4 remained rather constant. In contrast to Cxcr4, we found that transcript levels of the chemokine receptor Cxcr7 were decreased following day 3 after busulfan treatment. The expression pattern therefore rather resembles that of the spermatogonial marker gene Lin28a and is consistent with our protein data demonstrating that Cxcr7 is expressed by germ cells located at the basement membrane in normal adult mouse testes.
However, all studies revealing an intense loss of post-meiotic germ cells suffer from the fact that relative changes in somatic cell mRNA levels may be overestimated. In such scenarios any detectable change could simply reflect the fact that Sertoli cell transcription is unaffected while germ cell mRNA is diminished due to loss or inactivity of germ cell transcription.
Interestingly, performing immunofluorescence analyses on day 28 after busulfan treatment, we again observed Cxcr4 expression only in Lin28a-negative testicular cells. Furthermore, analysis on day 28 after busulfan treatment showed the expression of Cxcr7 on spermatogonia which repopulate seminiferous tubules. These findings were unexpected as the expression of Cxcr7 was only detected in somatic cells during germ cell migration of zebrafish [21], [22]. Our findings therefore suggest an additional function of Cxcr7 in mouse germ cells at a) later developmental stages and b) during testicular recovery in the mouse testis. Investigating the functional role of CXCR7 in human cell lines (HEK-293T and MDA-MB-231) two mechanisms have been suggested regarding the CXCL12 mediated action of CXCR7. These include active signalling [50], [51] and the modulation of CXCR4 activity via heterodimerization [42], [43], [52]. Interestingly, it was also demonstrated that cells showed enhanced growth and survival as well as adhesion properties following ligand binding to CXCR7 [53]. However, whether these mechanisms are relevant to the germ cell population in mammalian testes remains to be elucidated.
So far, studies in mammalian testes have focused on the interaction of Cxcl12 and Cxcr4 [14], [15]. We demonstrate for the first time that the chemokine receptor Cxcr7 is expressed throughout postnatal testicular development and recent studies suggest that this receptor may not act solely as a scavenger receptor for Cxcl12 but may rather be involved in the regulation of SSC growth and survival as well as cell adhesion [53]. Moreover, we suggest that the interaction between Cxcl12 and Cxcr7 may stimulate the spermatogenic repopulation of the seminiferous tubules following depletion. Further support for this suggestion is provided by the observed expression of the chemokine Cxcl12 during re-colonization of murine SSCs following germ cell transplantation [14]. Furthermore, we demonstrated that those germ cells repopulating the seminiferous tubules after induced germ cell depletion are immunopositive for Cxcr7. Our study therefore re-inforces the complexity of this system and future studies will aim to elucidate the functional role of the Cxcl12/Cxcr4/Cxcr7 interaction during testicular development and recovery.
Supporting Information
Testis weights following busulfan treatment. Adult mice were given a single injection of DMSO (•) or busulfan (▪ 38 mg/kg) and testis weights were measured on days 1, 3, 7, 21 and 28 (n = 10 per time point) after treatment. Results are expressed as mean ± SD. Busulfan groups marked with asterisks are significantly different (*, P = 0.01 to 0.05; **, P = 0.001 to 0.01 or ***, P<0.001).
(TIF)
Cxcr7 is expressed by undifferentiated spermatogonia during testicular germ cell development. The specific staining of the anti-CXCR7 antibody was validated by a peptide blocking experiment (A–C). Cxcr7 expression is restricted to Sall4-positive undifferentiated spermatogonia on days 7 (E–G) and 14 (I–K) of postnatal testicular development and in adult tissue (M–O). Incubation with corresponding IgG antibodies was used as negative control and a representative image is shown in (D, H, L, P). Scale bars represent 20 µm and 30 µm.
(TIF)
Representative micrographs of adult mouse testes after cytotoxic treatments. Micrographs showing PAS stained sections of testicular tissues on days 1 (A), 3 (B), 7 (C), 21 (D) and 28 (E) following DMSO injections. In contrast to the DMSO treatment group, which showed no depletion of germ cells, the testicular parenchyma of the busulfan treatment group still contained all germ cell types and Sertoli cells (arrowheads) on days 1 and 3 (F, G; Insert: K, L) but had lost spermatogonia (arrows) on day 7 (H; Insert: M)). Furthermore, spermatocytes were lost after 21 days (I; Insert: N) and on day 28 most tubules showed a Sertoli cell-only phenotype, with individual tubules containing spermatogonia (arrows) (J; Insert: O). Scale bars represent 10 µm and 50 µm.
(TIF)
Primer sequences used for real-time PCR, localization of respective proteins in the mouse testis and corresponding references.
(DOCX)
Qualitative assessment of the Cxcr7 expression in mouse testes during testicular germ cell development.
(DOCX)
Acknowledgments
We gratefully acknowledge Martin Heuermann and Günter Stelke (Centre of Reproductive Medicine and Andrology, Münster, Germany), for excellent animal caretaking as well as Jutta Salzig and Adelheid Kersebom for technical assistance.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data are within the paper or the supporting files.
Funding Statement
The study was primarily supported by a grant from the Innovative Medical Research of the Medical Faculty, University Münster (IMF, KO 111014). Additional funds for animal handling and histological aspects of the experimental work were provided by the DFG Sachbeihilfen SCHL394/9-1 and 11-2. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shetty G, Meistrich ML (2007) The missing niche for spermatogonial stem cells: Do blood vessels point the way? Cell Stem Cell 1:361–363. [DOI] [PubMed] [Google Scholar]
- 2. Yoshida S, Sukeno M, Nabeshima Y (2007) A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 317:1722–1726. [DOI] [PubMed] [Google Scholar]
- 3. Kostereva N, Hofmann MC (2008) Regulation of the spermatogonial stem cell niche. Reprod Domest Anim 43 Suppl 2386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, et al. (2000) Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287:1489–1493. [DOI] [PubMed] [Google Scholar]
- 5. Kubota H, Avarbock MR, Brinster RL (2004) Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 101:16489–16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kubota H, Avarbock MR, Brinster RL (2004) Culture conditions and single growth factors affect fate determination of mouse spermatogonial stem cells. Biol Reprod 71:722–731. [DOI] [PubMed] [Google Scholar]
- 7. Hess RA, Cooke PS, Hofmann MC, Murphy KM (2006) Mechanistic insights into the regulation of the spermatogonial stem cell niche. Cell Cycle 5:1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL (2006) Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A 103:9524–9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oatley JM, Oatley MJ, Avarbock MR, Tobias JW, Brinster RL (2009) Colony stimulating factor 1 is an extrinsic stimulator of mouse spermatogonial stem cell self-renewal. Development 136:1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dettin L, Ravindranath N, Hofmann MC, Dym M (2003) Morphological characterization of the spermatogonial subtypes in the neonatal mouse testis. Biol Reprod 69:1565–1571. [DOI] [PubMed] [Google Scholar]
- 11. Yomogida K, Yagura Y, Tadokoro Y, Nishimune Y (2003) Dramatic expansion of germinal stem cells by ectopically expressed human glial cell line-derived neurotrophic factor in mouse sertoli cells. Biol Reprod 69:1303–1307. [DOI] [PubMed] [Google Scholar]
- 12. Ebata KT, Zhang X, Nagano MC (2005) Expression patterns of cell-surface molecules on male germ line stem cells during postnatal mouse development. Mol Reprod Dev 72:171–181. [DOI] [PubMed] [Google Scholar]
- 13. Payne CJ, Gallagher SJ, Foreman O, Dannenberg JH, Depinho RA, et al. (2010) Sin3a is required by sertoli cells to establish a niche for undifferentiated spermatogonia, germ cell tumors, and spermatid elongation. Stem Cells 28:1424–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kanatsu-Shinohara M, Inoue K, Takashima S, Takehashi M, Ogonuki N, et al. (2012) Reconstitution of mouse spermatogonial stem cell niches in culture. Cell Stem Cell 11:567–578. [DOI] [PubMed] [Google Scholar]
- 15. Yang QE, Kim D, Kaucher A, Oatley MJ, Oatley JM (2013) CXCL12-CXCR4 signaling is required for the maintenance of mouse spermatogonial stem cells. J Cell Sci 126:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoon KA, Chae YM, Cho JY (2009) FGF2 stimulates SDF-1 expression through the erm transcription factor in sertoli cells. J Cell Physiol 220:245–256. [DOI] [PubMed] [Google Scholar]
- 17. Ponomaryov T, Peled A, Petit I, Taichman RS, Habler L, et al. (2000) Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest 106:1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Georgiou KR, Scherer MA, King TJ, Foster BK, Xian CJ (2012) Deregulation of the CXCL12/CXCR4 axis in methotrexate chemotherapy-induced damage and recovery of the bone marrow microenvironment. Int J Exp Pathol 93:104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ara T, Nakamura Y, Egawa T, Sugiyama T, Abe K, et al. (2003) Impaired colonization of the gonads by primordial germ cells in mice lacking a chemokine, stromal cell-derived factor-1 (SDF-1). Proc Natl Acad Sci U S A 100:5319–5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Molyneaux KA, Zinszner H, Kunwar PS, Schaible K, Stebler J, et al. (2003) The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 130:4279–4286. [DOI] [PubMed] [Google Scholar]
- 21. Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, et al. (2008) Control of chemokine-guided cell migration by ligand sequestration. Cell 132:463–473. [DOI] [PubMed] [Google Scholar]
- 22. Mahabaleshwar H, Boldajipour B, Raz E (2008) Killing the messenger: The role of CXCR7 in regulating primordial germ cell migration. Cell Adh Migr 2:69–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mahabaleshwar H, Tarbashevich K, Nowak M, Brand M, Raz E (2012) Beta-arrestin control of late endosomal sorting facilitates decoy receptor function and chemokine gradient formation. Development 139:2897–2902. [DOI] [PubMed] [Google Scholar]
- 24. Staton AA, Knaut H, Giraldez AJ (2011) miRNA regulation of Sdf1 chemokine signaling provides genetic robustness to germ cell migration. Nat Genet 43:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Comerford I, Litchfield W, Harata-Lee Y, Nibbs RJ, McColl SR (2007) Regulation of chemotactic networks by ‘atypical’ receptors. Bioessays 29:237–247. [DOI] [PubMed] [Google Scholar]
- 26. Ulvmar MH, Hub E, Rot A (2011) Atypical chemokine receptors. Exp Cell Res 317:556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cancellieri C, Vacchini A, Locati M, Bonecchi R, Borroni EM (2013) Atypical chemokine receptors: From silence to sound. Biochem Soc Trans 41:231–236. [DOI] [PubMed] [Google Scholar]
- 28. Nibbs RJ, Graham GJ (2013) Immune regulation by atypical chemokine receptors. Nat Rev Immunol 13:815–829. [DOI] [PubMed] [Google Scholar]
- 29. Dambly-Chaudiere C, Cubedo N, Ghysen A (2007) Control of cell migration in the development of the posterior lateral line: Antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eva C, Sprengel R (1993) A novel putative G protein-coupled receptor highly expressed in lung and testis. DNA Cell Biol 12:393–399. [DOI] [PubMed] [Google Scholar]
- 31. McIver SC, Loveland KL, Roman SD, Nixon B, Kitazawa R, et al. (2013) The chemokine CXCL12 and its receptor CXCR4 are implicated in human seminoma metastasis. Andrology 1:517–529. [DOI] [PubMed] [Google Scholar]
- 32. O’Shaughnessy PJ, Hu L, Baker PJ (2008) Effect of germ cell depletion on levels of specific mRNA transcripts in mouse sertoli cells and leydig cells. Reproduction 135:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zohni K, Zhang X, Tan SL, Chan P, Nagano MC (2012) The efficiency of male fertility restoration is dependent on the recovery kinetics of spermatogonial stem cells after cytotoxic treatment with busulfan in mice. Hum Reprod 27:44–53. [DOI] [PubMed] [Google Scholar]
- 34. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- 35. Brinkworth MH, Weinbauer GF, Schlatt S, Nieschlag E (1995) Identification of male germ cells undergoing apoptosis in adult rats. J Reprod Fertil 105:25–33. [DOI] [PubMed] [Google Scholar]
- 36. Ehmcke J, Joshi B, Hergenrother SD, Schlatt S (2007) Aging does not affect spermatogenic recovery after experimentally induced injury in mice. Reproduction 133:75–83. [DOI] [PubMed] [Google Scholar]
- 37. Pellegrino J, Castrillon DH, David G (2012) Chromatin associated Sin3A is essential for male germ cell lineage in the mouse. Dev Biol 369:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zheng K, Wu X, Kaestner KH, Wang PJ (2009) The pluripotency factor LIN28 marks undifferentiated spermatogonia in mouse. BMC Dev Biol 9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaytan F, Sangiao-Alvarellos S, Manfredi-Lozano M, Garcia-Galiano D, Ruiz-Pino F, et al. (2013) Distinct expression patterns predict differential roles of the miRNA-binding proteins, Lin28 and Lin28b, in the mouse testis: Studies during postnatal development and in a model of hypogonadotropic hypogonadism. Endocrinology 154:1321–1336. [DOI] [PubMed] [Google Scholar]
- 40. Gassei K, Orwig KE (2013) SALL4 expression in gonocytes and spermatogonial clones of postnatal mouse testes. PLoS One 8:e53976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, et al. (2010) Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc Natl Acad Sci U S A 107:628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, et al. (2007) Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A 104:14759–14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B (2009) CXCR7 heterodimerizes with CXCR4 and regulates CXCL12-mediated G protein signaling. Blood 113:6085–6093. [DOI] [PubMed] [Google Scholar]
- 44. Decaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, et al. (2011) CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem 286:32188–32197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Keulen CJ, de Rooij DG (1974) The recovery from various gradations of cell loss in the mouse seminiferous epithelium and its implications for the spermatogonial stem cell renewal theory. Cell Tissue Kinet 7:549–558. [DOI] [PubMed] [Google Scholar]
- 46. van Keulen CJ, de Rooij DG (1975) Spermatogenetic clones developing from repopulating stem cells surviving a high dose of an alkylating agent. Cell Tissue Kinet 8:543–551. [DOI] [PubMed] [Google Scholar]
- 47. Ventela S, Makela JA, Kulmala J, Westermarck J, Toppari J (2012) Identification and regulation of a stage-specific stem cell niche enriched by nanog-positive spermatogonial stem cells in the mouse testis. Stem Cells 30:1008–1020. [DOI] [PubMed] [Google Scholar]
- 48. Zohni K, Zhang X, Tan SL, Chan P, Nagano MC (2012) The efficiency of male fertility restoration is dependent on the recovery kinetics of spermatogonial stem cells after cytotoxic treatment with busulfan in mice. Hum Reprod 27:44–53. [DOI] [PubMed] [Google Scholar]
- 49. Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL (2006) Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 24:1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, et al. (2008) Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med 205:479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Z, Ma Q, Liu Q, Yu H, Zhao L, et al. (2008) Blockade of SDF-1/CXCR4 signalling inhibits pancreatic cancer progression in vitro via inactivation of canonical wnt pathway. Br J Cancer 99:1695–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Luker K, Gupta M, Luker G (2009) Bioluminescent CXCL12 fusion protein for cellular studies of CXCR4 and CXCR7. BioTechniques 47:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, et al. (2006) A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med 203:2201–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Testis weights following busulfan treatment. Adult mice were given a single injection of DMSO (•) or busulfan (▪ 38 mg/kg) and testis weights were measured on days 1, 3, 7, 21 and 28 (n = 10 per time point) after treatment. Results are expressed as mean ± SD. Busulfan groups marked with asterisks are significantly different (*, P = 0.01 to 0.05; **, P = 0.001 to 0.01 or ***, P<0.001).
(TIF)
Cxcr7 is expressed by undifferentiated spermatogonia during testicular germ cell development. The specific staining of the anti-CXCR7 antibody was validated by a peptide blocking experiment (A–C). Cxcr7 expression is restricted to Sall4-positive undifferentiated spermatogonia on days 7 (E–G) and 14 (I–K) of postnatal testicular development and in adult tissue (M–O). Incubation with corresponding IgG antibodies was used as negative control and a representative image is shown in (D, H, L, P). Scale bars represent 20 µm and 30 µm.
(TIF)
Representative micrographs of adult mouse testes after cytotoxic treatments. Micrographs showing PAS stained sections of testicular tissues on days 1 (A), 3 (B), 7 (C), 21 (D) and 28 (E) following DMSO injections. In contrast to the DMSO treatment group, which showed no depletion of germ cells, the testicular parenchyma of the busulfan treatment group still contained all germ cell types and Sertoli cells (arrowheads) on days 1 and 3 (F, G; Insert: K, L) but had lost spermatogonia (arrows) on day 7 (H; Insert: M)). Furthermore, spermatocytes were lost after 21 days (I; Insert: N) and on day 28 most tubules showed a Sertoli cell-only phenotype, with individual tubules containing spermatogonia (arrows) (J; Insert: O). Scale bars represent 10 µm and 50 µm.
(TIF)
Primer sequences used for real-time PCR, localization of respective proteins in the mouse testis and corresponding references.
(DOCX)
Qualitative assessment of the Cxcr7 expression in mouse testes during testicular germ cell development.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data are within the paper or the supporting files.