Abstract
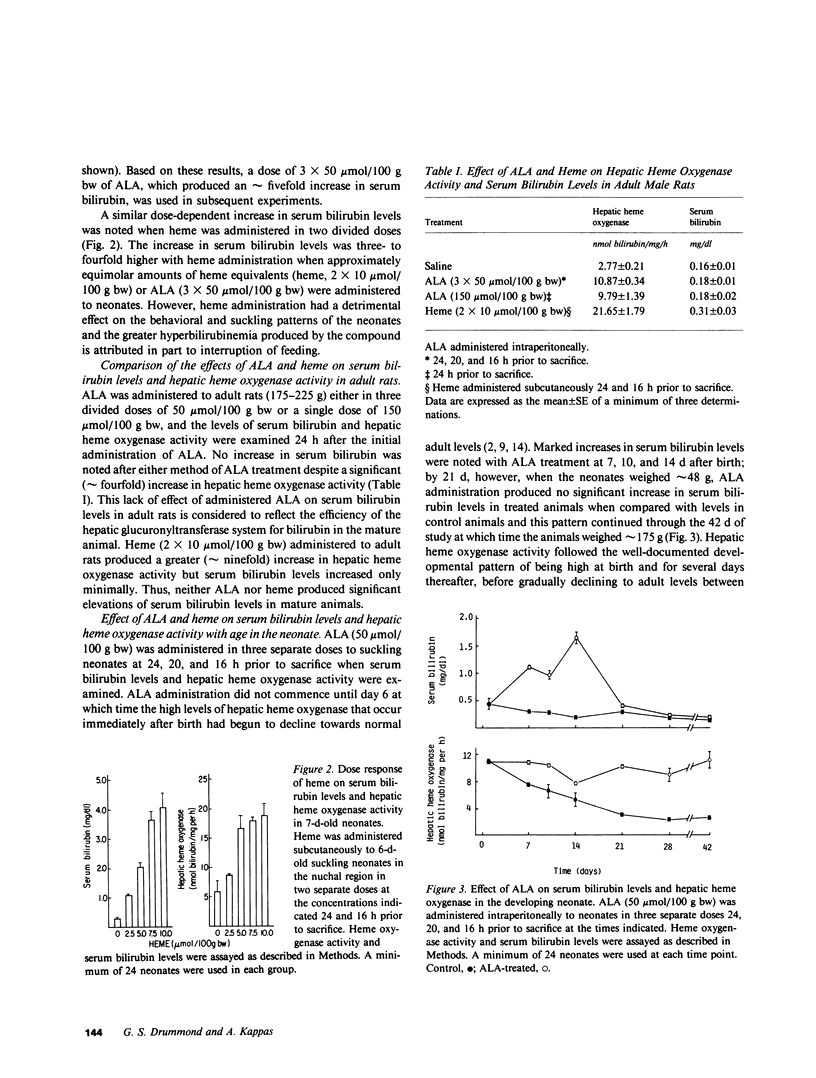
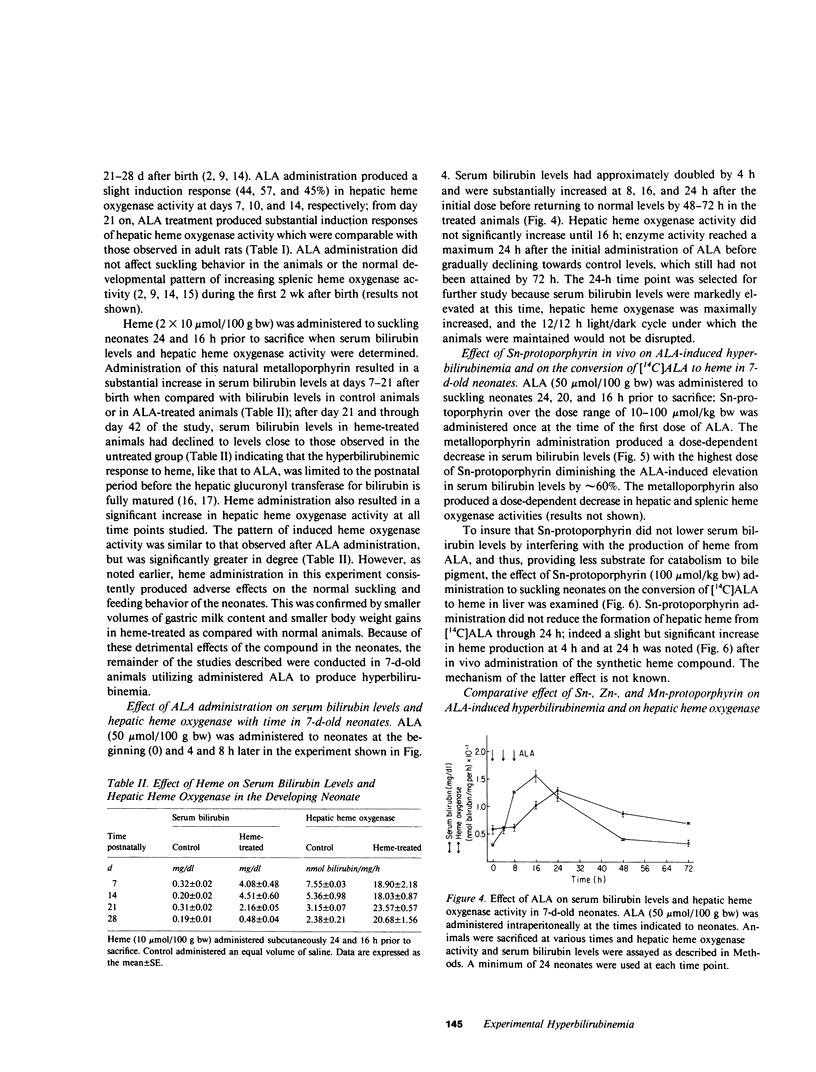
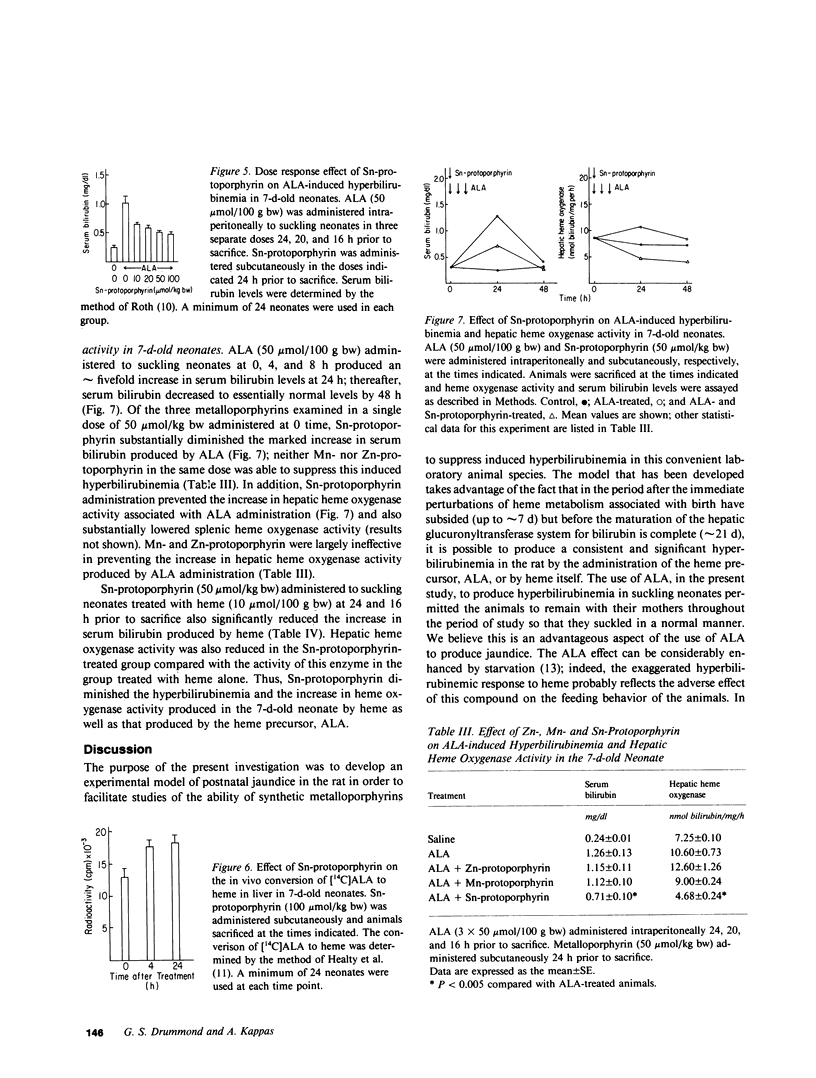
A model of experimental postnatal hyperbilirubinemia in the rat has been developed utilizing the heme precursor delta-aminolevulinic acid (ALA) to produce jaundice during a selective time period after birth. This time period is defined as that between 7 d postnatally, when the initial postpartum alterations of serum bilirubin and heme metabolism in the neonate have subsided, and 21 d, when the hepatic conjugation mechanism for the bile pigment appears fully developed. Administration of ALA in this time period led to a rapid, consistent, and significant dose-dependent increase in serum bilirubin levels in the newborn animals. Heme administration produced a qualitatively similar but enhanced effect. Both compounds, in addition, induced a dose-dependent increase in hepatic heme oxygenase activity concomitant with the increase in serum bilirubin levels. Neither compound increased serum bilirubin levels significantly when administered at or after 21 d postnatally. Administration of the synthetic metalloporphyrin, Sn-protoporphyrin, to ALA-treated neonates resulted in a dose-dependent decrease in serum bilirubin levels and hepatic heme oxygenase activity. Mn- and Zn-protoporphyrin in comparable doses did not significantly inhibit ALA-induced hyperbilirubinemia. Sn-protoporphyrin also inhibited the hyperbilirubinemia produced by heme in the suckling animals. ALA administration to newborn rats during the specific postnatal period described provides a simple and convenient model of experimental jaundice in the developing neonate which permits an examination of the potential ability of synthetic metalloporphyrins or other compounds to suppress induced hyperbilirubinemia in the newborn animal. The ability to induce a consistent and significant degree of jaundice in the postnatal rat by the method described may also be useful for other types of studies concerned with the biological disposition and effects of endogenously formed bilirubin in the neonate. The results of this study confirm in another model system the potent ability of Sn-protoporphyrin to suppress jaundice in the neonate, and suggest that suppression of heme oxidation by synthetic heme analogues may represent a useful therapeutic approach to the problem of severe hyperbilirubinemia in human premature newborn.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E., Drummond G. S., Freddara U., Sardana M. K., Sassa S. Porphyrogenic effects and induction of heme oxygenase in vivo by delta-aminolevulinic acid. Biochim Biophys Acta. 1981 Sep 4;676(3):289–299. doi: 10.1016/0304-4165(81)90162-8. [DOI] [PubMed] [Google Scholar]
- Anderson K. E., Simionatto C. S., Drummond G. S., Kappas A. Tissue distribution and disposition of tin-protoporphyrin, a potent competitive inhibitor of heme oxygenase. J Pharmacol Exp Ther. 1984 Feb;228(2):327–333. [PubMed] [Google Scholar]
- Brodersen R. Bilirubin transport in the newborn infant, reviewed with relation to kernicterus. J Pediatr. 1980 Mar;96(3 Pt 1):349–356. doi: 10.1016/s0022-3476(80)80671-8. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Chemoprevention of neonatal jaundice: potency of tin-protoporphyrin in an animal model. Science. 1982 Sep 24;217(4566):1250–1252. doi: 10.1126/science.6896768. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Prevention of neonatal hyperbilirubinemia by tin protoporphyrin IX, a potent competitive inhibitor of heme oxidation. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6466–6470. doi: 10.1073/pnas.78.10.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Suppression of hyperbilirubinemia in the rat neonate by chromium-protoporphyrin. Interactions of metalloporphyrins with microsomal heme oxygenase of human spleen. J Exp Med. 1982 Dec 1;156(6):1878–1883. doi: 10.1084/jem.156.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond G. S., Rosenberg D. W., Kappas A. Metal induction of haem oxygenase without concurrent degradation of cytochrome P-450. Protective effects of compound SKF 525A on the haem protein. Biochem J. 1982 Jan 15;202(1):59–66. doi: 10.1042/bj2020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halac E., Jr, Sicignano C. Re-evaluation of the influence of sex, age pregnancy, and phenobarbital on the activity of UDP-glucuronyl transferase in rat liver. J Lab Clin Med. 1969 Apr;73(4):677–685. [PubMed] [Google Scholar]
- Healey J. F., Bonkowsky H. L., Sinclair P. R., Sinclair J. F. Conversion of 5-aminolaevulinate into haem by liver homogenates. Comparison of rat and chick embryo. Biochem J. 1981 Sep 15;198(3):595–604. doi: 10.1042/bj1980595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappas A., Drummond G. S., Simionatto C. S., Anderson K. E. Control of heme oxygenase and plasma levels of bilirubin by a synthetic heme analogue, tin-protoporphyrin. Hepatology. 1984 Mar-Apr;4(2):336–341. doi: 10.1002/hep.1840040227. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine R. L., Fredericks W. R., Rapoport S. I. Entry of bilirubin into the brain due to opening of the blood-brain barrier. Pediatrics. 1982 Mar;69(3):255–259. [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Study of the developmental pattern of heme catabolism in liver and the effects of cobalt on cytochrome P-450 and the rate of heme oxidation during the neonatal period. J Exp Med. 1975 Jun 1;141(6):1400–1410. doi: 10.1084/jem.141.6.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D. Zinc . protoporphyrin is a selective inhibitor of heme oxygenase activity in the neonatal rat. Biochim Biophys Acta. 1981 Mar 18;673(3):339–350. doi: 10.1016/0304-4165(81)90465-7. [DOI] [PubMed] [Google Scholar]
- Pimstone N. R., Engel P., Tenhunen R., Seitz P. T., Marver H. S., Schmid R. Inducible heme oxygenase in the kidney: a model for the homeostatic control of hemoglobin catabolism. J Clin Invest. 1971 Oct;50(10):2042–2050. doi: 10.1172/JCI106697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. Dosage fluorimétrique de la bilirubine. Clin Chim Acta. 1967 Sep;17(3):487–492. doi: 10.1016/0009-8981(67)90225-2. [DOI] [PubMed] [Google Scholar]
- Sassa S., Drummond G. S., Bernstein S. E., Kappas A. Tin-protoporphyrin suppression of hyperbilirubinemia in mutant mice with severe hemolytic anemia. Blood. 1983 May;61(5):1011–1013. [PubMed] [Google Scholar]
- Schacter B. A., Joseph E., Firneisz G. Effect of cholestasis produced by bile duct ligation on hepatic heme and hemoprotein metabolism in rats. Gastroenterology. 1983 Feb;84(2):227–235. [PubMed] [Google Scholar]
- Song C. S., Moses H. L., Rosenthal A. S., Gelb N. A., Kappas A. The influence of postnatal development on drug-induced hepatic porphyria and the synthesis of cytochrome P-450. A biochemical and morphological study. J Exp Med. 1971 Nov 1;134(5):1349–1371. doi: 10.1084/jem.134.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970 Mar;75(3):410–421. [PubMed] [Google Scholar]
- Thaler M. M., Gemes D. L., Bakken A. F. Enzymatic conversion of heme to bilirubin in normal and starved fetuses and newborn rats. Pediatr Res. 1972 Mar;6(3):197–201. doi: 10.1203/00006450-197203000-00008. [DOI] [PubMed] [Google Scholar]
- Turkel S. B., Miller C. A., Guttenberg M. E., Moynes D. R., Godgman J. E. A clinical pathologic reappraisal of kernicterus. Pediatrics. 1982 Mar;69(3):267–272. [PubMed] [Google Scholar]
- Yoshinaga T., Sassa S., Kappas A. Purification and properties of bovine spleen heme oxygenase. Amino acid composition and sites of action of inhibitors of heme oxidation. J Biol Chem. 1982 Jul 10;257(13):7778–7785. [PubMed] [Google Scholar]



