Abstract
In this study, we used muscle and motor unit indices, derived from convenient surface electromyography (EMG) measurements, for examination of paretic muscle changes post stroke. For 12 stroke subjects, compound muscle action potential and voluntary surface EMG signals were recorded from paretic and contralateral first dorsal interosseous, abductor pollicis brevis, and abductor digiti minimi muscles. Muscle activation index (AI), motor unit number index (MUNIX), and motor unit size index (MUSIX) were then calculated for each muscle. There was a significant AI reduction for all the three muscles in paretic side compared with contralateral side, providing an evidence of muscle activation deficiency after stroke. The hand MUNIX (defined by summing the values from the three muscles) was significantly reduced in paretic side compared with contralateral side, whereas the hand MUSIX was not significantly different. Furthermore, diverse changes in MUNIX and MUSIX were observed from the three muscles. A major feature of the present examinations is the primary reliance on surface EMG, which offers practical benefits because it is noninvasive, induces minimal discomfort and can be performed quickly.
Index Terms: Chronic stroke, compound muscle action potential (CMAP), motor unit number index (MUNIX), motor unit size index (MUSIX), muscle activation, surface electromyography (EMG)
I. Introduction
Stroke, being a leading cause of adult disability and a third leading cause of death, has a detrimental effect on health-related quality of life. Following a stroke, patients may suffer from a variety of physical symptoms (such as spastic hypertonia, weakness, and impaired movement coordination) on the contralesional side of the body, among which weakness or inability to generate normal levels of muscle force has been recognized as one of the most serious impairment leading to disability in stroke patients [1]. Muscle strength deficits of stroke patients and their associations with performance on functional tasks have been extensively investigated. A study has shown a strong negative correlation between lower limb muscle strength deficits and gait performance [2]. The relation between strength deficits of upper limb muscles and arm performance has also been studied [3]–[6]. For example, hand grip strength was shown to be a good predictor of motor performance and therefore, serves a valuable marker for recovery of arm function for stroke patients [6].
To understand motor function impairment after stroke, a variety of electrophysiological studies have been performed to examine hemiparetic muscle changes at different levels, focusing on muscle fiber (single fiber electromyography (EMG) [7], [8], [14]), motor unit (concentric needle EMG [7], [9] [13], fine wire EMG [16], [17], and macro EMG [15]), and global muscle (surface EMG [10]–[12]) activities, respectively. Using incremental nerve stimulations, motor unit number estimation (MUNE) techniques that rely on laborious estimation of single motor unit size have been applied to assess loss of functional motor units in paretic muscles, as a result of spinal motoneuron degeneration after stroke [18]–[20]. With advances in both surface EMG recording and processing techniques, high-density surface electrodes have also found applications in revealing hemiparetic muscle changes at both the global muscle and motor unit levels [21]. However, most of the previous methods tend to be laborious, require expensive equipment, or require patients to tolerate needles.
Among a range of electrophysiological examinations, probably the most convenient and easy-to-apply methods are non-invasive measurements of voluntary surface EMG and compound muscle action potentials (CMAP) with conventional surface electrodes. In this study we used indices derived from such measurements to examine paretic muscle changes post stroke. Specifically, we investigated application of motor unit number index (MUNIX), motor unit size index (MUSIX) [22], [23], and muscle activation index (AI) [24] for examination of three hand muscles. We examined whether these indices were significantly different between paretic and contralateral hands. Such examination can provide useful data to help understand the underlying neuromuscular mechanisms of stroke induced motor impairment. This will also help guide development of appropriate strategies for stroke rehabilitation.
II. Methods
A. Subjects
Twelve subjects (7 male, 5 female, 59.9 ± 12.0 years) who sustained hemispheric stroke participated in this study. All the subjects were recruited by using the Clinical Neuroscience Research Registry at the Rehabilitation Institute of Chicago (Chicago, IL, USA). A screening examination and clinical assessment were performed to determine the eligibility for each stroke subject (Table I). Hand grip strength was measured bilaterally for each stroke subject using a portable Jamar Plus digital hand dynamometer (model EN-120604), and a comparison of grip strength of paretic and contralateral hands of the stroke subjects is presented as part of Table I. The study was approved by the Institutional Review Board of Northwestern University (Chicago, IL, USA). All the subjects gave their written consent before any assessment or recording procedures.
Table I. Stroke Subject Information.
| ID | Age | Sex | Duration | P(D) Side | FM | CM | Grip (P) | Grip (C) |
|---|---|---|---|---|---|---|---|---|
| 1 | 70 | M | 17.4 | L(R) | 21 | 3 | 4.8 | 30.5 |
| 2 | 68 | M | 8.9 | L(L) | 21 | 3 | 2.9 | 29.1 |
| 3 | 56 | F | 12.8 | L(L) | 6 | 2 | 2.6 | 20.8 |
| 4 | 64 | M | 14.9 | R(R) | 17 | 2 | 11.9 | 52.9 |
| 5 | 44 | M | 4.4 | R(R) | 58 | 5 | 24.0 | 68.0 |
| 6 | 81 | M | 16.4 | R(R) | 21 | 2 | 9.6 | 35.7 |
| 7 | 72 | M | 15.9 | R(R) | 47 | 5 | 8.6 | 26.3 |
| 8 | 61 | F | 14.2 | L(R) | 34 | 3 | 8.1 | 31.3 |
| 9 | 48 | M | 1.0 | R(L) | 48 | 6 | 23.1 | 50.7 |
| 10 | 57 | F | 1.1 | R(R) | 12 | 2 | 6.9 | 19.7 |
| 11 | 40 | F | 4.4 | L(R) | 11 | 2 | 9.3 | 29.6 |
| 12 | 58 | F | 24.7 | R(R) | 14 | 2 | 9.0 | 30.0 |
ID: subject identification. Age: years. Duration: years since the onset of stroke. P(D) Side: the side of hemiparesis with the side of hand dominance before stroke indicated in the parenthesis. FM: the Fugl-Meyer assessment scale of the paretic upper-limb (total score: 66). CM: the hand impairment part of the Chedoke-McMaster stroke assessment scale (from 1 to 7). Grip (P): the maximum grip strength (kilogram) of the paretic hand. Grip (C): the maximum grip strength (kilogram) of the contralateral hand.
B. Data Acquisition
The experiments were performed on both the affected and contralateral hands in all the stroke subjects. Three muscles were examined for each hand: the first dorsal interosseous (FDI), the abductor pollicis brevis (APB), and the abductor digiti minimi (ADM) muscles. A random order was used for examination of different muscles. Subjects were seated comfortably in a chair with the examined forearm placed in a natural position on a height-adjustable table. Subjects were instructed to relax at the wrist, elbow, and shoulder. A constant laboratory temperature (approximately 295 K) was maintained during the data collection.
Prior to the recording, each subject's skin over the ulnar and medial aspects of the wrist, the back of the hand and the palm, the index finger, the thumb, and the little finger were lightly abraded and cleaned with rubbing alcohol. A small amount of conductive electrode cream was applied to reduce skin–electrode impedance and then wiped to ensure not to leave any on the skin (to avoid short-circuiting the electrodes). The primary equipment used for this study was Sierra Wave EMG system (Cadwell Lab Inc, Kennewick, WA, USA). Electrode placement was similar to that for standard motor conduction studies [25]. Two 10-mm (diameter) silver/silver chloride disc surface electrodes were used to record surface EMG from the examined muscle. The active surface electrode was positioned over the examined muscle with the reference surface electrode placed on the second metacarpophalangeal joint for the FDI muscle, on the distal phalanx of the little finger for the ADM muscle, and on the metacarpal phalangeal joint of the thumb for the APB muscle, respectively. For each of the recordings, an adhesive large ground electrode was placed on the dorsum of the hand between the stimulus and recording sites. All surface electrode positions were further reinforced with surgical tapes to reduce movement during the recording. A remote handheld stimulator with a StimTroller was used to generate stimuli through a cathode (a 10-mm silver/silver chloride pole). The duration of each single pulse stimulus was 200 μs. The M wave was evoked with a cathode placed 2-cm proximal to the wrist crease over the ulnar nerve (for the FDI or ADM muscles) or the median nerve (for the APB muscle). For each examined muscle, different electrode positions were tested to ensure that the CMAP amplitude was maximized. A CMAP from each muscle was obtained by stimulation of the ulnar or median nerve at the wrist using intensity sufficient to elicit a maximum response. The stimulation intensity started around 15 mA, increasing approximately 20% above that amplitude each step, until the maximum motor response was reached. This was confirmed by no enlargement in the peak-to-peak amplitude of the M wave with further increased stimulation intensities.
After the CMAP recording, the voluntary surface EMG signals were recorded from the examined muscle with the electrodes maintained at the same position. The subject was instructed to generate an isometric muscle contraction at four to six different levels representing minimal to maximal effort (index finger abduction for the FDI muscle, thumb abduction for the APB muscle, and little finger abduction for the ADM muscle). The force levels were defined by the examiner providing resistance to the tested muscle. The different force levels were performed using a single trial with graded contraction consisting of four to six interference EMG epochs (each graded contraction lasting for at least 2 s). Two trials were collected for each muscle. Substantial rest was allowed during the experiment to avoid muscle fatigue.
For all the subjects, the CMAP and voluntary surface EMG recordings were sampled at 6.4 and 32 kHz, with a bandpass filter setting at 3 Hz–2 kHz and 10 Hz–10 kHz, respectively (highcut filter: 2-pole, 12 dB/octave; lowcut filter: 1-pole, 6 dB/octave). A system notch filter was used to remove the power line interference noise from the voluntary surface EMG but not for CMAP signals. All the signals were recorded by a differential ac amplifier and stored to a hard disk for offline analysis.
C. Data Analysis
1) MUNIX Calculation
The CMAP and different levels of surface interference pattern (SIP) EMG were used to compute the MUNIX for the examined muscle. The details of MUNIX calculation have been described in [22] and [23]. In brief, the area and power of the CMAP and the SIPs (for a one-second epoch) at different voluntary contraction levels were first calculated. These values were used to compute the “ideal case motor unit count (ICMUC),” defined as the ratio of the CMAP power multiplying SIP area to CMAP area multiplying SIP power. The relation between the ICMUC and SIP area was modeled as ICMUC = β(SIP area)α, and linear regression between logarithms of ICMUC and SIP area was used to estimate β and α. The MUNIX was calculated as the ICMUC value when the SIP area was 20 mV · ms, i.e., MUNIX = β(20)α. In MUNIX analysis, it is noted that very low amplitude voluntary surface EMG signals may give very high ICMUC values. To reduce this artifact, following Nandedkar et al. [23], three criteria were imposed to accept an SIP epoch: (1) SIP area > 20 mV · ms; (2) ICMUC < 100; and (3) SIP area/CMAP area > 1. In addition, only those CMAPs whose amplitude is greater than 0.5 mV were accepted for the MUNIX analysis. In addition to MUNIX, the MUSIX, defined as the ratio of the CMAP amplitude to the MUNIX, was also calculated.
2) Muscle AI Calculation
For each examined muscle, we calculated the average rectified value of the surface EMG signal (1 s duration) at the MVC level and normalized it to the CMAP amplitude to obtain the muscle activation index (AI). Such processing can minimize the peripheral effects and provide assessment of upper motoneuron activation [24].
3) Statistical Analysis
We measured the CMAP, MUNIX, MUSIX, and muscle AI in each of the paretic and contralateral muscles of stroke subjects, and further obtained hand index of each parameter by summing or averaging the measurements from the three muscles. Repeated measures ANOVA (RM- ANOVA) using linear mixed model (LMM) was performed to examine the differeces of the aformentioned parameters between the paretic and cotralateral sides (SPSS Inc., Chicago, IL, USA). It has been discussed that the LMM is more powerful to analyze repeated measures observations than other models, such as generalized linear model (GLM) [26]. Akaike's Information Criteria (AIC) was used as a criterion of goodness-of-fit to determine the best covariance structure for the RM-ANOVA model [27]. We found that compound symmetry covariance structure was more appropriate to analyze the data than other covariance structures. Pairwise comparison using the Bonferroni correction with family confidence coefficient 0.95 was calculated for the significant effects in the RM-ANOVA posthoc tests. In addition, correlation analysis was conducted to determine the relationship between the relative reduction of hand grip strength and the relative alterations in each of the examined parameters. All data were presented in the form of mean ± standard error in the rest of the paper unless specified.
III. Experimental Results
Hand MUNIX estimation involved calculation of MUNIX values in the FDI, APB, and ADM muscles. Examples of the individual muscle's MUNIX calculation in the paretic and contralateral hands of a stroke subject (Subject 9) are presented in Fig. 1. It was observed that the surface EMG signals at MVC for each examined muscle were much lower in the paretic side than the contralateral side. Likewise, the CMAP amplitude of the three examined muscles was also lower in the paretic hand than in the contralateral hand. The MUNIX estimates, however, demonstrated reduced values in the paretic FDI and APB muscles and slightly higher MUNIX values in the paretic ADM muscle compared with the contralateral side. We noted that the MUSIX values were lower for the paretic FDI and ADM muscles and higher for the paretic APB muscle compared with the contralateral side. By summing the values of the three muscles to obtain the hand CMAP and motor unit index, we found that the hand CMAP amplitude and MUNIX were lower in the paretic side compared with the contralateral side (paretic hand CMAP = 27.8 mV, contralateral hand CMAP = 35.2 mV; paretic hand MUNIX = 500, contralateral hand MUNIX = 589). The hand MUSIX was slightly lower in the paretic side than the contralateral side (paretic hand MUSIX = 169.9 μV, contralateral hand MUSIX = 180.7 μV).
Fig. 1.
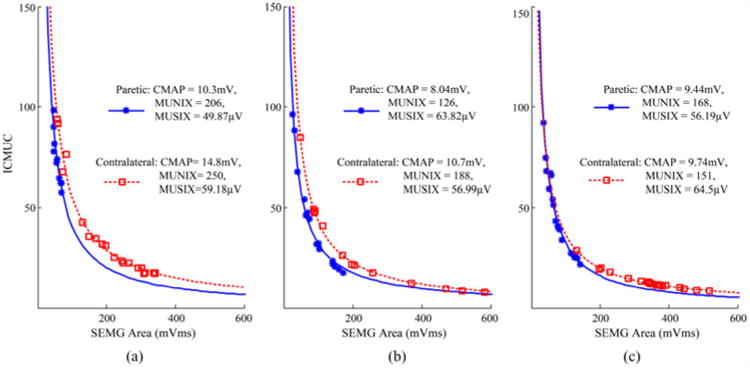
Computation of MUNIX in (a) FDI, (b) APB, and (c) ADM muscles in both the paretic and contralateral sides of a stroke subject (Subject 9). Paretic: solid lines with stars; contralateral: dotted lines with squares. MUNIX is defined as the ICMUC value (Y-axis) when the surface EMG area (X-axis) equals 20 mV ms.
When we averaged the values of 12 stroke subjects [see Fig. 2(a), (b)], the LMM indicated a significant reduction of the hand CMAP amplitude and hand MUNIX values in the paretic side compared with the contralateral side (paretic hand CMAP: 22.7 ± 4.3 mV, contralateral hand CMAP: 28.0 ± 2.6 mV, p < 0.01; paretic hand MUNIX: 401 ± 77, contralateral hand MUNIX: 463 ± 69, p < 0.05).
Fig. 2.
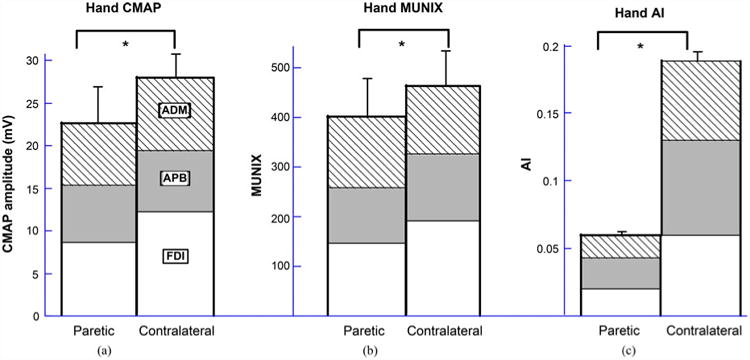
Comparison of (a) hand CMAP amplitude; (b) hand MUNIX; and (c) hand AI values between paretic and contralateral sides of 12 stroke subjects. The bars represent standard errors. The asterisk (*) indicates significant difference between the two groups. For detailed comparison of specific muscles please refer to the text.
Statistical analysis of individual muscle's CMAP and motor unit indices, however, showed diverse results. For the FDI muscle, the CMAP amplitude and MUNIX values were substantially lower in the paretic side compared with the contralateral side (paretic FDI CMAP: 8.7 ± 1.1 mV, contralateral FDI CMAP: 12.4 ± 0.6 mV, p < 0.01; paretic FDI MUNIX: 147 ± 21, contralateral FDI MUNIX: 191 ±17, p < 0.05). The MUSIX values in the paretic muscle were lower than the contralateral side, but no significance existed (paretic FDI MUSIX: 60.9 ± 3.1 μV, contralateral FDI MUSIX: 70.0 ± 6.2 μ V, p > 0.2).
In contrast, for the APB muscle, there was no significant difference in either the mean CMAP amplitude or the MUNIX values between the paretic and contralateral muscles (paretic APB CMAP: 6.7±0.6 mV, contralateral APB CMAP: 7.2 ± 0.6 mV, p > 0.4; paretic APB MUNIX: 112 ± 10, contralateral APB MUNIX: 135 ± 14, p > 0.2). The MUSIX values in the paretic muscle were higher than the contralateral side, but no significance existed (paretic APB MUSIX: 59.9 ± 2.0 μV, contralateral APB MUSIX: 55.5 ± 2.7μV, p > 0.4).
Analysis of the ADM muscles indicated a decrease of CMAP amplitude in the paretic side (paretic ADM CMAP: 7.3 ± 0.7 mV, contralateral ADM CMAP: 8.5 ± 0.5 mV, p = 0.05). The MUNIX values from the paretic and contralateral muscles were comparable (paretic ADM MUNIX: 140 ± 21, contralateral ADM MUNIX: 138 ± 10, p > 0.9). The MUSIX values in the paretic muscle were lower than the contralateral side, but no significance existed (paretic ADM MUSIX: 55.4 ± 3.1 μV, contralateral ADM MUSIX: 64.1 ± 5.0 μV, p > 0.2).
The average muscle AI was calculated for all subjects across the FDI, APB, and ADM muscles [see Fig. 2(c)]. A consistent observation was made that for each examined muscle, the AI was significantly reduced in the paretic side compared with the contralateral side (paretic FDI AI = 0.0198 ± 0.0036, contralateral FDI AI = 0.0595 ± 0.0166, p < 0.05; paretic APB AI = 0.0229 ± 0.003, contralateral APB AI = 0.0696 ± 0.0054, p < 0.001; paretic ADM AI = 0.0167 ± 0.0029, contralateral ADM AI = 0.0586 ± 0.006, p < 0.01). Comparison of the hand AI (averaged from the three muscles) between paretic and contralateral sides revealed significant lower values in the paretic hand (paretic hand AI = 0.0198 ± 0.0022, contralateral hand AI = 0.0626 ± 0.0085, p < 0.01).
As Fig. 3 shows, the correlation between hand MUNIX and hand CMAP amplitude demonstrated a moderate to strong linear relation in both the paretic and contralateral sides (paretic: R2 = 0.91, p < 0.01; contralateral: R2 = 0.38, p < 0.05). No covariate effect from age, duration after stroke onset, chedoke or Fugl-Meyer assessment, and grip force (p > 0.5 for all) was found on any response variables including the CMAP, MUNIX, MUSIX, and AI.
Fig. 3.
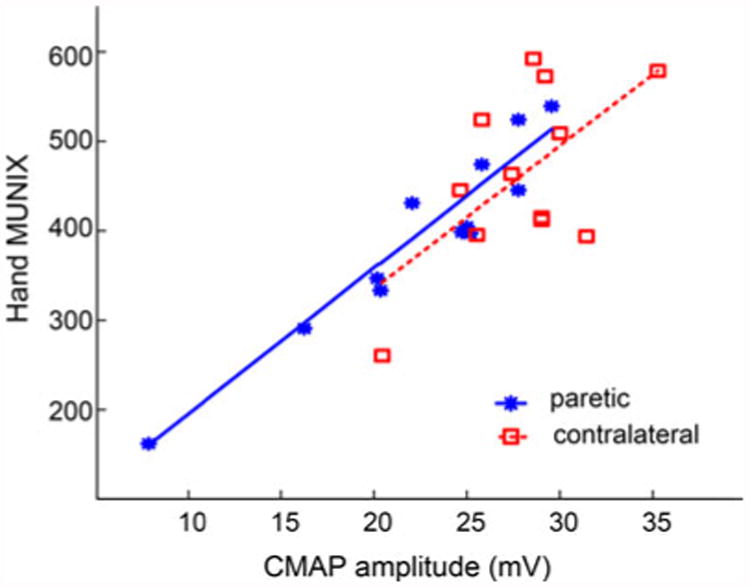
Correlations between the hand CMAP amplitude and MUNIX values for both paretic and contralateral sides of stroke subjects.
Table II presents a summary of comparison in the examined parameters between paretic and contralateral muscles.
Table II. Summary of Results.
| FDI | APB | ADM | |
|---|---|---|---|
| CMAP | P < C(*) | P < C | P < C |
| MUNIX | P < C(*) | P < C | P ∼C |
| MUSIX | P < C | P > C | P < C |
| Muscle AI | P < C(*) | P < C(*) | P < C(*) |
P: paretic side; C: contralateral side. The asterisk (*) indicates significant difference between the two groups.
IV. Discussion
We used muscle and motor unit indices, derived from convenient surface EMG measurements, for examination of paretic muscle changes post stroke. While various electrophysiological methods (such as needle EMG [7]–[9], [13]–[17], high-density surface EMG [21], MUNE [18]–[20], etc.) have been used for examination of hemiparetic muscles, these methods are laborious, require expensive equipment, or require patients to tolerate needles.
In the current study, we chose to use voluntary surface EMG and CMAP recordings, which are noninvasive, simple to perform, and well tolerated by patients. The data analysis procedures for deriving motor unit or muscle indices are also straightforward and quick to implement. For example, the MUNIX technique only requires minimum amounts of electrical stimulation to acquire CMAP, and thus can overcome laborious and time-consuming estimation of the single motor unit potential size (using either incremental nerve stimulation or spike triggered averaging techniques), required by the traditional MUNE methods [28], [29]. Because of this advantage, the MUNIX technique has recently achieved increasing applications, among which most studies focused on detecting motoneuron loss and measuring disease progression in amyotrophic lateral sclerosis [30]–[38]. The validity of the MUNIX measurement in assessing motoneuron diseases has been confirmed by experimental comparisons between MUNE and MUNIX results [39], [40], as well as a simulation approach that relies on motoneuron pool and surface EMG models [41], [42]. By systematically varying the model inputs, it was demonstrated that the MUNIX estimates closely correlate with the CMAP, while variation of other factors (such as motor unit recruitment and rate coding strategies, adjustment of motor unit recruitment range, reduction of motor unit firing rates, etc.) is relatively not sensitive to MUNIX estimation. This supports that MUNIX estimates can approximately characterize motor unit number changes if only varying the number of input motor units to the model (while keeping the motoneuron pool and muscle parameters unchanged). One advantage of the MUNIX measurement over the CMAP is that compared with the latter, the former is more sensitive to motor unit loss with compensatory muscle fiber reinnervation [23], [41]. However, the simulation results also indicated reduction of the input action potential amplitude would substantially underestimate the motor unit numbers, implying potential limitations of the MUNIX methods (because it may be unclear whether the MUNIX reduction is induced by loss of motor units or loss of muscle fiber size). In such a situation, MUSIX measurement, as performed in this study, is of much importance. Reduction of MUNIX in combination with enlarged MUSIX can provide evidence of loss of motor units. If MUNIX is reduced with lack of MUSIX changes, the drop of MUNIX may be either from actual motor unit loss (without sufficient compensatory muscle fiber reinnervation) or/and from muscle fiber atrophy [42].
Given the above, it is important to perform a complementary MUNIX and MUSIX examination to assess the complex neural and muscular changes after stroke. Our study was performed on three major hand muscles. It was found that for the tested stroke subjects the hand CMAP amplitude (as a summation of three tested muscles) was significantly lower in the paretic side than the contralateral side. There was a linear correlation between CMAP and MUNIX values for both paretic and contralateral hands, which is consistent to previous MUNIX findings [23], [35]. The hand MUNIX values were found to be significantly lower in paretic side than in contralateral side. However, it is worth noting that different findings were observed for each of the three muscles.
For the FDI muscle, we found that both CMAP and MUNIX values were significantly reduced in the paretic side compared with the contralateral side. However, the MUSIX values of the paretic FDI muscles were smaller than those of the contralateral FDI muscles. These findings did not provide secure evidence of spinal motoneuron degeneration for the tested stroke subjects, because the reduction of MUNIX or CMAP values may be due to muscle fiber atrophy, and/or actual motor unit (most likely big motor units) loss without a sufficient compensatory muscle fiber reinnervation process.
For the APB muscle, it was found that the CMAP and MUNIX values in the paretic side were lower than those in the contralateral side, whereas the MUSIX value of the paretic side was higher than the contralateral side. No significant difference was observed for any of the comparisons. It appears that for the tested strokes subjects, compared with the FDI muscle the APB muscle was less affected in terms of CMAP or MUNIX measurements. The reduced MUNIX in combination with slightly enlarged MUSIX values as observed from the paretic APB muscles provided limited evidence of spinal motoneuron degeneration after stroke.
For the ADM muscle, it was found that the CMAP values were reduced in paretic side compared with the contralateral side (p = 0.05). Surprisingly, the mean MUNIX values were similar for paretic and contralateral muscles. As a result, the MUSIX values were smaller in the paretic muscles compared with the contralateral muscles. We note that the AI of the paretic ADM muscle was the lowest among all the examined muscles. We speculate that the dramatically reduced AI is a potential factor for relatively high MUNIX values of the paretic ADM muscles. There are different factors that may contribute to muscle AI reduction including decreased motor unit firing rates and partial paralysis. In the latter case, motoneurons that are still alive and have a functional connection to the muscle can be activated by electrical stimulation, but cannot be activated voluntarily due to a deficit in descending drive. These motoneurons would make a contribution to the CMAP, but not to the SIP. Although previous simulation work suggested that systematic reduction of motor unit peak firing rates would not have a significant impact on the MUNIX estimation [42], it is not clear how partial paralysis might affect the MUNIX estimation. This remains a topic for further investigation.
In contrast to different observations in MUNIX measurements for the three tested muscles, consistent findings were obtained in muscle AI. For all the three muscles, we found that there was a significant AI reduction in the paretic side, which was approximately 1/3 the values from the contralateral side, among which the ADM muscle had the most reduced AI. The significantly lower AI in paretic muscles may be due to reduced motor unit firing rates as revealed previously [16], [17], and/or inactivation of part of the motor unit pool (i.e., partial paralysis).
The consistent observation of significantly reduced AI values in all the three muscles supports that a fundamental influence of stroke on the neuromuscular system is an impairment in the ability to voluntarily activate limb muscles not only in acute and sub-acute patients, but also in chronic patients. Many patients in this study had stroke more than 10 years ago, yet their AI reduction is still severely affected.
Although paretic hand MUNIX and muscle AI were significantly lower compared with contralateral hands, we did not find that relative alterations in these parameters were correlated to the relative reduction of hand grip strength. There are several factors that may contribute to this observation. For example, one factor is diffuse muscle coactivation during grip strength testing [43]. Besides distal hand muscles, maximum grip strength includes contributions from proximal muscles in the arm, which were not considered in the current study. Another factor is related to complex neural and muscular changes post stroke, which may be present in different degrees, influence the measured indices, and compromise their correlation with hand grip strength. In addition, the parameters in this study were estimated from each separate muscle; however, the possible impairment of the coordination of different muscles to generate the maximal grip strength was not considered [44]. Finally, the technical limitation of the MUNIX measurement should be acknowledged. For example, only one direction of voluntary contraction was performed for each tested muscle. For multifunctional muscles, different contraction tasks may influence MUNIX estimations, especially for those selectively affected muscles [45].
V. Conclusion
This study applied muscle and motor unit indices, derived from convenient surface EMG and CMAP recordings, for examination of three hand muscles (FDI, APB, and ADM) of 12 stroke subjects. For all the three muscles, consistent findings were observed that there was a significant reduction of the muscle AI (ratio of the maximum voluntary EMG to the CMAP) in paretic side compared with contralateral side. In contrast, analysis of the three muscles indicated diverse changes in MUNIX and MUSIX measurements. Combining the three muscles, the hand CMAP and MUNIX were significantly reduced in paretic side compared with contralateral side, whereas the hand MUSIX was not significantly different. The current results provide an evidence of muscle activation deficiency after stroke, and suggest that there might be varying degree of motor unit loss or muscle fiber atrophy (or both) in different muscles after stroke. A primary advantage of these analyses is that they offer practical benefits because of their noninvasive and convenient character. A limitation of the study is that the current MUNIX analyses are difficult to differentiate among complex neuromuscular changes, thus, providing elusive evidence about spinal motoneuron degeneration after stroke.
Acknowledgments
The authors would like to thank W. Z. Rymer, M.D., Ph.D., for his support and useful discussions on this study.
This work was supported by the National Institutes of Health of the US Department of Health and Human Services under grants R24HD050821 and R01NS080839.
Biographies

Xiaoyan Li received the B.S. degree in electrical engineering from Anhui University, and the M.S. degree in biomedical engineering from the University of Science and Technology of China, both in Hefei, China. She received the second M.S. degree in computer sciences from Loyola University Chicago, in 2002, and the Ph.D. degree in bioengineering from the University of Illinois at Chicago, in 2008.
Later she was a Postdoctoral Research Fellow in the Institute for Neural Computation ofthe University of California at San Diego, CA, and in the Department of Physical Medicine and Rehabilitation of Northwestern University, Chicago, IL, respectively, and a Research Associate at the Sensory Motor Performance Program of the Rehabilitation Institute of Chicago, IL. She became a Research Assistant Professor in Physical Medicine and Rehabilitation of Northwestern University, in 2013. She is currently an Assistant Professor in the Department of Physical Medicine and Rehabilitation of the University of Texas Health Science Center at Houston. Her research interests include motor control, neurological disorders and rehabilitation.

Jie Liu received the B.S. and M.S. degrees in mechanical manufacturing and automation from Harbin Institute of Technology, Harbin, Heilongjiang, China, in 1998 and 2000, respectively, and the Ph.D. degree in mechanical engineering from Beijing University of Aeronautics and Astronautics, Beijing, China, in 2003.
He was a Marie Curie Transfer of Knowledge Fellow at Unilever R&D, Port Sunlight, UK, and a Postdoctoral Fellow in the BrainGate Project at Brown University, Providence, RI. He is currently a Research Associate at the Rehabilitation Institute of Chicago, Chicago, IL. His research interests include electromyography signal processing, control of robots and assistive devices.

Sheng Li received the Bachelor of Medicine degree in medicine from Beijing Medical University, Beijing, China, in 1993 and the Ph.D. degree in kinesiology from Pennsylvania State University, University Park, PA, in 2002 and subsequently completed a postdoctoral research fellowship in neurorehabilitation at Rehabilitation Institute of Chicago, in 2004.
He became an Assistant Professor in 2004 at the University of Montana. He then returned to residency training from the Department of Physical Medicine and Rehabilitation, University of Texas Medical School-Houston from 2009 to 2013. He is currently an Associate Professor in Physical Medicine and Rehabilitation at the University of Texas Medical School-Houston, and an Attending Physician at TIRR Memorial Hermann Hospital.

Ying-Chih Wang received the doctoral degree from the University of Florida, Gainesville, in 2007.
She was a Postdoctoral Research Fellow from 2007 to 2010 at the Sensory Motor Performance program of the Rehabilitation Institute of Chicago. She is currently an Assistant Professor at the University of Wisconsin-Milwaukee, WI, and an Adjunct Research Assistant Professor at the Northwestern University, Chicago, IL. Her research interests include developing quantitative functional outcome measures and performing comparative effectiveness research with new rehabilitation technologies.

Ping Zhou (S′01–M′05–SM′07) received the B.S. degree in electrical engineering (1995), and the M.S. degree in biomedical engineering (1999) from the University of Science and Technology of China, Hefei, China. He received the Ph.D. degree in biomedical engineering from Northwestern University, Evanston, IL, in 2004.
From 1999 to 2014, he was progressively a research assistant, research associate, and (full and part time) research faculty at the Rehabilitation Institute of Chicago, Chicago, IL. He was also an Adjunct Research Assistant and later Associate Professor in Physical Medicine and Rehabilitation of Northwestern University from 2006 to 2014. He currently holds a Visiting Adjunct Associate Professor position in Physical Medicine and Rehabilitation at the University of Texas Health Science Center at Houston, TX. He is Director of the Neuromyoelectric Engineering for Rehabilitation laboratory in TIRR Memorial Hermann Research Center located in Houston. He is also an Adjunct Professor in Biomedical Engineering program of the University of Science and Technology of China. His research interests include biomedical signal (in particular, EMG) processing, motor unit pathophysiology, electrodiagnosis, myoelectric control, and assistive devices for neurorehabilitation.
Contributor Information
Xiaoyan Li, Email: xiaoyan25@gmail.com, Department of Physical Medicine and Rehabilitation, University of Texas Health Science Center at Houston and TIRR Memorial Hermann Research Center, Houston, TX 77030, USA.
Jie Liu, Email: jie.liu2009@gmail.com, Sensory Motor Performance Program, Rehabilitation Institute of Chicago, Chicago, IL 60611, USA.
Sheng Li, Email: sheng.li@uth.tmc.edu, Department of Physical Medicine and Rehabilitation, University of Texas Health Science Center at Houston and TIRR Memorial Hermann Research Center, Houston, TX 77030, USA.
Ying-Chih Wang, Email: inga.wang.melnychuk@gmail.com, Department of Occupational Science and Technology, University of Wisconsin-Milwaukee, Milwaukee, WI 53202 USA.
Ping Zhou, Email: dr.ping.zhou@ieee.org, Department of Physical Medicine and Rehabilitation, University of Texas Health Science Center at Houston and TIRR Memorial Hermann Research Center, Houston, TX77030 USA, and also with the Biomedical Engineering Program, University of Science and Technology of China, Hefei 230027, China.
References
- 1.Bourbonnais D, Vanden Noven S. Weakness in patients with hemiparesis. Amer J Occup Theory. 1989 May;43(5):313–319. doi: 10.5014/ajot.43.5.313. [DOI] [PubMed] [Google Scholar]
- 2.Bohannon RW. Strength deficits also predict gait performance in patients with stroke. Percept Mot Skills. 1991 Aug;73(1):146. doi: 10.2466/pms.1991.73.1.146. [DOI] [PubMed] [Google Scholar]
- 3.Harris JE, Eng JJ. Paretic upper-limb strength best explains arm activity in people with stroke. Phys Theory. 2007 Jan;87(1):88–97. doi: 10.2522/ptj.20060065. [DOI] [PubMed] [Google Scholar]
- 4.Harris JE, Eng JJ. Strength training improves upper-limb function in individuals with stroke: A meta-analysis. Stroke. 2010 Jan;41(1):136–140. doi: 10.1161/STROKEAHA.109.567438. [DOI] [PubMed] [Google Scholar]
- 5.Boissy P, Bourbonnais D, Carlotti MM, Gravel D, Arsenault BA. Maximal grip force in chronic stroke subjects and its relationship to global upper extremity function. Clin Rehabil. 1999 Aug;13(4):354–362. doi: 10.1191/026921599676433080. [DOI] [PubMed] [Google Scholar]
- 6.Sunderland A, Tinson D, Bradley L, Hewer RL. Arm function after stroke. An evaluation of grip strength as a measure of recovery and a prognostic indicator. J Neurol Neurosurg Psychiatry. 1989 Nov;52(11):1267–1272. doi: 10.1136/jnnp.52.11.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dattola R, Girlanda P, Vita G, Santoro M, Roberto ML, Toscano A, Venuto C, Baradello A, Messina C. Muscle rearrangement in patients with hemiparesis after stroke: An electrophysiological and morphological study. Eur Neurol. 1993;33:109–114. doi: 10.1159/000116915. [DOI] [PubMed] [Google Scholar]
- 8.Chang CW. Evident trans-synaptic degeneration of motor neurons after stroke: A study of neuromuscular jitter by axonal microstimulation. Electroencephalogr Clin Neurophysiol. 1998;109:199–202. doi: 10.1016/s0924-980x(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 9.Lukacs M. Electrophysiological signs of changes in motor units after ischaemic stroke. Clin Neurophysiol. 2005;116:1566–1570. doi: 10.1016/j.clinph.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Toffola ED, Sparpaglione D, Pistorio A, Buonocore M. Myoelectric manifestations of muscle changes in stroke patients. Arch Phys Med Rehabil. 2001;82:661–665. doi: 10.1053/apmr.2001.22338. [DOI] [PubMed] [Google Scholar]
- 11.Zhou P, Suresh NL, Rymer WZ. Model based sensitivity analysis of EMG-force relation with respect to motor unit properties: Applications to muscle paresis in stroke. Ann Biomed Eng. 2007;35:1521–1531. doi: 10.1007/s10439-007-9329-3. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Suresh A, Zhou P, Rymer WZ. Alterations in the peak amplitude distribution of the surface electromyogram poststroke. IEEE Trans Biomed Eng. 2013 Mar;60(3):845–852. doi: 10.1109/TBME.2012.2205249. [DOI] [PubMed] [Google Scholar]
- 13.Brown WF, Snow R. Denervation in hemiplegic muscles. Stroke. 1990;21:1700–1704. doi: 10.1161/01.str.21.12.1700. [DOI] [PubMed] [Google Scholar]
- 14.Lukacs M, Vecsei L, Beniczky S. Changes in muscle fiber density following a stroke. Clin Neurophysiol. 2009;120:1539–1542. doi: 10.1016/j.clinph.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Lukacs M, Vecsei L, Beniczky S. Large motor units are selectively affected following a stroke. Clin Neurophysiol. 2008;119:2555–2558. doi: 10.1016/j.clinph.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995;18:1101–1114. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- 17.Chou LW, Palmer JA, Binder-Macleod S, Knight CA. Motor unit rate coding is severely impaired during forceful and fast muscular contractions in individuals post stroke. J Neurophysiol. 2013 Jun;109(12):2947–2954. doi: 10.1152/jn.00615.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arasaki K, Igarashi O, Ichikawa Y, Machida T, Shirozu I, Hyodo A, Ushijima R. Reduction in the motor unit number estimate (MUNE) after cerebral infarction. J Neurol Sci. 2006;250:27–32. doi: 10.1016/j.jns.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Hara Y, Masakado Y, Chino N. The physiological functional loss of single thenar motor units in the stroke patients: When does it occur? Does it progress? Clin Neurophysiol. 2004;115:97–103. doi: 10.1016/j.clinph.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 20.McComas AJ, Sica RE, Upton AR, Aguilera N. Functional changes in motoneurones of hemiparetic patients. J Neurol Neurosurg Psychiatry. 1973;36:183–193. doi: 10.1136/jnnp.36.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallenberg LA, Hermens HJ. Motor unit properties of biceps brachii in chronic stroke patients assessed with high-density surface EMG. Muscle Nerve. 2009 Feb;39(2):177–185. doi: 10.1002/mus.21090. [DOI] [PubMed] [Google Scholar]
- 22.Nandedkar SD, Nandedkar DS, Barkhaus PE, Stalberg EV. Motor unit number index (MUNIX) IEEE Trans Biomed Eng. 2004 Dec;51:2209–2211. doi: 10.1109/TBME.2004.834281. [DOI] [PubMed] [Google Scholar]
- 23.Nandedkar SD, Barkhaus PE, Stalberg EV. Motor unit number index (MUNIX): Principle, method, and findings in healthy subjects and in patients with motor neuron disease. Muscle Nerve. 2010 Nov;42(5):798–807. doi: 10.1002/mus.21824. [DOI] [PubMed] [Google Scholar]
- 24.Haughton JF, Little JW, Powers RK, Robinson LR, Goldstein B. M/RMS: An EMG method for quantifying upper motoneuron and functional weakness. Muscle Nerve. 1994;17:936–942. doi: 10.1002/mus.880170814. [DOI] [PubMed] [Google Scholar]
- 25.Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. 4th. ch. 5. New York, NY, USA: Oxford Univ. Press; 2013. pp. 74–98. [Google Scholar]
- 26.Littell RC. SAS for Mixed Models. 2nd. Cary, NC, USA: SAS Institute, Inc.; 2006. [Google Scholar]
- 27.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Control. 1974 Dec;19(6):716–723. [Google Scholar]
- 28.McComas AJ, Fawcett PR, Campbell MJ, Sica RE. Electrophysiological estimation of the number of motor units within a human muscle. J Neurol Neurosurg Psychiatry. 1971;34:121–131. doi: 10.1136/jnnp.34.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bromberg MB. Updating motor unit number estimation (MUNE) Clin Neurophysiol. 2007;118:1–8. doi: 10.1016/j.clinph.2006.07.304. [DOI] [PubMed] [Google Scholar]
- 30.Neuwirth C, Nandedkar S, Stalberg E, Weber M. Motor unit number index (MUNIX): A novel neurophysiological technique to follow disease progression in amyotrophic lateral sclerosis. Muscle Nerve. 2010;42:379–384. doi: 10.1002/mus.21707. [DOI] [PubMed] [Google Scholar]
- 31.Drey M, Groösch C, Neuwirth C, Bauer JM, Sieber CC. The motor unit number index (MUNIX) in sarcopenic patients. Exp Gerontol. 2013 Apr;48(4):381–384. doi: 10.1016/j.exger.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Kaya RD, Nakazawa M, Hoffman RL, Clark BC. Interrelationship between muscle strength, motor units, and aging. Exp Gerontol. 2013 Sep;48(9):920–925. doi: 10.1016/j.exger.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Jahanmiri-Nezhad F, Rymer WZ, Zhou P. An examination of the motor unit number index (MUNIX) in muscles paralyzed by spinal cord injury. IEEE Trans Inf Technol Biomed. 2012 Nov;16(6):1143–1149. doi: 10.1109/TITB.2012.2193410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandedkar SD, Barkhaus PE, Stålberg EV. Reproducibility of MUNIX in patients with amyotrophic lateral sclerosis. Muscle Nerve. 2011 Dec;44(6):919–922. doi: 10.1002/mus.22204. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Wang YC, Suresh NL, Rymer WZ, Zhou P. Motor unit number reductions in paretic muscles of stroke survivors. IEEE Trans Inf Technol Biomed. 2011 Jul;15(4):505–512. doi: 10.1109/TITB.2011.2140379. [DOI] [PubMed] [Google Scholar]
- 36.Neuwirth C, Nandedkar S, Stålberg E, Barkhaus PE, Carvalho Md, Furtula J, Dijk JP, Baldinger R, Castro J, Costa J, Otto M, Sandberg A, Weber M. Motor unit number index (MUNIX): A novel neurophysiological marker for neuromuscular disorders; test-retest reliability in healthy volunteers. Clin Neurophysiol. 2011 Sep;122(9):1867–1872. doi: 10.1016/j.clinph.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Sandberg A, Nandedkar SD, Stålberg E. Macro electromyography and motor unit number index in the tibialis anterior muscle: Differences and similarities in characterizing motor unit properties in prior polio. Muscle Nerve. 2011 Mar;43(3):335–341. doi: 10.1002/mus.21878. [DOI] [PubMed] [Google Scholar]
- 38.Ahn SW, Kim SH, Kim JE, Kim SM, Kim SH, Park KS, Sung JJ, Lee KW, Hong YH. Reproducibility of the motor unit number index (MUNIX) in normal controls and amyotrophic lateral sclerosis patients. Muscle Nerve. 2010 Nov;42(5):808–13. doi: 10.1002/mus.21765. [DOI] [PubMed] [Google Scholar]
- 39.Furtula J, Johnsen B, Christensen PB, Pugdahl K, Bisgaard C, Christensen MK, Arentsen J, Frydenberg M, Fuglsang-Frederiksen A. MUNIX and incremental stimulation MUNE in ALS patients and control subjects. Clin Neurophysiol. 2013 Mar;124(3):610–618. doi: 10.1016/j.clinph.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 40.Boekestein WA, Schelhaas HJ, van Putten MJ, Stegeman DF, Zwarts MJ, van Dijk JP. Motor unit number index (MUNIX) versus motor unit number estimation (MUNE): A direct comparison in a longitudinal study of ALS patients. Clin Neurophysiol. 2012 Aug;123(8):1644–1649. doi: 10.1016/j.clinph.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 41.van Dijk JP, van de Ven WJM, Stegeman DF. presented at the XVIII Congress Int Soc Electrophysiol Kinesiol. Aalborg, Denmark: 2010. Evaluation of the motor unit number index (MUNIX) as a measure for motor unit loss. [Google Scholar]
- 42.Li X, Rymer WZ, Zhou P. A simulation-based analysis of motor unit number index (MUNIX) technique using motoneuron pool and surface electromyogram models. IEEE Trans Neural Syst Rehabil Eng. 2012 May;20(3):297–304. doi: 10.1109/TNSRE.2012.2194311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bromberg MB, Larson WL. Relationships between motor-unit number estimates and isometric strength in distal muscles in ALS/MND. J Neurol Sci. 1996 Aug;(139):38–42. doi: 10.1016/0022-510x(96)00074-3. [DOI] [PubMed] [Google Scholar]
- 44.Lee SW, Triandafilou K, Lock BA, Kamper DG. Impairment in task-specific modulation of muscle coordination correlates with the severity of hand impairment following stroke. PLoS One. 2013 Jul;8(7):e68745. doi: 10.1371/journal.pone.0068745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou P, Li X, Rymer WZ. Computing motor unit number index of the first dorsal interosseous muscle with two different contraction tasks. Med Eng Phys. 2012 Oct;34(8):1209–1212. doi: 10.1016/j.medengphy.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


