Abstract
Purpose
To summarize current work identifying inflammatory components that underlie associations between obesity-associated type 2 diabetes (T2D) and coronary artery disease (CAD).
Recent findings
Recent studies implicate immune cells as drivers of pathogenic inflammation in human T2D. Inflammatory lymphocytes characterize unhealthy adipose tissue (AT), but regional adipose volume, primarily visceral and pericardial fat; also predict severity and risk for obesity-associated CAD. Having a greater understanding of shared characteristics between inflammatory cells from different AT depots and a more accessible tissue such as blood will facilitate progress towards clinical translation of our appreciation of obesity as an inflammatory disease.
Summary
Obesity predisposes inflammation and metabolic dysfunction through multiple mechanisms, but these mechanisms remain understudied in humans. Studies of obese subjects have identified disproportionate impacts of specific T cell subsets in metabolic diseases like T2D. Based on demonstration that AT inflammation is depot-specific, analysis of adiposity by waist-to-hip ratio or magnetic resonance imaging (MRI) will increase interpretive value of lymphocyte-focused studies and aid clinicians in determining which obese individuals are at highest risk for CAD. New tools to combat obesity-associated CAD and other co-morbidities will stem from identification of immune cell-mediated inflammatory networks that are amenable to pharmacological interventions.
Keywords: coronary artery disease, obesity, inflammation, T cell, B cells, type 2 diabetes
Introduction
The prevalence of obesity continues to increase worldwide and with current trajectories obesity will burden healthcare systems for decades [1*]. Obesity often triggers the inflammation that is believed to increase risk for many co-morbidities, with greatest risk stemming from the simmering inflammation that underlies type 2 diabetes (T2D) and coronary artery disease (CAD)[2**, 3]. Obesity-associated inflammation is largely due to overproduction of pro-inflammatory cytokines by macrophages [4, 5], B cells and T cells [6–10**], all of which are recruited to expanding AT. Immune cell-mediated inflammation reinforces a pro-inflammatory balance of adipokines, or adipocyte cytokines, which are also increased in expanding AT [11]. This combination of cytokines from adipose-associated immune cells plus adipokines promotes metabolic dysregulation that includes insulin resistance (IR) and T2D [12*, 13]. The pro-inflammatory cytokine profile in obesity likely spills over into the circulation, where increased serum cytokines predict increased CAD risk [14, 15], pulmonary diseases [16–18] and cancer [19]. Inflammation may thereby link seemingly disparate co-morbidities of obesity.
Although mouse models have enabled substantial progress over the last decade in describing immune compartment contributions to metabolic dysfunction, an understanding of the role inflammation plays in human obesity and associated metabolic disease remains rudimentary. Recent findings from human studies reviewed herein highlight ongoing progress in our understanding of inflammation as a driver of obesity-associated disease with emphasis on CAD.
Obesity-associated inflammation and immune cells in T2D
A major advance in metabolic research came with the understanding of obesity as a chronic, low-grade inflammatory state, which differs from the immune response to infection but involves well-understood modulators of nutrient storage and efflux pathways [8, 20, 21*]. It was observations that activated macrophages within AT produce the majority of adipose-associated pro-inflammatory cytokines, and that these cytokines promote IR [4, 5] which provoked the current focus on multiple types of immune cells in metabolic research and founded the field of “Immunometabolism”. Extensive genetic, dietary and pharmacologic interventions in mouse models in parallel with observations from human studies identified a causal link between obesity-associated inflammation and metabolic disease [22, 23]. Such studies also revealed a role for macrophages in tissue remodeling within AT of lean individuals that contrasts with pro-inflammatory functions of newly recruited and/or in situ proliferating macrophages of obese AT [24*]. Other myeloid immune cells including neutrophils, eosinophils and mast cells also play roles in promoting inflammatory responses and IR in obese AT [25–27].
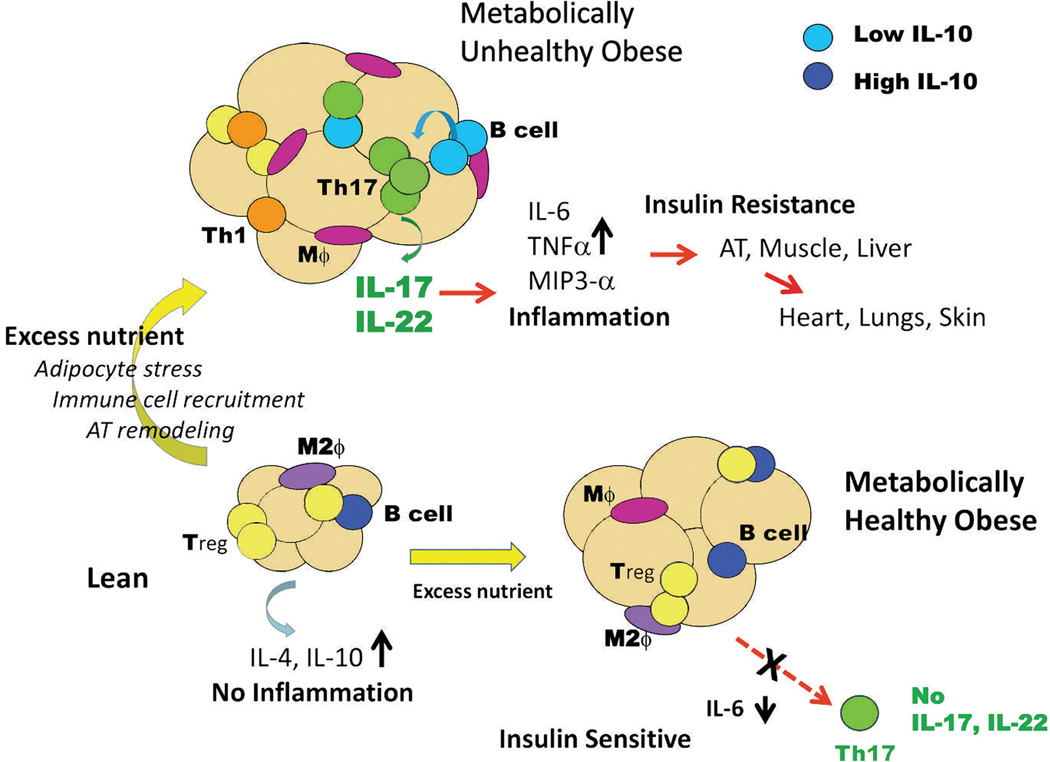
Classically designated “adaptive” immune cells, including B cells, CD8+ and CD4+ T cells, also infiltrate AT and increase in number in response to obesity [28*, 29] (Figure 1). Both B and T cells release and/or stimulate release of pro-inflammatory cytokines in obesity-associated IR/T2D, bolstering local and systemic inflammation. In addition to the B cell-intrinsic changes in T2D humans and obese/IR mice, B cells stimulate inflammatory cytokine production by CD4+ and CD8+ T cells [6, 10]. Thus B cells promote T2D-associated inflammation through direct (cell-intrinsic) and indirect (T cell-mediated) mechanisms. Further work with human samples showed that contact between B and T cells is required for maximal pro-inflammatory CD4+ Th17 cell function in samples from T2D but not from obese, non-diabetic subjects. Although anti-inflammatory functions of B cells and regulatory T cells (Tregs) have also been well-defined, these functions appear to be diminished in obesity [8, 30–32]. For example, B cells from lean, ‘metabolically healthy’ humans and mice release significant amounts of the anti-inflammatory cytokine IL-10, but B cell IL-10 is severely down-regulated in response to obesity/IR/T2D [10, 33]. This shift to a potentially pathogenic, pro-inflammatory B cell cytokine profile may mechanistically underlie demonstrations that B cell-null mice fed a high-fat diet (HFD) are equally obese but less prone to obesity-associated IR and other metabolic disturbances. Anti-inflammatory CD4+ T regulatory cells (Tregs) are similarly underrepresented in obese/IR mice and T2D humans, in part due to lower numbers of Tregs [8, 34] but also likely due to suppression of anti-inflammatory IL-10 production [35*], although clarification is needed in follow-up studies with human samples. Despite overall similarities in roles for B and T cells in human and mouse T2D, a more comprehensive assessment of T cells in obesity suggests fundamental differences in obesity-associated inflammation between humans and mice, obese mice have predominantly CD8 and Th1 inflammatory responses with minor changes in Th17 cells [8, 9], whereas in obese humans the Th17 axis dominates T cell-mediated inflammation [10, 33, 36, 37**].
Figure 1.
Role for immune cells in inflammatory response of metabolically healthy obese (insulin sensitive) and metabolically unhealthy (insulin resistant) obese adipose tissue. Blue= IL-10hi B cell, Light blue= IL-10lo B cell, Orange= Th1 cell, Dark Green=Th17 cell, Yellow=regulatory T cells (Tregs) Purple oval= anti-inflammatory M2 macrophages (M2ϕ), pink oval=pro-inflammatory M1 macrophage (Mϕ). Anti-inflammatory immune cells dominate metabolically healthy tissue, but decrease in metabolically unhealthy tissue. In contrast, pro-inflammatory immune cells dominate metabolically unhealthy tissue. Original artwork.
Th17, in obesity and T2D
The importance of T cell cytokines such as IFNγ and IL-17 in obesity is less appreciated than thoroughly examined, classical “diabetogenic” cytokines (e.g., TNF-α, IL-6 and IL-1β) [22]. Th17 cells, the dominant source of IL-17, are instead recognized primarily for roles in clearance of select pathogens, and for detrimental effects in autoimmune diseases such as multiple sclerosis (mouse EAE) [38* 39]. Data showing that IL-6 and IL-1β drive Th17 differentiation [40, 41*] is part of an emerging appreciation of Th17 expansion in obesity-associated IR/T2D. Recently, leptin, an adipokine generally increased in obesity/IR, was shown to also support Th17 expansion [42*, 43]. Overall, the relationship between increased IL-6/IL-1β/leptin and increased Th17 differentiation, coupled with the importance of Th17s in autoimmune diseases, may explain clinical evidence that obesity predisposes people to increased risk for inflammation-mediated autoimmune diseases including psoriasis, rheumatoid arthritis [44], lupus [45] and multiple sclerosis [7, 46].
Despite these likely mechanistic links between obesity-associated inflammation and Th17 cells, the Th17 signature cytokines IL-17 and IL-22 have been more or less dismissed in mouse models of obesity and T2D, in part because IL-17+ cells (likely Th17s) infiltrate murine subcutaneous rather than visceral AT in response to HFD [47], or Th17s dominate only after obesity/IR is established [48]. IL-17 gene deletion caused an abnormal weight gain on both low-fat (LF) or HFD compared to WT controls, precluding a straight-forward interpretation of roles for Th17 cells in obese knockout animals [49]. This lack of strong data for IL-17/Th17 dominance in obesity-associated IR in mice has moderated excitement over possible functions of Th17s in human obesity/IR/T2D, although several reports have shown IL-17 and IL-22 induced IR in chief metabolic regulators such as hepatocytes, adipocytes and myocytes [37, 49, 50].
Evidence of a role for Th17s in human disease includes demonstrations that plasma Th17 cytokines and in vitro Th17 activity tightly correlate with measures of glycated hemoglobin A1c levels of T2D patients [33, 36, 51*]. Two recent studies also showed increased Th17 function (i.e. IL-17 and IL-22 production) AT-associated immune cells from well-characterized obese/insulin sensitive (IS) versus obese/IR subjects [37, 52**]. Taken together, this work suggested some aspects of human immunometabolism should be more thoughtfully examined with animal models that more closely recapitulate human physiology. One example of such an approach is long term (9 month) HFD feeding of genetically homogeneous C57BL6 mice, which surprisingly revealed 4 different metabolic response groups. These groups include the lean/IR and obese/IS groups that are generally absent (or even culled) following standard 12–16 wk feeding protocols [53]. Measuring cytokines from these metabolic groups of mice or investigation of strains of mice with resistance to diet-induced obesity [54] could facilitate progress in understanding mechanisms underlying immune cell/cytokine involvement in patients.
Obesity, inflammation and associated coronary artery disease (CAD)
Western diet together with obesity, T2D, hypertension, dyslipidemia, physical inactivity, and increasing average age, are believed to be primary causes of CAD and CAD-associated heart failure, the leading cause of death in T2D individuals [55]. The role of inflammation in CAD has been appreciated for decades, with a relatively early appreciation that local immune cell infiltrates characterize CAD and can specifically predict risk for fatal outcome [56, 57]. Immune cell infiltrates occur downstream of endothelial dysfunction, which promotes lipoprotein transcytosis from the plasma into the vessel intima. Subsequent immune cell infiltration/activation thereby links endothelial changes to cardiovascular disease/CAD (Reviewed in [58, 59]. Teasing out the complex interactions amongst obesity, CAD and T2D is a daunting endeavor, but the appreciation of inflammation as a critical mediator primes the field to expand the findings above thus spur fundamentally new clinical approaches.
Documented roles for macrophages, T cells and B cells in CAD suggest that obesity-associated inflammation bridges immune cell function and CAD [60–66]. Macrophages were the first immune cell recognized to play critical roles in the development of atherosclerosis, from early fatty streak formation through progression to vulnerable plaques. Mechanisms of macrophage involvement in CAD include the ability to form foam cells, and to secrete high amounts of pro-inflammatory, pro-CAD cytokines such as TNFα, IL-1β, IL-6 and IL-8 [60, 67–72].
Pro-inflammatory T cells also play critical roles in the endothelial dysfunction that precedes CAD [61–65]. IFNγ, a pro-inflammatory cytokine produced by both CD4+ and CD8+ T cells, is critical for the development of atherosclerosis. Furthermore, inhibition of Th1 cells, thus IFNγ and other CD4+-associated cytokines, ameliorates disease in mouse models (Reviewed in [73], independently indicating Th1s promote CAD. Similarly, IL-17, the major cytokine produced by Th17s, is important for the recruitment of macrophages to developing atherosclerotic lesions [61, 74, 75*], and both CD4+ and CD8+ T cells characterize unstable plaques (71–74). Thus, multiple lines of evidence suggest that pro-inflammatory T cells support obesity-associated atherosclerosis and may link CAD to T2D.
B cells are more recently appreciated mediators of CAD, although their roles are more complex than the pro-atherogenic functions of macrophages and T cells. B cell depletion protects against CAD in model animals [66], suggesting B cells are pathogenic. In contrast, some studies show that B cells protect against atherosclerosis [76]. The latter interpretation is consistent with the demonstration that removal of the normal splenic reservoir of B cells renders patients more susceptible to CAD [77]. Although exact mechanisms are not known, these seemingly contradictory findings on roles for B cells in atherosclerosis may be explained by demonstrations that B cells can initially protect against inflammatory disease, but then can change their activity, promoting pathogenesis [10, 34, 78]. For example, the loss of B cell IL-10 in T2D discussed above, coupled with a T2D-associated increase in the ability of B cells to produce CAD-associated IL-8 [72, 79] is consistent with a disease-associated gain of pathogenic B cell function. B cells also promote pericardial AT expansion and inflammation in a mouse model of obesity-associated IR [10, 34]. Taken together, these reports suggest that B cells from obese/IR subjects support CAD-associated inflammation through multiple mechanisms such as 1. promoting pro-inflammatory cytokine production including high IL-8 and low IL-10 release [34, 69, 80, 81], 2. by supporting AT expansion in obesity [10], and 3. promoting pro-inflammatory Th17 cells [6, 10, 82]. Both the activities of individual immune cells and the cross-talk amongst immune cell subsets raise the clinically critical possibility that immunomodulatory drugs, such as the generally safe B cell depletion drug rituximab [83, 84] may have unappreciated efficacy in the prevention of obesity-associated CAD [85].
The role of pericardial AT (pAT) in local inflammation and CAD
Although adipose depots all increase in volume with obesity, fat deposits in different anatomical regions show depot-associated levels of immune cell infiltration and inflammation, and thus differentially associate with disease. To generalize, subcutaneous AT is more metabolically “protective”, while pericardial and other visceral depots are highly correlated with risk for obesity-associated disease, including CAD [86–88]. The recognition that pAT physiology, which includes pAT inflammation, is a critical mediator of CAD significantly departs from the outdated assumption that pAT expansion is an uninteresting epiphenomenon of both obesity-independent and obesity-associated CAD.
Numerous analyses point to the importance of pAT expansion and concomitant inflammation in obesity/IR-associated CAD. pAT from subjects with CAD has increased inflammatory hallmarks compared to pAT from subjects undergoing non-CAD heart procedures [72, 89–91]. Notably, pAT volume also associates with systemic inflammation, as measured by IL-6 and C-reactive protein (CRP) levels [92]. Also, CAD is more tightly associated with the amount of pAT in lean individuals than with a variety of more “accepted” CAD risk factors, including body fat distribution [93]. pAT volume is a strong independent risk factor for CAD severity [88], and positively associates with calcified coronary plaque [94, 95] which suggests that pAT may exert local toxic effects on the coronary vasculature [96]. Thus it is unsurprising that the amount of pAT negatively correlates with cardiac output and stroke volume [97]. Additionally, epidemiological studies show that relatively high pAT volume (>300cm3) associates with a 4-fold increased risk of CAD, whereas smoking and T2D increase CAD risk 1.6- and 3-fold, respectively [98]. Prospective studies revealed that the volume of epicardial AT, the depot that literally coats the myocardium, is predictive of obstructive CAD even before patients develop overt pathology [99*–101]. Finally, perhaps the most convincing human evidence linking obesity, inflammation and CAD is analysis of monozygotic twins discordant for obesity. This study found that the obese twin had a greater epicardial AT volume, and that CRP, one surrogate for systemic inflammation, was the only one of several factors measured that significantly associated with epicardial AT volume. These studies support the conclusion that inflammation and epicardial AT volume predict risk for CAD [102*, 103] and highlight the urgency of a more comprehensive analysis of pAT physiology focused on inflammation, to advance the long-term goal of countering CAD pathogenesis.
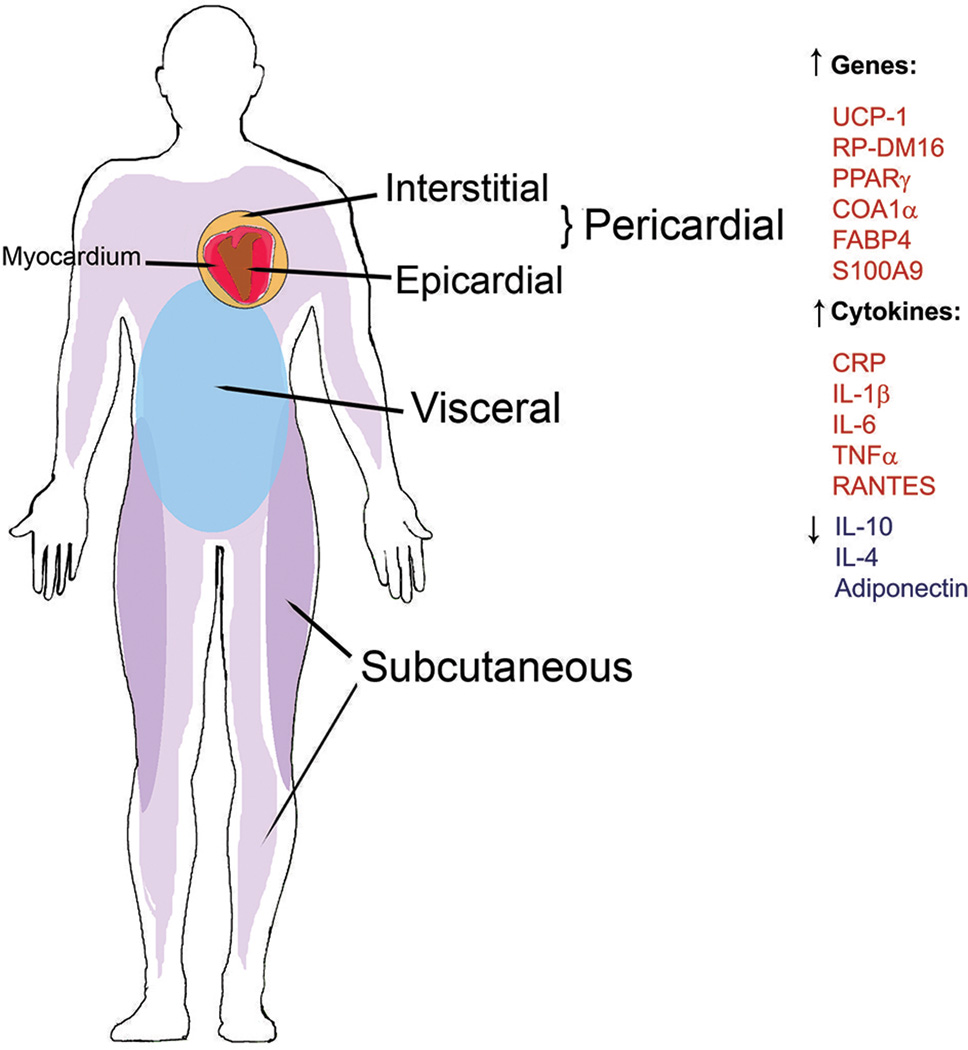
Together the associations between pAT inflammation and CAD/impaired cardiovascular function, plus the known roles of obesity in AT volume and pro-inflammatory immune cell function [4–7, 10, 33, 34, 47, 72, 79, 89, 91, 104] frame the idea that obesity-associated changes in pAT physiology link obesity and CAD, and are further exacerbated in the presence of IR. However, one gap in the pAT analyses is that “pAT” is often imprecisely defined, and can denote the epicardial AT that coats the heart, the interstitial AT that coats the outside of the pericardial membrane (thus does not directly touch the heart) or both. Both heart-proximal depots (Figure 2) are linked to local and systemic inflammation, although each is also somewhat distinct from the other [72, 89–92, 105, 106*]. Epicardial AT may disproportionately impact CAD due to a shared circulation with the myocardium [107]. Regardless, a comparison of physiology of epicardial fat, interstitial fat and blood from the same individual is essential to comprehensively understand inflammation in heart-proximal AT, and will be vital for identifying pAT signatures in a more accessible tissue.
Figure 2.
Depiction of regional adipose tissue (AT) deposition in human body and obesity-associated gene and cytokine profiles. AT depots that preferentially expand in metabolically unhealthy (inflammatory) responses include pericardial (epicardial and interstitial) and visceral depots. AT that preferentially expands in metabolically healthy obese individuals includes widespread subcutaneous depots. Shown are up- or down-regulated genes, adipokines, or cytokines associated with obesity comorbidities, T2D and CAD. Original artwork.
A clinically important, yet unappreciated aspect of studies on obesity, inflammation and CAD is the possibility that fundamentally different mechanisms drive CAD in lean/IS compared to obese/IS or obese/IR subjects. This novel prospect is raised by our recent work showing mechanisms of periodontitis (PD), another common comorbidity in obesity/IR, significantly differ between lean/IS and obese/IR mice [108]. Lean mice developed PD through a B cell-independent process, while PD development in obese/IR mice was highly B cell-dependent. Obesity-associated inflammatory responses in PD and CAD are inadequately studied in humans, although one cross-sectional study of obese subjects with and without PD identified that both obesity and PD increased risk for cardiovascular disease [109*]. Future identification of correlates of CAD over a range of metabolic status with subsequent mechanistic analyses will be needed to determine whether the currently similar standard of care for CAD in lean and obese/IR patients is the best approach.
Conclusion
Our developing appreciation of links among obesity, inflammation and CAD will require multiple complementary approaches to leverage new concepts into translatable outcomes. Careful characterization of human subjects, particularly analysis of AT distribution by measures as simple as waist: hip ratio, will be needed to stratify subjects that are most likely obese/metabolically healthy from those that are obese/metabolically unhealthy [93, 110]. Notably, subjects with T2D are most effectively analyzed as a unique cohort, rather than being lumped into the “obese/IR” category. Use of models that more closely parallel human disease will increase the translational significance of studies. A call for simple analysis of metabolic status in all clinical drug trials (for example, HbA1c) would identify drugs with potential efficacy against obesity-associated CAD, while incurring minimal extra cost. Similarly, collection of (at least) serum and peripheral blood mononuclear cells from all obesity or CAD-associated clinical trials would provide samples for testing of new possibilities, like Th17 dominance. Concepts developed in human sample studies, with further testing in models to refine concepts, will integrate the strengths of both bench and bedside research to allow the field to exploit our understanding of obesity-associated inflammation for clinical gains over the short term.
Key points.
Obese AT causes a shift towards pro-inflammatory Th17 immune response in humans, which differs from the Th1/CD8+ T cell dominance of murine obesity/insulin resistance.
Pro-inflammatory T cell responses may underlie increased risk for obesity associated CAD.
Classifying subjects within obese cohorts such as by depot-specific immune responses or specific adipose depot volume will assist in developing criteria for obesity-associated inflammation, CAD risk and severity.
Acknowledgements
Work herein was supported by NIH R21DK089270, NIH R56 DK096525, NIH U01 CA182898 and NIH R56 DK090455.
Footnotes
Disclosure
The authors have no conflict of interests.
References
- 1.Skinner A, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the united states, 1999–2012. JAMA Pediatrics. 2014;21 doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 2. Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Progress in cardiovascular diseases. 2014;56:369–381. doi: 10.1016/j.pcad.2013.10.016. This review provides a clear overview of obesity contributions to cardiovascular disease with focus on abdominal AT inflammation. Authors emphasize that simplifying therapeutic objectives by directed study of higher risk obese individuals might be beneficial in CVD prevention and relatively simple measures such as visceral/ectopic adiposity and activity level could identify such patients in the clinic.
- 3. Alexopoulos N, Katritsis D, Raggi P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis. 2014;233:104–112. doi: 10.1016/j.atherosclerosis.2013.12.023. Authors have compiled and summarized research studies that link intra-abdominal and epicardial AT to CAD. They recommend that non-invasive CT, MRI or echocardiagraphy could be employed to assess these new markers of CAD risk.
- 4.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winer S, Paltser G, Chan Y, et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 8.Feuerer M, Herrero L, Cipolletta D, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 10. Defuria J, Belkina AC, Jagannathan-Bogdan M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. This study revealed a critical disease-associated interplay between B and T cells, with B cells promoting IR by multiple mechanisms such as: release of proinflammatory cytokines, stimulaton of AT growth/expansion, altering T cell ratios; increasing Th17/Th1 over Treg. A major strength was to apply findings from the mouse model to identify functional similarities of peripheral B and T cells from human T2D subjects.
- 11.Duffaut C, Galitzky J, Lafontan M, Bouloumie A. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem Biophys Res Commun. 2009;384:482–485. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 12. Aroor AR, DeMarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism Clinical and Experimental. 2013;62:1543–1552. doi: 10.1016/j.metabol.2013.07.001. This review gives brief, but detailed summaries of inflammatory mediators involved in insulin resistance and their impact on cardiovascular disease.
- 13.Yang M, Deng J, Yang l, et al. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am J Pathol. 2012;180 doi: 10.1016/j.ajpath.2012.03.010. In Press. [DOI] [PubMed] [Google Scholar]
- 14.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–214. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 15.Cheng KH, Chu CS, Lee KT, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes (Lond) 2008;32:268–274. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland ER. Linking obesity and asthma. Ann N Y Acad Sci. 2014;1311:31–41. doi: 10.1111/nyas.12357. [DOI] [PubMed] [Google Scholar]
- 17.Davidson WJ, Mackenzie-Rife KA, Witmans MB, et al. Obesity negatively impacts lung function in children and adolescents. Pediatr Pulmonol. 2013 doi: 10.1002/ppul.22915. [DOI] [PubMed] [Google Scholar]
- 18.Cavailles A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. European respiratory review: an official journal of the European Respiratory Society. 2013;22:454–475. doi: 10.1183/09059180.00008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lashinger LM, Ford NA, Hursting SD. Interacting inflammatory and growth factor signals underlie the obesity-cancer link. J Nutr. 2014;144:109–113. doi: 10.3945/jn.113.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu X, Grijalva A, Skowronski A, et al. Obesity Activates a Program of Lysosomal-Dependent Lipid Metabolism in Adipose Tissue Macrophages Independently of Classic Activation. Cell Metabolism. 2014;18:816–830. doi: 10.1016/j.cmet.2013.11.001. Results reported in this study define a non-inflammatory lipid lipolysis role for macrophages within adipose tissue that actually has an effect on whole AT metabolism. These data point to a novel aspect of immune cell function within AT that might have an unrecognized contribution to obesity/metabolic dysfunction and may open a new avenue for treatment development.
- 22.Cancello R, Clement K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG: an international journal of obstetrics and gynaecology. 2006;113:1141–1147. doi: 10.1111/j.1471-0528.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 23.Zeyda M, Farmer D, Todoric J, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;9:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 24. Amano SU, Cohen JL, Vangala P, et al. Local Proliferation of Macrophages Contributes to Obesity-Associated Adipose Tissue Inflammation. Cell Metab. 2013 doi: 10.1016/j.cmet.2013.11.017. A recent study that revealed another aspect of macrophage biology with possible implications for drug targeting of AT inflammatory response.
- 25.Elgazar-Carmon V, Rudich A, Hadad N, Levy R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Wu D, Molofsky AB, Liang HE, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q, Sundar K, Mishra PK, et al. Helminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanisms. Infect Immun. 2009 doi: 10.1128/IAI.01170-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrante AW., Jr Macrophages, fat, the emergence of immunometabolism. The Journal of Clinical Investigation. 2013;123:4992–4993. doi: 10.1172/JCI73658. This review includes a recent perspective on and implications of immune cell roles in both obese and non-obese AT.
- 29.Nikolajczyk BS, Jagannathan-Bogdan M, Denis GV. The outliers become a stampede as immunometabolism reaches a tipping point. Immunol Rev. 2012;249:253–275. doi: 10.1111/j.1600-065X.2012.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duffaut C, Zakaroff-Girard A, Bourlier V, et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608–1614. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 31.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1304–1310. doi: 10.1161/ATVBAHA.108.165100. [DOI] [PubMed] [Google Scholar]
- 32.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 33.Jagannathan-Bogdan M, McDonnell ME, Shin H, et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–1172. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jagannathan M, McDonnell M, Liang Y, et al. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia. 2010;53:1461–1471. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Han JM, Patterson SJ, Speck M, et al. Insulin Inhibits IL-10-Mediated Regulatory T Cell Function: Implications for Obesity. J Immunol. 2014;192:623–629. doi: 10.4049/jimmunol.1302181. This study is the first to identify a direct effect of insulin upon regulatory T cell IL-10 cytokine production and reveals additional complexity of immune response in obese AT. If confirmed in humans such effects have implications for potential avenues of drug development.
- 36.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 2009;33:151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 37. Fabbrini E, Cella M, McCartney SA, et al. Association Between Specific Adipose Tissue CD4 + T-Cell Populations and Insulin Resistance in Obese People. Gastroenterology. 2013 doi: 10.1053/j.gastro.2013.04.010. Results appearing in this study advance the field of immunometabolism in several ways: by stratifying obese subjects, investigating multiple metabolic cell types and comparing detailed metabolic profiles, authors identified a skewed T cell bias towards Th17 that was associated with metabolic disease rather than obesity per se. These findings suggest new studies should include examining the Th17 pathway.
- 38. Bedoya SK, Lam B, Lau K, Larkin J., 3rd Th17 cells in immunity and autoimmunity. Clin Dev Immunol. 2013;2013:986789. doi: 10.1155/2013/986789. Readers interested in learning more about known roles for Th17 cells in human pathology will find timely summaries in this review.
- 39.Fukaya T, Someya K, Hibino S, et al. Loss of Sprouty4 in T cells ameliorates experimental autoimmune encephalomyelitis in mice by negatively regulating IL-1β receptor expression. Biochemical and Biophysical Research Communications. doi: 10.1016/j.bbrc.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Korn TBE, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 41.Hebel KRM, Kosak B, Chang HD, et al. IL-1β and TGF-β act antagonistically in induction and differentially in propagation of human proinflammatory precursor CD4+ T cells. J Immunol. 2011;187:5627–5635. doi: 10.4049/jimmunol.1003998. [DOI] [PubMed] [Google Scholar]
- 42. Yu Y, Liu Y, Shi FD, et al. Cutting edge: Leptin-induced RORgammat expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J Immunol. 2013;190:3054–3058. doi: 10.4049/jimmunol.1203275. Experiments of the study utilize mice and human samples and demonstrate a role for leptin in stimulating differentiation of Th17 cells providing further evidence of a potential link between obesity and susceptibility to autoimmune disese.
- 43.Amarilyo G, Iikuni N, Shi FD, et al. Leptin promotes lupus T-cell autoimmunity. Clin Immunol. 2013;149:530–533. doi: 10.1016/j.clim.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Russolillo Anna IS, Peluso Rosario, et al. Obesity and psoriatic arthritis: from pathogenesis to clinical outcome and management. Rheumatology (Oxford) 2013;52:62–67. doi: 10.1093/rheumatology/kes242. [DOI] [PubMed] [Google Scholar]
- 45.Rizk AGT, Nassef S, Abdallah A. The impact of obesity in systemic lupus erythematosus on disease parameters, quality of life, functional capacity and the risk of atherosclerosis. Int J Rheum Dis. 2012;15:261–267. doi: 10.1111/j.1756-185X.2011.01698.x. [DOI] [PubMed] [Google Scholar]
- 46.Hedström AK, Lima Bomfim I, Barcellos L, et al. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology. 2014;82:865–782. doi: 10.1212/WNL.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bertola A, Ciucci T, Rousseau D, et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes. 2012;61:2238–2247. doi: 10.2337/db11-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuniga LA, Shen WJ, Joyce-Shaikh B, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947–6959. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang Y, Bian Z, Zhao L, et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol. 2011;166:281–290. doi: 10.1111/j.1365-2249.2011.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sumarac-Dumanovic M, Jeremic D, Pantovic A, et al. Therapeutic improvement of glucoregulation in newly diagnosed type 2 diabetes patients is associated with a reduction of IL-17 levels. Immunobiology. 2013;218:1113–1118. doi: 10.1016/j.imbio.2013.03.002. This study demonstrates that fairly simple measures of serum cytokine levels along with metabolic profiles thoughtfully obtained prior to and after inititation of treatment for a metabolic disease, can facilitate decisions about research focus areas.
- 52. Dalmas E, Venteclef N, Caer C, et al. T cell-derived IL-22 amplifies IL-1beta-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014 doi: 10.2337/db13-1511. This study has also identified a Th17 pathway cytokine, IL-22 and IL-1beta to be critical mediators of inflammatory responses identified within obese diabetic AT of human subjects. Such recent findings support further investigation and focus on this immune pathway in humans.
- 53.Burcelin Rmy CV, Dacosta Anabela. Roy-Tirell Alexandrai, Thorens Bernard. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab. 2002;282:E834–E842. doi: 10.1152/ajpendo.00332.2001. [DOI] [PubMed] [Google Scholar]
- 54.Lee KTY, Karunakaran S, Ho MM, Clee SM. PWD/PhJ and WSB/EiJ Mice Are Resistant to Diet-Induced Obesity But Have Abnormal Insulin Secretion. Endocrinology. 2011;152:3005–3017. doi: 10.1210/en.2011-0060. [DOI] [PubMed] [Google Scholar]
- 55.Kones RRU. Prevention of cardiovascular disease: updating the immensity of the challenge and the role of risk factors. Hosp Pract. 1995;42(2014):92–100. doi: 10.3810/hp.2014.02.1096. [DOI] [PubMed] [Google Scholar]
- 56.Kohchi K, Takebayashi S, Hiroki T, Nobuyoshi M. Significance of adventitial inflammation of the coronary artery in patients with unstable angina: results at autopsy. Circulation. 1985;71:709–716. doi: 10.1161/01.cir.71.4.709. [DOI] [PubMed] [Google Scholar]
- 57.Stratford N, Britten K, Gallagher P. Inflammatory infiltrates in human coronary atherosclerosis. Atherosclerosis. 1986;59:271–276. doi: 10.1016/0021-9150(86)90122-x. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z, Nakayama T. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010;2010:535918. doi: 10.1155/2010/535918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schafer KKS. Update on the cardiovascular risk in obesity: endocrine and paracrine role of the adipose tissue. Hell J Cardiol. 2011;52:327–336. [PubMed] [Google Scholar]
- 60.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith E, Prasad KM, Butcher M, et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110:675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eid RE, Rao DA, Zhou J, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao Z, Wu Y, Cheng M, et al. Activation of Th17/Th1 and Th1, but not Th17, is associated with the acute cardiac event in patients with acute coronary syndrome. Atherosclerosis. 2011;217:518–524. doi: 10.1016/j.atherosclerosis.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 65.Methe H, Brunner S, Wiegand D, et al. Enhanced T-helper-1 lymphocyte activation patterns in acute coronary syndromes. J Am Coll Cardiol. 2005;45:1939–1945. doi: 10.1016/j.jacc.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 66.Ait-Oufella H, Herbin O, Bouaziz JD, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med. 2010;207:1579–1587. doi: 10.1084/jem.20100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gerrity RG. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981;103:191–200. [PMC free article] [PubMed] [Google Scholar]
- 68.Gerrity RG. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981;103:181–190. [PMC free article] [PubMed] [Google Scholar]
- 69.Kanda T, Hirao Y, Oshima S, et al. Interleukin-8 as a sensitive marker of unstable coronary artery disease. Am J Cardiol. 1996;77:304–307. doi: 10.1016/s0002-9149(97)89400-3. [DOI] [PubMed] [Google Scholar]
- 70.Lachman LB, Page SO, Metzgar RS. Purification of human interleukin 1. J Supramol Struct. 1980;13:457–466. doi: 10.1002/jss.400130405. [DOI] [PubMed] [Google Scholar]
- 71.Matthews N. Tumour-necrosis factor from the rabbit. II. Production by monocytes. Br J Cancer. 1978;38:310–315. doi: 10.1038/bjc.1978.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sacks HS, Fain JN, Cheema P, et al. Depot-specific overexpression of proinflammatory, redox, endothelial cell, and angiogenic genes in epicardial fat adjacent to severe stable coronary atherosclerosis. Metab Syndr Relat Disord. 2011;9:433–439. doi: 10.1089/met.2011.0024. [DOI] [PubMed] [Google Scholar]
- 73.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 74.Taleb S, Herbin O, Ait-Oufella H, et al. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2691–2698. doi: 10.1161/ATVBAHA.107.149567. [DOI] [PubMed] [Google Scholar]
- 75. Chalubinski M, Wojdan K, Dorantowicz R, et al. Comprehensive insight into immune regulatory mechanisms and vascular wall determinants of atherogenesis - emerging perspectives of immunomodulation. Arch Med Sci. 2013;9:159–165. doi: 10.5114/aoms.2013.33355. This review covers work describing how additional cellular players, including Th17 and Treg, in the etiology of cardiovascular disease need to be incorporated into current thinking about therapeutics.
- 76.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–753. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Witztum JL. Splenic immunity and atherosclerosis: a glimpse into a novel paradigm? J Clin Invest. 2002;109:721–724. doi: 10.1172/JCI15310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matsushita T, Yanaba K, Bouaziz JD, et al. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sacks HS, Fain JN, Cheema P, et al. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care. 2011;34:730–733. doi: 10.2337/dc10-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jagannathan M, Hasturk H, Liang Y, et al. TLR Cross-Talk Specifically Regulates Cytokine Production by B Cells from Chronic Inflammatory Disease Patients. J Immunol. 2009;183:7461–7470. doi: 10.4049/jimmunol.0901517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noronha AM, Liang Y, Hetzel JT, et al. Hyperactivated B cells in human inflammatory bowel disease. J Leukoc Biol. 2009;86:1007–1016. doi: 10.1189/jlb.0309203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van de Veerdonk FL, Lauwerys B, Marijnissen RJ, et al. The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum. 2011;63:1507–1516. doi: 10.1002/art.30314. [DOI] [PubMed] [Google Scholar]
- 83.Salliot C, Dougados M, Gossec L. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis. 2009;68:25–32. doi: 10.1136/ard.2007.083188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Linos E. Balancing the risks and benefits of rituximab. JAMA Internal Medicine. 2013;173:920–937. doi: 10.1001/jamainternmed.2013.743. [DOI] [PubMed] [Google Scholar]
- 85.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Payne GA, Kohr MC, Tune JD. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. British Journal of Pharmacology. 2012;165:659–669. doi: 10.1111/j.1476-5381.2011.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Konishi M, Sugiyama S, Sugamura K, et al. Association of pericardial fat accumulation rather than abdominal obesity with coronary atherosclerotic plaque formation in patients with suspected coronary artery disease. Atherosclerosis. 2010;209:573–578. doi: 10.1016/j.atherosclerosis.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 88.Taguchi R, Takasu J, Itani Y, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157:203–209. doi: 10.1016/s0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]
- 89.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 90.Iacobellis G, Pistilli D, Gucciardo M, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 91.Fain JN, Sacks HS, Bahouth SW, et al. Human epicardial adipokine messenger RNAs: comparisons of their expression in substernal, subcutaneous, and omental fat. Metabolism. 2010;59:1379–1386. doi: 10.1016/j.metabol.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 92.Tadros TM, Massaro JM, Rosito GA, et al. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity (Silver Spring) 2010;18:1039–1045. doi: 10.1038/oby.2009.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Denis GV, Hamilton JA. Healthy obese persons: How can they be identified and do metabolic profiles stratify risk? Current Opinion in Endocrinology, Diabetes and Obesity. 2013;20:369–376. doi: 10.1097/01.med.0000433058.78485.b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ding J, Kritchevsky SB, Harris TB, et al. The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914–1919. doi: 10.1038/oby.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McClain J, Hsu F, Brown E, et al. Pericardial adipose tissue and coronary artery calcification in the Multi-ethnic Study of Atherosclerosis (MESA) Obesity (Silver Spring) 2013;21:1056–1063. doi: 10.1002/oby.20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 97.Ruberg FL, Chen Z, Hua N, et al. The relationship of ectopic lipid accumulation to cardiac and vascular function in obesity and metabolic syndrome. Obesity (Silver Spring) 2010;18:1116–1121. doi: 10.1038/oby.2009.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Greif M, Becker A, von Ziegler F, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:781–786. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 99. Neeland IJ, Gupta S, Ayers CR, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;77:1835–1844. doi: 10.1161/CIRCIMAGING.113.000532. Authors report recent findings of fat distribution assessed by both MRI and dual enegy x-ray absorptiometry in a cross-sectional analysis of more than 2700 subjects in the Dallas Heart Study. Their attention to regional AT differences and detailed morphometry revealed that within obese subjects significant associatiation between visceral AT and concentric remodeling of the heart was observed, while sub-cutaneous lower body AT was protective for LV function, whereas no such association between hemodynamics and sub-cutaneous abdominal AT was found.
- 100.Divers J, Wagenknecht LE, Bowden DW, et al. Regional adipose tissue associations with calcified atherosclerotic plaque: African American-diabetes heart study. Obesity (Silver Spring) 2010;18:2004–2009. doi: 10.1038/oby.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Imai A, Komatsu S, Ohara T, et al. Visceral abdominal fat accumulation predicts the progression of noncalcified coronary plaque. Atherosclerosis. 2012;222:524–529. doi: 10.1016/j.atherosclerosis.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 102. Liang Kae-Woei TI-C, Lee Wen-Jane, Lee I-Te, et al. MRI Measured Epicardial Adipose Tissue Thickness at the Right AV Groove Differentiates Inflammatory Status in Obese Men With Metabolic Syndrome. Obesity (Silver Spring) 2012;20:525–532. doi: 10.1038/oby.2011.155. This study reports how MRI may be utilized to assess cardiovascular risk in patients by specific measurement of the epicardial AT, right atrioventricular groove.
- 103.Granér M, Seppälä-Lindroos A, Rissanen Aila, et al. Epicardial Fat, Cardiac Dimensions, and Low-Grade Inflammation in Young Adult Monozygotic Twins Discordant for Obesity. Am J Cardial. 2012;109:1295–1302. doi: 10.1016/j.amjcard.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 104.Wassel CL, Laughlin GA, Araneta MR, et al. Associations of pericardial and intrathoracic fat with coronary calcium presence and progression in a multiethnic study. Obesity (Silver Spring) 2013;21:1704–1712. doi: 10.1002/oby.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring) 2009;17:625. doi: 10.1038/oby.2008.575. author reply 6–7. [DOI] [PubMed] [Google Scholar]
- 106. Sacks HS, Fain JN, Bahouth SW, et al. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab. 2013;98:E1448–E1455. doi: 10.1210/jc.2013-1265. In the interest of defining additional brown fat-like therapeutic targets, this study determined gene expression profiles of heart-associated fat depots. In particular, epicardial AT had relatively higher UCP-1 gene transcripts compared to visceral paracardial fat, consistent with a "beige" fat cell lineage.
- 107.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 108.Zhu M, Belkina AC, Defuria J, et al. B cells promote obesity-associated periodontis and oral pathology-associated inflammation. J Leukoc Biol. 2014 doi: 10.1189/jlb.4A0214-095R. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pires JR, Santos Id, de Camargo LF, et al. Framingham cardiovascular risk in patients with obesity and periodontitis. J Indian Soc Periodontol. 2014;18:14–18. doi: 10.4103/0972-124X.128193. One of only a few recent human studies in which metabolic and PD parameters were evaluated for cardiac risk in obese subjects. Using the Framingham Score, both obesity and PD positively correlated with increased risk for CVD.
- 110.Rossi R, Iaccarino D, Nuzzo A, et al. Influence of body mass index on extent of coronary atherosclerosis and cardiac events in a cohort of patients at risk of coronary artery disease. Nutrition, Metabolism & Cardiovascular Diseases. 2011;21:86–93. doi: 10.1016/j.numecd.2009.09.001. [DOI] [PubMed] [Google Scholar]