Abstract
Introduction:
HIV and malaria infections occur in the same individuals, particularly in sub-Saharan Africa. We examined whether daily multivitamin supplementation (vitamins B complex, C, and E) or vitamin A supplementation altered malaria incidence in HIV-infected women of reproductive age.
Methods:
HIV-infected pregnant Tanzanian women recruited into the study were randomly assigned to daily multivitamins (B complex, C, and E), vitamin A alone, both multivitamins and vitamin A, or placebo. Women received malaria prophylaxis during pregnancy and were followed monthly during the prenatal and postpartum periods. Malaria was defined in 2 ways: presumptive diagnosis based on a physician's or nurse's clinical judgment, which in many cases led to laboratory investigations, and periodic examination of blood smears for malaria parasites.
Results:
Multivitamin supplementation compared with no multivitamins significantly lowered women's risk of presumptively diagnosed clinical malaria (relative risk: 0.78, 95% confidence interval: 0.67 to 0.92), although multivitamins increased their risk of any malaria parasitemia (relative risk: 1.24, 95% confidence interval: 1.02 to 1.50). Vitamin A supplementation did not change malaria incidence during the study.
Conclusions:
Multivitamin supplements have been previously shown to reduce HIV disease progression among HIV-infected women, and consistent with that, these supplements protected against development of symptomatic malaria. The clinical significance of increased risk of malaria parasitemia among supplemented women deserves further research, however. Preventive measures for malaria are warranted as part of an integrated approach to the care of HIV-infected individuals exposed to malaria.
Key Words: randomized controlled trial; multivitamin (vitamins B complex, C, E) supplementation; vitamin A supplementation; HIV-infected women; malaria incidence
INTRODUCTION
Millions of pregnancies occur each year in world regions affected by malaria transmission.1 Malaria in pregnancy may cause maternal anemia, maternal mortality, premature pregnancy termination,2 and low birth weight or death of the offspring.3 Furthermore, malaria-endemic regions have some of the highest burdens of HIV infection globally,4,5 with HIV and malaria infections often occurring in the same individuals. HIV-related immunosuppression increases malaria susceptibility,6 whereas malaria may transiently increase plasma viral load.7 Both infections may interact detrimentally in pregnancy to increase risks of pregnancy complications and adverse infant outcomes.8–11
Multivitamin supplements, including vitamins B complex, C, and E, slow down disease progression and reduce the occurrence of HIV-associated complications such as dysentery and acute upper respiratory infections in HIV-infected women.12 Whether multivitamins alter malaria susceptibility in HIV-infected women is unknown. Studies conducted among children suggest multivitamins could reduce incidence of clinical malaria,13 although a possible increase in malaria parasitemia was reported by a Gambian randomized trial of thiamine, riboflavin, vitamin C, and iron supplementation.14 Other studies suggest that individual vitamins may protect against clinical malaria, for instance, vitamin A supplementation reduced clinical malaria incidence among children in one study.15
In this paper, we aim to determine the effect of multivitamin supplementation on malaria incidence in HIV-infected women. Multivitamin supplementation in HIV-infected individuals improves CD4 T-cell counts,12,16 and the resulting sustained CD4 T-cell functioning12,17 could improve antibody response and protection against malaria.18,19 In addition, we examine the effect of vitamin A supplementation on malaria incidence in HIV-infected women, given protection previously observed in children. To the best of our knowledge, randomized trials that addressed these questions are few and this is the first trial to examine the effect of vitamin interventions on malaria risk in HIV-infected individuals.
METHODS
A randomized controlled trial was conducted in Dar es Salaam, Tanzania, from April 1995 until August 2003,12,20 during which 1078 HIV-infected pregnant women were randomly assigned using a factorial design to receive a daily oral dose of 1 of 4 regimens: placebo, vitamin A alone (30 mg β-carotene with 5000 IU preformed vitamin A), multivitamins without vitamin A (20 mg vitamins B1, 20 mg B2, 25 mg B6, 100 mg niacin, 50 μg B12, 500 mg C, 30 mg E, and 800 μg folic acid), or multivitamins with vitamin A at the listed doses. At delivery, women received an additional 200,000 IU oral vitamin A if their assigned regimen included vitamin A, or placebo if otherwise. Participants and study staff were blinded to regimen assignments. During the trial implementation, the Data Safety and Monitoring Board noted a significantly higher risk of mother-to-child transmission of HIV among women assigned to vitamin A supplementation,21 whereas multivitamin supplementation reduced the risks of fetal loss, low birth weight, and severe prematurity.16,21
Thus, the protocol was modified to provide multivitamin supplements for the duration of pregnancy to all participants who subsequently became pregnant during the study (starting after May 1998), and to exclude vitamin A supplements during pregnancy (from September 2000) until the trial concluded. As we wanted to understand the effects of multivitamin supplementation, and vitamin A supplementation, on malaria, we decided a priori to restrict analyses to contributed person-time that had been unaffected by the protocol modifications. These analyses therefore include time from enrollment to just before the start of a second pregnancy during the study (314 women had a second pregnancy), or until the study end, loss to follow-up, or death—whichever occurred first—if no second pregnancy occurred [median (interquartile range) length of follow-up for women with only 1 pregnancy during the study, 41.0 (8.6–75.3) months].
During pregnancy, all women received 120 mg ferrous iron and 5 mg folate daily and weekly malaria prophylaxis (500 mg chloroquine phosphate) in accordance with standard prenatal care in Tanzania at the time. Malaria is endemic in Dar es Salaam, with year round stable transmission. Iron–folate supplements and prophylactic chloroquine were discontinued when pregnancy ended. Women diagnosed with malaria were treated according to the Tanzanian standard of care. Antiretroviral therapy (ART) was unavailable in Tanzania during the study period; most study participants were in the early clinical stages of HIV infection22 and did not qualify for ART at baseline.
During monthly clinic visits, study nurses evaluated women for symptoms and history of illnesses, while study physicians conducted physical examinations, made presumptive diagnoses, and requested investigations when indicated. Routine blood smears were universally performed at baseline, the first postnatal visit, and every 12 months, while women suspected to have malaria at monthly visits had additional blood films at their physicians' discretion. Thick and thin films stained with 5% Giemsa solution were examined microscopically by 2 laboratory technologists for the presence of asexual Plasmodium falciparum, P. malariae, P. vivax, or P. ovale parasites. Discordant reports were resolved by a senior laboratory technologist. We calculated malaria parasite densities per microliter of blood from the number of parasites per 200 leukocytes, assuming a leukocyte count of 8000 cells per microliter.23
Statistical Analysis
Our main outcomes were first new episode of any malaria parasitemia and first presumptive diagnosis of clinical malaria during the study. We also examined intervention effects on the first new episode of ≥10,000 parasites per microliter. Presumptive clinical malaria was defined as having either physician-diagnosed malaria (diagnosis of malaria made by study physicians after considering symptoms and clinical examination findings), nurse-diagnosed malaria (reports of recent high-grade fever with associated chills, rigors, and sweating and/or axillary temperatures of 37.5°C or higher recorded by study nurses), or both. Women with any parasitemia at enrollment were included in the study after a 30-day malaria-free period, documented by slides.
We tested differences in baseline characteristics between intervention groups using t tests or Wilcoxon rank-sum tests for continuous variables, and χ2 tests or Fisher's exact tests for categorical variables, as appropriate. Cox proportional hazards regression models,24 with months since randomization as the time scale, were used to estimate the effects of treatment on time to first malaria episodes. We examined the effects of multivitamins without vitamin A, vitamin A alone, and multivitamins with vitamin A, compared with placebo, on the risk of outcomes and tested for the interaction of multivitamins with vitamin A using a likelihood ratio test. As there was no significant interaction between multivitamins and vitamin A supplementation in relation to the risk of malaria, the intervention groups were collapsed to increase statistical power; multivitamin supplementation was compared with no multivitamins, and vitamin A was compared with no vitamin A (interaction test P values were 0.59 in relation to risk of any parasitemia and 0.24 in relation to risk of presumptive clinical malaria). We also explored the possibility of modification of the multivitamin effects by the following factors: gravidity (primigravida or multigravida), season (rainy or dry season), pregnancy status (pregnant versus no longer pregnant), time since delivery (≤3 versus >3 months), baseline WHO HIV disease stage22 (stage 1, 2, or 3), and baseline CD4 T-cell count (<200, 200 to <350, ≥350 cells/μL).
All analyses used SAS software version 9.2 (SAS Institute, Cary, NC, USA).25 The Human Subjects Committee of the Harvard School of Public Health and the Research and Publications Committee of Muhimbili University of Health and Allied Sciences gave institutional review board approval for the randomized trial; women provided informed consent for participation in the trial.
RESULTS
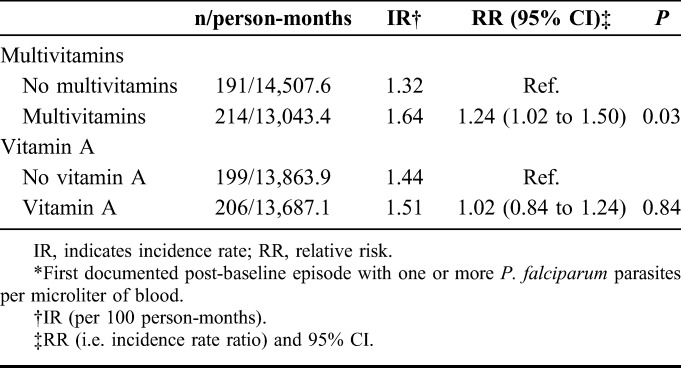
Of the 1078 trial participants, 1050 were eligible for this study, contributing a total of 27,551 months of follow-up. Twenty-eight women were excluded from analyses—27 were lost to follow-up immediately after enrollment and 1 had protracted malaria parasitemia starting at baseline. Five hundred and twenty-eight women received multivitamins and 529 received vitamin A supplements. At baseline (Table 1), most women were in the second trimester of pregnancy, with 84% in the first WHO clinical stage22 of HIV infection. Nearly one-third of the women had CD4 T-cell counts of at least 500 per microliter. Women reported low daily dietary intake of iron and folate, but moderately high daily vitamin C intake, compared with the recommended intakes for pregnant women. More than half had moderate or severe anemia and plasma vitamin E levels were low, suggesting that many had multiple micronutrient deficiencies. The baseline characteristics of women in the intervention groups were similar. Plasmodium falciparum caused malaria parasitemia during the study, and the median time to first episode of any parasitemia after baseline was 18.1 months (interquartile range 6.4–32.3 months), whereas the median time to the first presumptive clinical malaria episode was 9.5 months (interquartile range 2.9–22.8 months).
TABLE 1.
Baseline Characteristics of Study Participants by Intervention Group*
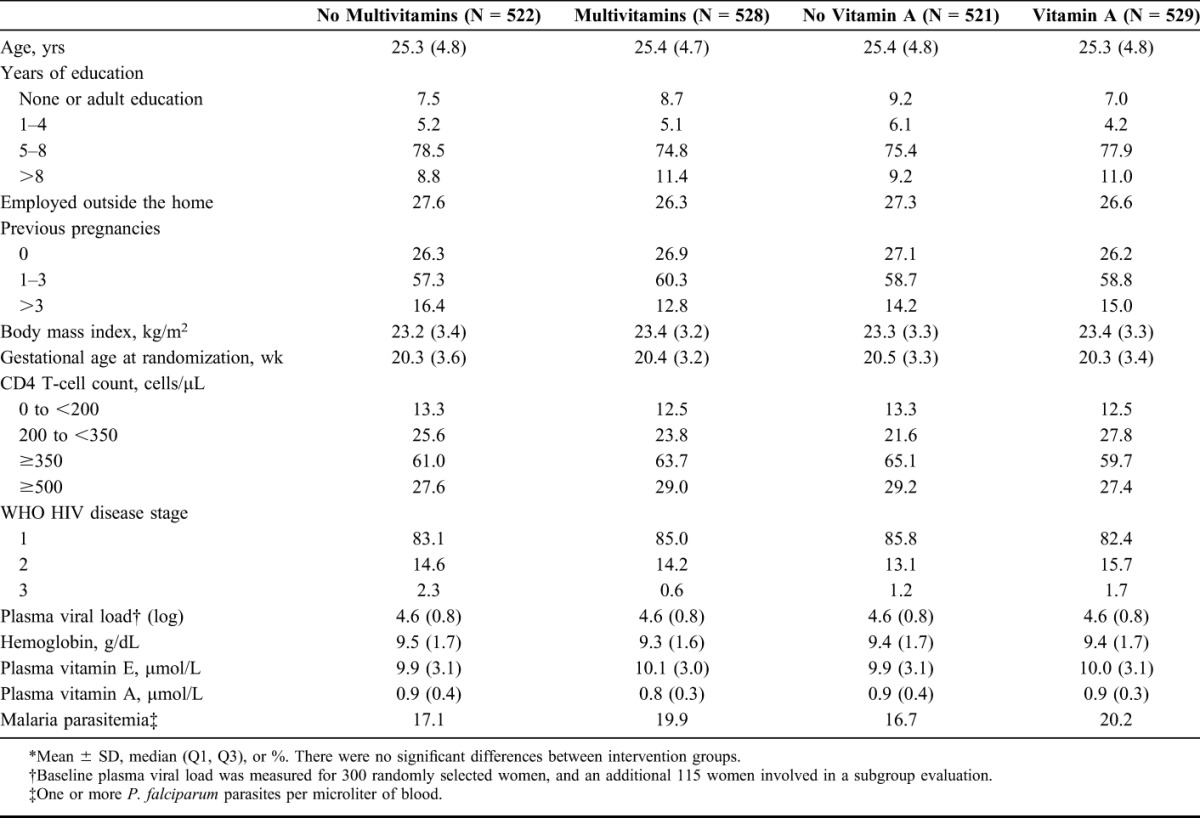
Supplementation with vitamins B complex, C, and E appeared protective against presumptive clinical malaria (Table 2). Women receiving multivitamins compared with no multivitamins had a lower risk of developing clinical malaria [22% lower, 95% confidence interval (CI): 8% to 33% lower]—results were similar when physician-diagnosed and nurse-diagnosed malaria were considered separately. Vitamin A supplementation compared with no vitamin A did not significantly alter the risk of developing clinical malaria [relative risk (RR) 0.97, 95% CI: 0.82 to 1.14].
TABLE 2.
Effects of Multivitamin Supplementation and Vitamin A Supplementation on the Risk of Presumptive Clinical Malaria*
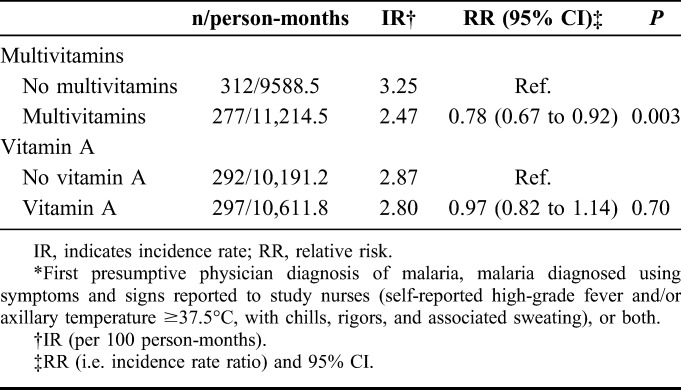
Multivitamin supplementation significantly increased the risk of any malaria parasitemia by 24% (95% CI: 2% to 50%) relative to no multivitamins (Table 3), although at baseline, the mean parasite counts for the 2 groups were similar (P = 0.48). When we considered the severity of parasitemia, malaria parasite densities of ≥10,000 per microliter were infrequent—40 women had malaria episodes with ≥10,000 parasites per microliter. The effect estimate was consistent with that of any malaria parasitemia, although not statistically significant (severe parasitemia RR 1.23, 95% CI: 0.66 to 2.29). Vitamin A supplementation compared with no vitamin A did not significantly alter the risk of malaria parasitemia (RR 1.02, 95% CI: 0.84 to 1.24).
TABLE 3.
Effects of Multivitamin Supplementation and Vitamin A Supplementation on the Risk of Any Malaria Parasitemia*
Exploration of the modification of the effect of multivitamin supplementation on the risk of malaria parasitemia and of presumptive clinical malaria by different factors found no modification by pregnancy status, time since delivery, number of previous pregnancies, season, baseline WHO HIV disease stage, or baseline CD4 T-cell counts (P values for the interaction test ranged from 0.19 to 0.96 and 0.13 to 0.87, respectively, for malaria parasitemia and presumptive clinical malaria).
DISCUSSION
We found that daily multivitamin supplementation that included vitamins B complex, C, and E reduced the risk of presumptive clinical malaria diagnosis among HIV-infected women living in a malaria-endemic region. We also found increased risk of developing any malaria parasitemia for women receiving multivitamin supplementation. The effects were similar regardless of pregnancy status, number of previous pregnancies, season, and WHO HIV disease stage. Vitamin A supplementation, however, did not change women's risks of presumptive clinical malaria or malaria parasitemia.
Our study suggests that multivitamin supplementation could have prevented clinical malaria episodes, as presumptive diagnoses of clinical malaria were less frequent among HIV-infected women assigned to multivitamins. Multivitamin supplementation was previously found to result in significant reduction in mortality, increased CD4 T-cell counts, and reduced plasma viral load among these women.12 Other studies have also found vitamin supplementation to be associated with prolonged AIDS-free survival. The use of vitamin B supplements by HIV-infected individuals was associated with delayed progression to AIDS and delayed mortality in South Africa,26 and improved survival in the United States.27 A randomized controlled trial in Thailand found that multiple micronutrient supplements (vitamins B complex, A, C, E, D, K, and minerals) lowered mortality rates among HIV-infected individuals who were severely immunosuppressed, when compared with placebo,28 whereas a trial involving 49 HIV-infected individuals reported a trend towards reduced viral loads for those assigned to vitamins C and E compared with placebo.29 A recent randomized controlled trial in Botswana also found that a supplement comprising vitamins B complex, C, E, and selenium had morbidity-reducing effects among 878 HIV-infected ART-naive adults.17 However, although we found multivitamin supplementation to protect against clinical malaria, symptoms diagnosed as clinical malaria could also have been caused by infections other than malaria, in which case, the protective multivitamin effects we observed would have resulted from a reduced incidence of HIV-related infections, and not necessarily reductions in clinical malaria incidence.
CD4 T cells play a role in the development of acquired immunity against malaria,18,19 and the multivitamin-induced improvements in immune function could have resulted in reduced rates of clinical disease. However, it is possible that other infectious conditions were misidentified as malaria among these HIV-infected women—presumptive diagnoses relied on symptoms and signs that could have non-malaria causes in HIV-infected individuals.30 Although our study is the first randomized trial to examine the relationship of vitamin supplementation with malaria incidence in HIV-infected women, the offspring of our study participants were investigated for malaria incidence during the first 2 years of life.13 Relative to children of placebo recipients, children whose mothers received multivitamins alone had significantly reduced clinical malaria rates (71% reduction in malaria incidence) and children whose mothers received both multivitamins and vitamin A had lower rates of high malaria parasitemia defined as ≥5000 parasites per microliter (a 2%–33% reduction in incidence), although main effects of multivitamins were not statistically significant for both outcomes. These children were indirectly exposed to maternal supplements, and protection against malaria observed among children could have been because women who received multivitamin supplements had better health outcomes, which may have improved their capacity to care for their children. For example, children with healthier mothers may have been more likely to access malaria-prevention measures like insecticide-treated nets (ITNs), as access required travel to ITN distribution points.
Multivitamin supplementation increased women's risks of malaria parasitemia in this study. Several components of the multivitamin supplement may have encouraged malaria parasite growth, especially after women stopped receiving malaria prophylaxis because their pregnancies ended. Increased iron levels after the absorption-enhancing influence of vitamin C in the human gut31 could have increased parasite growth32—the iron absorption hypothesis is supported by previous findings that multivitamins improved hemoglobin concentrations of study participants.33 The antioxidant functions of vitamins C and E could have reduced parasite susceptibility to oxidative stressors,34 and folic acid could have contributed to DNA synthesis in parasites capable of using exogenous folate.35 Our finding of increased malaria parasitemia among recipients of multivitamin supplementation is consistent with findings from another randomized trial, which reported a tendency toward increased malaria parasite densities among children who received a supplement containing thiamine, riboflavin, vitamin C, and iron (our multivitamin supplement included these micronutrients, excepting iron) compared with the placebo,14 although the elevation in malaria parasitemia seen in that study may have been because the intervention included iron.
Supplementation with vitamin A did not significantly alter malaria incidence in our study. This is in contrast with the results of the vitamin A supplementation trial in children that found supplementation significantly reduced P. falciparum clinical episodes.15 Earlier vitamin A supplementation trials found no beneficial effects on P. falciparum density, probable malaria illness, or malaria-associated mortality.36
Our study is a randomized controlled trial, in which study participants and staff were unaware of intervention assignments, thus could not alter their behavior in favor of any study regimen. The factorial design enabled us to separately examine multivitamin and vitamin A effects, as well as the interactions between the two. However, misclassification of febrile episodes as clinical malaria outcomes may have occurred during the study. Additionally, the infrequency of routine blood film samples may have reduced our ability to detect some episodes of asymptomatic parasitemia. The potential for outcome misclassification is complicated by the fact that malaria parasitemia may be unaccompanied by symptoms in endemic areas18,37 and that clinical malaria can be associated with undetectable parasitemia, for instance when parasites are sequestered in internal organs.18
This study addresses health problems impacting millions globally, since malaria and HIV co-infection occur in many countries, especially in sub-Saharan Africa.37,38 HIV infection increases the risk of malaria. It is associated with reduced malaria immunity6,39—clinical malaria maybe severe or fatal in the nonimmune40,41—and with suboptimal response to malaria treatment in adults.42,43 Yet access to malaria-prevention interventions is still inadequate in many countries. While 52 countries affected by malaria are on track to reduce their malaria incidence by 75%, in keeping with World Health Assembly and Roll Back Malaria targets for 2015, these countries account for only 4% of global malaria cases.5
Our findings are especially generalizable to countries where ART is provided only to individuals with advanced HIV infection. Although some countries are adopting lifelong coverage of ART for all HIV-infected pregnant women regardless of disease severity (option B+),44 other countries continue to recommend lifelong ART only for those with advanced disease, and provide ART prophylaxis to pregnant women with less severe disease, to prevent mother-to-child transmission of HIV during the prenatal and perinatal periods (option A).45 As was previously reported, multivitamin supplementation during pregnancy and in the postpartum period is beneficial for HIV-infected women, reducing HIV disease progression12 and improving the hematologic status of women and their offspring.33 Multivitamin supplementation also improves pregnancy outcomes of HIV-infected women, reducing the risks of fetal death, low birth weight, and severe preterm birth,16 as well as reducing the incidence of clinical malaria13 among their offspring. Multivitamin supplements costing as little as a few cents per person per day could benefit those who are ineligible for ART, by slowing HIV progression and reducing complications, possibly including malaria. Malaria-prevention measures, including vector control methods and use of ITNs, are recommended by the WHO for all individuals at risk of malaria.5
Further research is needed on the effects of multivitamin supplementation in the context of adequate ART. Also, studies that isolate the effects of individual vitamins would improve our understanding of causal mechanisms. Daily use of multivitamin supplements by HIV-infected women in our study improved clinical outcomes, including reducing symptomatic malaria infections. The clinical significance of increased malaria parasitemia warrants additional research, but in light of evidence to date, multivitamin supplementation is appropriate as part of an integrated approach to the care of HIV-infected individuals that includes malaria-prevention measures.
ACKNOWLEDGMENTS
The authors would like to thank the women who participated in the study, without them the study could not occur. The authors also thank all study staff, including nurses, physicians, midwives, supervisors, laboratory staff, and administrative staff, who contributed time and effort to conducting the study. Finally, the authors thank the leadership of the Muhimbili University of Health and Allied Sciences, Muhimbili National Hospital, the City of Dar es Salaam Regional Health Authority, and the Tanzanian National AIDS Control Program for providing institutional support for the study.
Footnotes
C.D. was funded in part by NICHD (K24HD058795).
The authors have no other funding or conflicts of interest to disclose.
REFERENCES
- 1.Dellicour S, Tatem AJ, Guerra CA, et al. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med. 2010;7:e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGready R, Lee SJ, Wiladphaingern J, et al. Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population-based study. Lancet Infect Dis. 2012;12:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steketee RW, Nahlen BL, Parise ME, et al. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1–2 suppl):28–35. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. HIV/AIDS. Fact sheet N°360. Available at: http://www.who.int/mediacentre/factsheets/fs360/en/. Accessed February 25, 2014.
- 5.World Health Organization. World Malaria Report 2013. Geneva, Switzerland: World Health Organization; 2013. Available at: http://www.who.int/malaria/publications/world_malaria_report_2013/en/. Accessed February 25, 2014. [Google Scholar]
- 6.Whitworth J, Morgan D, Quigley M, et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet. 2000;356:1051–1056. [DOI] [PubMed] [Google Scholar]
- 7.Kublin JG, Patnaik P, Jere CS, et al. Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet. 2005;365:233–240. [DOI] [PubMed] [Google Scholar]
- 8.Ayisi JG, van Eijk AM, ter Kuile FO, et al. The effect of dual infection with HIV and malaria on pregnancy outcome in western Kenya. AIDS. 2003;17:585–594. [DOI] [PubMed] [Google Scholar]
- 9.Briand V, Badaut C, Cot M. Placental malaria, maternal HIV infection and infant morbidity. Ann Trop Paediatr. 2009;29:71–83. [DOI] [PubMed] [Google Scholar]
- 10.ter Kuile FO, Parise ME, Verhoeff FH, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-Saharan Africa. Am J Trop Med Hyg. 2004;71(2 suppl):41–54. [PubMed] [Google Scholar]
- 11.Ticconi C, Mapfumo M, Dorrucci M, et al. Effect of maternal HIV and malaria infection on pregnancy and perinatal outcome in Zimbabwe. J Acquir Immune Defic Syndr. 1999;34:289–294. [DOI] [PubMed] [Google Scholar]
- 12.Fawzi WW, Msamanga GI, Spiegelman D, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med. 2004;351:23–32. [DOI] [PubMed] [Google Scholar]
- 13.Villamor E, Msamanga G, Saathoff E, et al. Effects of maternal vitamin supplements on malaria in children born to HIV-infected women. Am J Trop Med Hyg. 2007;76:1066–1071. [PubMed] [Google Scholar]
- 14.Bates CJ, Powers HJ, Lamb WH, et al. Effect of supplementary vitamins and iron on malaria indices in rural Gambian children. Trans R Soc Trop Med Hyg. 1987;81:286–291. [DOI] [PubMed] [Google Scholar]
- 15.Shankar AH, Genton B, Semba RD, et al. Effect of vitamin A supplementation on morbidity due to Plasmodium falciparum in young children in Papua New Guinea: a randomized trial. Lancet. 1999;354:203–209. [DOI] [PubMed] [Google Scholar]
- 16.Fawzi WW, Msamanga GI, Spiegelman D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet. 1998;351:1477–1482. [DOI] [PubMed] [Google Scholar]
- 17.Baum MK, Campa A, Lai S, et al. Effect of micronutrient supplementation on disease progression in asymptomatic, antiretroviral-naive, HIV-infected adults in Botswana: a randomized clinical trial. JAMA. 2013;310:2154–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36 Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plebanski M, Hill AV. The immunology of malaria infection. Curr Opin Immunol. 2000;12:437–441. [DOI] [PubMed] [Google Scholar]
- 20.Fawzi WW, Msamanga GI, Spiegelman D, et al. Rationale and design of the Tanzania Vitamin and HIV Infection Trial. Control Clin Trials. 1999;20:75–90. [DOI] [PubMed] [Google Scholar]
- 21.Fawzi WW, Msamanga GI, Hunter D, et al. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS. 2002;16:1935–1944. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Interim proposal for a WHO Staging System for HIV infection and Disease [in English, French]. Wkly Epidemiol Rec. 1990;65:221–224. [PubMed] [Google Scholar]
- 23.World Health Organization. Basic Laboratory Methods in Medical Parasitology. Geneva, Switzerland: World Health Organization; 1991. Available at: http://whqlibdoc.who.int/publications/9241544104_%28part1%29.pdf. Accessed February 25, 2014. [Google Scholar]
- 24.Cox DR. Regression models and lifetables. J R Stat Soc (B). 1972;34:187–220. [Google Scholar]
- 25.SAS [computer program]. Version 9.2. Cary, NC: SAS Institute; 2010.
- 26.Kanter AS, Spencer DC, Steinberg MH, et al. Supplemental vitamin B and progression to AIDS and death in black South African patients infected with HIV. J Acquir Immune Defic Syndr. 1999;21:252–253. [DOI] [PubMed] [Google Scholar]
- 27.Tang AM, Graham NM, Saah AJ. Effects of micronutrient intake on survival in human immunodeficiency virus type 1 infection. Am J Epidemiol. 1996;143:1244–1256. [DOI] [PubMed] [Google Scholar]
- 28.Jiamton S, Pepin J, Suttent R, et al. A randomized trial of the impact of multiple micronutrient supplementation on mortality among HIV-infected individuals living in Bangkok. AIDS. 2003;17:2461–2469. [DOI] [PubMed] [Google Scholar]
- 29.Allard JP, Aghdassi E, Chau J, et al. Effects of vitamin E and C supplementation on oxidative stress and viral load in HIV-infected subjects. AIDS. 1998;12:1653–1659. [DOI] [PubMed] [Google Scholar]
- 30.Anglaret X, Dakoury-Dogbo N, Bonard D, et al. Causes and empirical treatment of fever in HIV-infected adult outpatients, Abidjan, Cote d'Ivoire. AIDS. 2002;16:909–918. [DOI] [PubMed] [Google Scholar]
- 31.Johnston CS. Vitamin C. In: Erdman JW, Jr, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed Hoboken, NJ: John Wiley & Sons, Inc.; 2012:248–260. [Google Scholar]
- 32.Doherty CP. Host-pathogen interactions: the role of iron. J Nutr. 2007;137:1341–1344. [DOI] [PubMed] [Google Scholar]
- 33.Fawzi WW, Msamanga GI, Kupka R, et al. Multivitamin supplementation improves hematologic status in HIV-infected women and their children in Tanzania. Am J Clin Nutr. 2007;85:1335–1343. [DOI] [PubMed] [Google Scholar]
- 34.Levander OA, Ager AL., Jr Malarial parasites and antioxidant nutrients. Parasitology. 1993;107(suppl):S95–S106. [DOI] [PubMed] [Google Scholar]
- 35.Metz J. Folic acid metabolism and malaria. Food Nutr Bull. 2007;28(4 suppl):S540–S549. [DOI] [PubMed] [Google Scholar]
- 36.Binka FN, Ross DA, Morris SS, et al. Vitamin A supplementation and childhood malaria in northern Ghana. Am J Clin Nutr. 1995;61:853–859. [DOI] [PubMed] [Google Scholar]
- 37.Flateau C, Le Loup G, Pialoux G. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis. 2011;11:541–556. [DOI] [PubMed] [Google Scholar]
- 38.Slutsker L, Marston BJ. HIV and malaria: interactions and implications. Curr Opin Infect Dis. 2007;20:3–10. [DOI] [PubMed] [Google Scholar]
- 39.Patnaik P, Jere CS, Miller WC, et al. Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural Malawi. J Infect Dis. 2005;192:984–991. [DOI] [PubMed] [Google Scholar]
- 40.Cohen C, Karstaedt A, Frean J, et al. Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin Infect Dis. 2005;41:1631–1637. [DOI] [PubMed] [Google Scholar]
- 41.Grimwade K, French N, Mbatha DD, et al. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS. 2004;18:547–554. [DOI] [PubMed] [Google Scholar]
- 42.Kamya MR, Gasasira AF, Yeka A, et al. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. J Infect Dis. 2006;193:9–15. [DOI] [PubMed] [Google Scholar]
- 43.Van Geertruyden JP, Mulenga M, Mwananyanda L, et al. HIV-1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malaria. J Infect Dis. 2006;194:917–925. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2013. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/en/. Accessed February 25, 2014. [PubMed] [Google Scholar]
- 45.World Health Organization. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Recommendations for a Public Health Approach (2010 Version). Geneva, Switzerland: World Health Organization; 2010. Available at: http://www.who.int/hiv/pub/mtct/antiretroviral2010/en/. Accessed February 25, 2014. [PubMed] [Google Scholar]


