Abstract
Purpose
The effects of ovarian function suppression (OFS) on survival and patient-reported outcomes were evaluated in a phase III trial in which premenopausal women were randomly assigned to tamoxifen with or without OFS.
Patients and Methods
Premenopausal women with axillary node-negative, hormone receptor–positive breast cancer tumors measuring ≤ 3 cm were randomly assigned to tamoxifen alone versus tamoxifen plus OFS; adjuvant chemotherapy was not permitted. Primary end points were disease-free survival (DFS) and overall survival (OS). Secondary end points included toxicity and patient-reported outcomes. Patient-reported outcome data included health-related quality of life, menopausal symptoms, and sexual function. These were evaluated at baseline, 6 months, 12 months, and then annually for up to 5 years after registration.
Results
In all, 345 premenopausal women were enrolled: 171 on tamoxifen alone and 174 on tamoxifen plus OFS. With a median follow-up of 9.9 years, there was no significant difference between arms for DFS (5-year rate: 87.9% v 89.7%; log-rank P = .62) or OS (5-year rate: 95.2% v 97.6%; log-rank P = .67). Grade 3 or higher toxicity was more common in the tamoxifen plus OFS arm (22.4% v 12.3%; P = .004). Patients treated with tamoxifen plus OFS had more menopausal symptoms, lower sexual activity, and inferior health-related quality of life at 3-year follow-up (P < .01 for all). Differences diminished with further follow-up.
Conclusion
When added to tamoxifen, OFS results in more menopausal symptoms and sexual dysfunction, which contributes to inferior self-reported health-related quality of life. Because of early closure, this study is underpowered for drawing conclusions about the impact on survival when adding OFS to tamoxifen.
INTRODUCTION
Ovarian function was related to breast cancer in 1882, when Nunn1 reported cancer regression 6 months after menopause. The first known therapeutic oophorectomy occurred in 1895 on a woman with recurrent breast cancer.2,3 Pharmacologic advances involving luteinizing hormone-releasing hormone (LHRH) agonists and improved surgical technique permitted the study of ovarian function suppression (OFS) in the adjuvant setting. Several trials demonstrate benefits from ovarian suppression or surgical oophorectomy equaling or exceeding benefits from cytotoxic chemotherapies in patients with hormone receptor–positive breast cancer.4–9 A meta-analysis10 by the Early Breast Cancer Trialists' Group suggests that OFS reduces breast cancer recurrence and mortality in the absence of other therapies. However, a second meta-analysis8 suggests that LHRH agonists alone do not improve recurrence or mortality, although adding them to other therapies reduces both recurrence and death following recurrence. Some consider OFS to be standard of care.11
Questions about OFS persist, and its role remains uncertain. It is not the recommended standard of care in North America, where tamoxifen alone is considered standard adjuvant endocrine therapy for premenopausal women.12 In the absence of definitive survival benefits, patient-reported outcomes (PROs) such as health-related quality of life (HRQoL) should be considered when use of OFS is considered. Understanding the additional toxicity from OFS beyond that of tamoxifen is complicated. Some published studies assess OFS versus cytotoxic chemotherapy.5,6,13–15 Other studies assess the impact of OFS in settings in which women are essentially past cytotoxic chemotherapy (which induces ovarian failure in premenopausal women).7,16–18 Only a few studies contain a tamoxifen alone arm.19,20 Furthermore, most published trials of OFS have not reported PROs such as HRQoL, hot flashes, and sexual dysfunction. The SOFT (Suppression of Ovarian Function Trial) study will address the value that OFS adds to standard adjuvant endocrine therapy in premenopausal women. SOFT randomly assigned women to tamoxifen alone versus tamoxifen plus OFS or aromatase inhibitor plus OFS. That phase III trial has completed accrual, but definitive results have not been reported for the tamoxifen versus tamoxifen plus OFS arms.21 SOFT included a significant proportion of women (54%) receiving adjuvant chemotherapy.21
The Eastern Cooperative Oncology Group (ECOG) undertook a phase III trial comparing tamoxifen versus tamoxifen plus OFS in premenopausal women with node-negative, hormone receptor–positive primary invasive breast cancers who did not receive adjuvant chemotherapy (Phase III Comparison of Tamoxifen v Tamoxifen With Ovarian Ablation in Premenopausal Women With Axillary Node–Negative Receptor-Positive Breast Cancer ≤ 3 cm; E-3193, INT-0142). Primary objectives included comparing the overall survival (OS) and disease-free survival (DFS) between the two arms. Secondary objectives included toxicity and PROs such as comparison of menopausal symptoms, sexual function, and HRQoL between treatment arms. The trial was terminated before reaching the enrollment goal because of slow accrual; however, it completed the necessary accrual to meet the PRO objectives. We report the study results here.
PATIENTS AND METHODS
Patient Population
Eligible patients were premenopausal women with node-negative, estrogen receptor (ER) –positive and/or progesterone receptor (PgR) –positive primary invasive breast cancers. Primary tumors had to be ≤ 3 cm in greatest diameter. Randomization was required within 84 days of definitive breast surgery (mastectomy or breast conservation). Premenopausal status was defined as a menstrual period within the past 6 months without prior oophorectomy, or in the case of prior hysterectomy, as age 55 years or younger with one or both ovaries remaining and an estradiol level in the normal premenopausal range. Patients could not have received prior systemic therapy for breast cancer, aside from ≤ 12 weeks of tamoxifen. Patients were ineligible if they had any evidence of locally advanced or metastatic disease at diagnosis. The institutional review boards at each registering institution approved the protocol. All patients provided written informed consent.
Treatment
In this open-label, randomized phase III trial, patients were randomly assigned 1:1 to tamoxifen alone or tamoxifen plus OFS by using permuted blocks within strata with dynamic balancing across main institutions and their affiliated networks. Stratification factors included hormone receptor status, tumor size, and type of OFS. The treatment schedule for the tamoxifen alone arm was tamoxifen 20 mg orally per day for 5 years; the schedule for the tamoxifen plus OFS arm was tamoxifen 20 mg orally per day for 5 years combined with OFS. OFS was per patient or physician choice and could consist of LHRH analog (goserelin 3.6 mg depot every 4 weeks for 5 years beginning within 4 weeks of random assignment; leuprolide acetate 3.75 mg every 4 weeks for 5 years beginning within 4 weeks of random assignment), surgical ablation (done within 12 weeks of random assignment), or radiation ovarian ablation (20 Gy in 10 fractions within 12 weeks of random assignment22). No dose reductions were permitted. Other adjuvant systemic therapies including chemotherapy were not permitted.
End Points and Assessments
The primary end points were disease-free survival (DFS) and overall survival (OS). Secondary end points included toxicity and difference in Functional Assessment of Cancer Therapy-Breast (FACT-B) scores at 6 and 12 months. Additional PROs were prespecified as exploratory end points. DFS was defined as time from random assignment to the earliest of disease recurrence, new primary breast cancer, or death as a result of any cause, censoring patients without recurrence or death at the date of last disease assessment known to be disease free. OS was defined as time from random assignment to death as a result of any cause, with living patients censored at last evaluation date. Disease recurrence and survival were assessed every 6 months for the first 5 years and yearly thereafter. Adverse events were assessed on the same schedule by using National Cancer Institute Common Toxicity Criteria, version 1.23
Participants were surveyed to evaluate menopausal symptoms, sexual function, and HRQoL. Assessment of menopausal symptoms was conducted with questions from the Postmenopausal Estrogen/Progestin Intervention checklist,24 which consisted of 15 questions devoted to various symptoms. Assessment of sexual function was conducted by using the Sexual Activity Questionnaire,25 which consisted of five questions about sexual activity. Assessment of HRQoL was measured by using FACT-B (Version 3), which consisted of 27 general questions pertaining to patients with all cancers plus nine additional questions specific to patients with breast cancer.26 Assessments of PROs were performed at baseline, at 6 and 12 months, and yearly thereafter for up to 5 years following registration.
Statistical Analysis
The target enrollment was 1,600 eligible patients, with 90% power to detect 33% reduction in the hazard of recurrence from adding OFS to tamoxifen (5-year DFS rate of 83.0% v 88.3%) by using a log-rank test with a one-sided type I error rate of 0.025. This design also gives 81% power to detect a 33% reduction in the hazard of death (5-year OS of 90.0% for tamoxifen alone v 93.2% for tamoxifen plus OFS) by using a log-rank test with a one-sided type I error rate of 0.025. For PROs, 110 patients per arm give 90% power to detect a difference of five points in the FACT-B total score at 6 and 12 months by using a two-sample t test with an overall type I error rate of 0.05 (Bonferroni adjustment was made for these comparisons), assuming the common standard deviation of 10.5. The target sample size for PRO end points was 367 patients, assuming a 40% loss in PROs by 12 months, for an effective sample size of 220.
All analyses were conducted in all eligible patients following the intent-to-treat principle. Associations between categorical or binary variables were examined by using the χ2 and Fisher's exact tests.27 The distribution of OS and DFS was estimated by using the Kaplan-Meier method28 and compared between treatment arms by the log-rank test. Cox proportional hazards model29 was used to estimate the treatment effect after adjusting for baseline covariates. A two-sample t test was used to compare PRO scores or score changes between treatment arms. Analysis of variance test was used to compare PRO scores based on type of OFS. Multivariable linear mixed effect models with unstructured covariance matrices were constructed to estimate the time profile for PRO end points and to assess the treatment difference over time, assuming that any missing data were missing at random. A sensitivity analysis was conducted by using the lognormal survival model for analyzing longitudinal data that incorporates the nonignorable censoring mechanism.30 A bootstrap method31 was applied to estimate the SEs of the regression coefficients in the lognormal survival models. All P values were for two-sided tests; P < .05 was considered statistically significant except for FACT-B comparisons at 6 and 12 months (P < .025 was used based on Bonferroni adjustment). No adjustment was made for multiple comparisons for other end points. SAS 9.0 (SAS Institute, Cary, NC) and STATA 11.2 (STATA, College Station, TX) were used for analyses.
RESULTS
Patient Demographics and Disease Characteristics
A total of 345 participants were enrolled between September 1994 and November 1997, 171 on tamoxifen alone and 174 on tamoxifen plus OFS. The final analysis, including all clinical outcomes and PRO end points, was conducted in March 2003 with median follow-up time of 5.86 years. However, participants were observed for recurrence and survival until June 2007 (median follow-up, 9.9 years; range, 0.2 to 12.3 years). DFS and OS analyses were updated in July 2011 and are presented here.
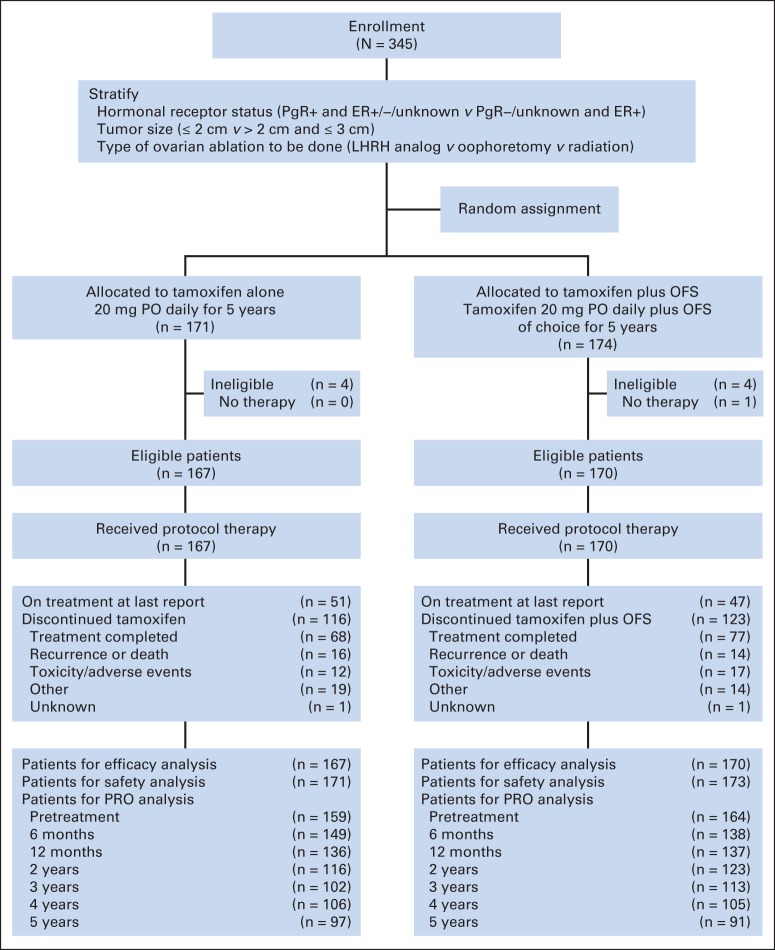
The CONSORT diagram is provided in Figure 1. Of 345 registered participants, eight (four receiving tamoxifen and four receiving tamoxifen plus OFS) were ineligible; one patient (tamoxifen plus OFS) withdrew after random assignment and thus never started the assigned treatments. By the intent-to-treat principle, the total number of analyzable participants is the 337 eligible patients. The two arms were well balanced with respect to known patient characteristics (Table 1). At study entry, the median age was 45 years (range, 26 to 55 years). Most participants were white (91%) with tumors ≤ 2 cm (83%), which were both estrogen receptor–positive and progesterone receptor–positive (88%). Participants randomly assigned to tamoxifen plus OFS generally chose to undergo surgical ablation of ovarian function via oophorectomy (42%), followed by medical suppression with an LHRH agonist (36%).
Fig 1.
CONSORT diagram for Tamoxifen and Ovarian Ablation in Treating Patients With Node-Negative, Receptor-Positive Breast Cancer (E-3193) trial. ER, estrogen receptor; LHRH, luteinizing hormone-releasing hormone; OFS, ovarian function suppression; PgR, progesterone receptor; PO, orally; PRO, patient-reported outcome.
Table 1.
Patient Demographic and Disease Characteristics (n = 337 analyzable participants)
| Characteristic | Tamoxifen Alone (n = 167) |
Tamoxifen + OFS (n = 170) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 46 | 44 | ||
| Range | 26-55 | 30-54 | ||
| 26-40 | 22 | 13.2 | 36 | 21.2 |
| 41-50 | 117 | 70.1 | 111 | 65.3 |
| 51-55 | 28 | 16.8 | 23 | 13.5 |
| Race/ethnicity | ||||
| White | 154 | 92.2 | 154 | 90.6 |
| Black | 7 | 4.2 | 9 | 5.3 |
| Other | 5 | 3.6 | 7 | 4.1 |
| Unknown | 1 | 0 | ||
| Hormone receptors | ||||
| ER positive, PgR positive | 147 | 88 | 149 | 87.7 |
| ER positive, PgR negative | 13 | 7.8 | 14 | 8.2 |
| ER positive, PgR unknown | 1 | 0.6 | 2 | 1.2 |
| ER negative, PgR positive | 4 | 2.4 | 4 | 2.4 |
| ER unknown, PgR positive | 2 | 1.2 | 1 | 0.6 |
| Tumor size, cm | ||||
| ≤ 1.0 | 22 | 13.2 | 15 | 8.8 |
| 1.1-2.0 | 117 | 70.1 | 126 | 74.1 |
| > 2.0 | 28 | 16.8 | 29 | 17.1 |
| Type of OFS | ||||
| LHRH | — | — | 61 | 36 |
| Oophorectomy | — | — | 72 | 42 |
| Radiation | — | — | 22 | 13 |
| Refused OFS | — | — | 15 | 9 |
| Surgical procedure | ||||
| Breast conservation | 97 | 58.1 | 112 | 65.9 |
| Mastectomy | 70 | 41.9 | 58 | 34.1 |
Abbreviations: ER, estrogen receptor; LHRH, luteinizing hormone-releasing hormone; OFS, ovarian function suppression; PgR, progesterone receptor.
Treatment
As of March 2003, 116 participants (69%) receiving tamoxifen and 123 (72%) receiving tamoxifen plus OFS reported discontinuing treatment; the reasons cited for treatment discontinuation were similar between the arms. The most common reason cited was treatment completion (68 [41%] receiving tamoxifen and 77 [45%] receiving tamoxifen plus OFS; P = .441). Twenty-nine patients (8.6%; 12 receiving tamoxifen, 17 receiving tamoxifen plus OFS) discontinued treatment because of adverse events and 30 patients (8.9%; 16 receiving tamoxifen, 14 receiving tamoxifen plus OFS) discontinued as a result of recurrence or death. In addition to patients who discontinued treatment, 15 patients (9.0%) receiving tamoxifen plus OFS refused OFS and four (2.4%) receiving tamoxifen also received OFS.
DFS and OS
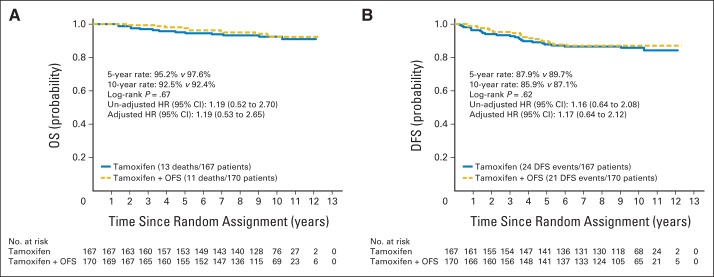
As of June 2007 (when follow-up for survival and recurrence stopped), 45 DFS events (tamoxifen, 24; tamoxifen plus OFS, 21) and 24 deaths had been observed among 337 eligible participants (Fig 2). The 5-year OS rate for tamoxifen was 95.2% (95% CI, 90.5% to 97.6%) compared with a rate of 97.6% (95% CI, 93.6% to 99.1%) for tamoxifen plus OFS (log-rank P = .67; Fig 2A). The adjusted hazard ratio (HR) was 1.19 (95% CI, 0.52 to 2.70) for tamoxifen versus tamoxifen plus OFS. The 5-year DFS rate for tamoxifen was 87.9% (95% CI, 81.9% to 92.0%) compared with the rate of 89.7% (95% CI, 83.9% to 93.5%) for tamoxifen plus OFS (log-rank P = .62; Fig 2B). The adjusted HR was 1.17 (95% CI, 0.64 to 2.12) for tamoxifen versus tamoxifen plus OFS.
Fig 2.
Comparison of (A) overall survival (OS) and (B) disease-free survival (DFS) by treatment in analyzable patients. HR, hazard ratio; OFS, ovarian function suppression.
Appendix Tables A1 and A2 (online only) display the results for the prespecified subgroup analysis based on OFS type and race. The estimated HRs for DFS and OS were not statistically significant, based on OFS type (Appendix Table A1). Among white women, the HR was 1.18 (95% CI, 0.63 to 2.21; P = .60) for DFS for tamoxifen versus tamoxifen plus OFS, and among nonwhite women, the HR was 1.02 (95% CI, 0.13 to 7.96; P = .98; Appendix Table A2).
Toxicity
The most common grade 3 or higher toxicities were hot flashes, weight gain, neuropsychiatric adverse effects, such as anxiety or depression, and neurologic adverse effects, such as somnolence and confusion (Table 2). The proportion of grade 3 or greater toxicity was higher for tamoxifen plus OFS compared with tamoxifen (22.4% v 12.3%; P = .004). No lethal adverse events were reported in the study.
Table 2.
Grade 3 or Higher Toxicities by Treatment Arm
| Toxicity Type | Tamoxifen Alone (n = 171) Grade |
Tamoxifen + OFS (n = 174) Grade |
||||||
|---|---|---|---|---|---|---|---|---|
| 3 |
4 |
3 |
4 |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Hot flashes | 8 | 4.7 | — | 28 | 16.1 | — | ||
| Neuropsychiatric* | 2 | 1.2 | 2 | 1.2 | 4 | 2.3 | — | |
| Neuroclinical† | 1 | 0.6 | — | 4 | 2.3 | — | ||
| Allergy | — | — | 1 | 0.6 | — | |||
| Weight gain | 4 | 2.3 | — | 6 | 3.4 | — | ||
| Vaginal dryness | — | — | 1 | 0.6 | — | |||
| Changes in libido | — | — | 1 | 0.6 | — | |||
| Anemia | — | — | — | 1 | 0.6 | |||
| Clotting disorders | — | — | 1 | 0.6 | — | |||
| Itching | 1 | 0.6 | — | — | — | |||
| Night sweats | — | — | 1 | 0.6 | — | |||
| Dizziness | 1 | 0.6 | — | — | — | |||
| Liver | — | — | 1 | 0.6 | — | |||
| Edema | — | — | 1 | 0.6 | — | |||
| Hypoglycemia | — | — | 1 | 0.6 | — | |||
| Pain | — | — | 1 | 0.6 | — | |||
| Insomnia | 2 | 1.2 | — | — | — | |||
| Endometriosis | 1 | 0.6 | — | — | — | |||
| Amenorrhea | 1 | 0.6 | — | — | — | |||
| Hypertension | — | — | 1 | 0.6 | — | |||
| Highest grade | 16 | 11.1 | 2 | 1.2 | 38 | 21.8 | 1 | 0.6 |
Abbreviation: OFS, ovarian function suppression.
Included anxiety and depression.
Included somnolence; confusion; hallucinations; coordination issues such as tremors, nystagmus, ataxaia, and dysdiadokinesis; and headache.
PROs
Overall, compliance with PRO assessments was high and was similar between the two arms in all follow-up visits in the study (Fig 1). More than 93% of the patients submitted the PRO forms at baseline in both arms. At months 6 and 12, the retention rate was 83% and 79%, respectively, in the overall sample; thus, the prespecified effective sample size of 220 patients at 12 months was achieved. Combining all time points, a total of 1,889 surveys (68.2%) were completed. Among missing surveys, 433 (15.6%) were missing because of institutional error, 153 (5.5%) because of patient refusal, and 46 (1.7%) because of patient expiration.
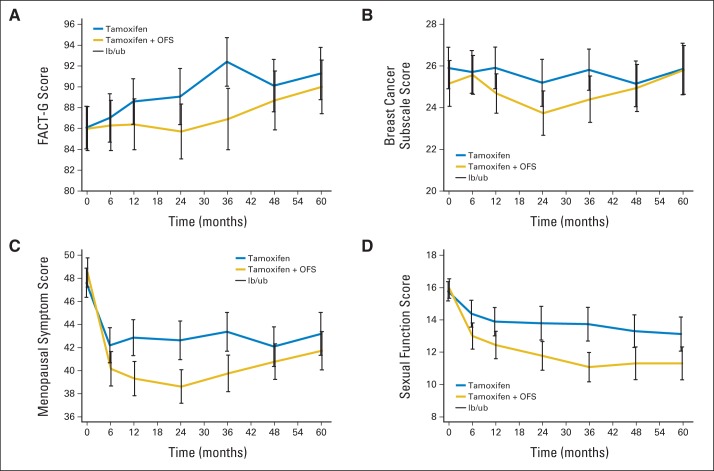
Table 3 and Figure 3 display the mean scores for PRO end points by treatment arm at each visit time in analyzable patients per protocol. Patients receiving tamoxifen plus OFS reported worse HRQoL as measured by the mean scores on both the FACT-General (FACT-G) and FACT-B cancer subscales compared with those receiving tamoxifen alone at all time points (Figs 3A and 3B). These differences became more pronounced over time and reached statistical and clinical significance for FACT-G at year 3 (difference, 6.39). This difference decreased over time. Women receiving tamoxifen plus OFS had more menopausal symptoms at all time points compared with women receiving tamoxifen alone, with statistically significant differences at 1, 2, and 3 years of follow-up (Fig 3C, Table 3). Sexual activity was lower among women receiving tamoxifen plus OFS compared with women receiving tamoxifen alone at all follow-up time points after month 6 (Fig 3D, Table 3), and differences were statistically significant. Appendix Table A3 (online only) displays PRO score changes between follow-up and baseline visits by arm. The tamoxifen plus OFS arm persistently had more differences from baseline starting at 6 months in menopausal and sexual activity compared with the tamoxifen alone arm.
Table 3.
Comparison of PRO End Points Between Tamoxifen Alone and Tamoxifen Plus OFS in Analyzable Participants
| End Points | Tamoxifen Alone |
Tamoxifen + OFS |
Difference in Mean | P (t test) | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Mean | SD | No. | Mean | SD | |||
| FACT-B* | ||||||||
| Baseline | 103† | 113.30 | 16.87 | 102 | 110.26 | 19.48 | 3.04 | .23 |
| Month 6 | 134 | 112.28 | 19.72 | 128 | 112.35 | 18.12 | −0.07 | .98 |
| Year 1 | 130 | 114.47 | 17.74 | 132 | 110.96 | 18.52 | 3.51 | .12 |
| Year 2 | 109 | 114.02 | 19.70 | 113 | 109.39 | 19.35 | 4.63 | .08 |
| Year 3 | 99 | 118.18 | 15.92 | 104 | 110.46 | 20.49 | 7.72 | .003 |
| Year 4 | 98 | 114.65 | 17.52 | 96 | 113.14 | 19.24 | 1.52 | .57 |
| Year 5 | 93 | 117.04 | 17.51 | 84 | 116.24 | 15.49 | 0.80 | .75 |
| FACT-G‡ | ||||||||
| Baseline | 158 | 86.24 | 13.18 | 164 | 85.79 | 13.91 | 0.45 | .76 |
| Month 6 | 149 | 86.98 | 14.61 | 137 | 86.26 | 14.57 | 0.72 | .68 |
| Year 1 | 136 | 88.52 | 13.24 | 137 | 86.18 | 14.61 | 2.34 | .17 |
| Year 2 | 116 | 89.19 | 14.93 | 123 | 85.50 | 15.06 | 3.69 | .06 |
| Year 3 | 102 | 92.47 | 12.15 | 113 | 86.54 | 16.08 | 5.39 | .003 |
| Year 4 | 106 | 90.10 | 13.37 | 104 | 88.57 | 14.85 | 1.54 | .43 |
| Year 5 | 97 | 91.30 | 12.87 | 91 | 89.88 | 12.62 | 1.42 | .45 |
| Breast subscale§ | ||||||||
| Baseline | 104† | 25.98 | 5.12 | 102 | 25.13 | 5.68 | 0.85 | .26 |
| Month 6 | 134 | 25.68 | 6.10 | 129 | 25.56 | 5.47 | 0.12 | .88 |
| Year 1 | 130 | 25.88 | 5.82 | 132 | 24.72 | 5.60 | 1.16 | .10 |
| Year 2 | 109 | 25.22 | 6.03 | 113 | 23.75 | 5.85 | 1.47 | .07 |
| Year 3 | 99 | 25.87 | 5.07 | 104 | 24.38 | 5.89 | 1.49 | .06 |
| Year 4 | 98 | 25.18 | 5.57 | 97 | 24.84 | 5.75 | 0.34 | .67 |
| Year 5 | 94 | 25.85 | 6.21 | 85 | 25.78 | 5.62 | 0.07 | .94 |
| Menopausal symptoms‖ | ||||||||
| Baseline | 148 | 47.61 | 7.99 | 157 | 48.71 | 7.99 | −1.10 | .23 |
| Month 6 | 139 | 42.13 | 9.16 | 128 | 40.21 | 8.59 | 1.92 | .08 |
| Year 1 | 131 | 42.85 | 9.04 | 136 | 39.40 | 8.71 | 3.45 | .002 |
| Year 2 | 114 | 42.59 | 9.13 | 118 | 38.74 | 8.06 | 3.85 | .001 |
| Year 3 | 97 | 43.37 | 8.41 | 113 | 39.78 | 8.59 | 3.59 | .003 |
| Year 4 | 105 | 41.98 | 8.98 | 103 | 40.86 | 8.11 | 1.12 | .35 |
| Year 5 | 97 | 43.22 | 9.46 | 90 | 41.83 | 8.08 | 1.39 | .28 |
| Sexual function¶ | ||||||||
| Baseline | 128 | 15.83 | 3.40 | 145 | 15.97 | 3.77 | −0.14 | .75 |
| Month 6 | 118 | 14.45 | 4.58 | 115 | 13.08 | 4.38 | 1.37 | .020 |
| Year 1 | 110 | 14.00 | 4.59 | 123 | 12.50 | 4.79 | 1.50 | .016 |
| Year 2 | 98 | 13.83 | 5.25 | 106 | 11.87 | 4.68 | 1.96 | .005 |
| Year 3 | 81 | 13.84 | 4.77 | 103 | 11.20 | 4.73 | 2.64 | < .001 |
| Year 4 | 85 | 13.26 | 4.76 | 85 | 11.38 | 4.73 | 1.87 | .011 |
| Year 5 | 80 | 13.18 | 4.85 | 78 | 11.45 | 4.49 | 1.73 | .022 |
Abbreviation: FACT-B, Functional Assessment of Cancer Therapy-Breast; FACT-G, FACT-General; OFS, ovarian function suppression; PRO, patient-reported outcome; SD, standard deviation.
Out of possible score 144, higher score indicates better HRQL.
On the original version of the baseline survey, the last page containing the breast subscale was missing. Thus, the FACT-B and breast subscales are unavailable for some patients. This was corrected in early 1995.
Out of possible score of 108, higher scores indicate better HRQL.
Out of possible score of 36, higher scores indicate fewer breast cancer-specific symptoms.
Out of possible score of 60, higher scores indicate fewer complaints or difficulty.
Out of possible score of 20, higher scores indicate better sexual activity.
Fig 3.
Patient-reported outcome scores for (A) Functional Assessment of Cancer Therapy-General (FACT-G), (B) breast cancer subscale, (C) menopause symptom, and (D) sexual function assessments and 95% CIs over time by treatment arm in analyzable patients. Vertical lines represent 95% CIs. lb/ub, lower border/upper border; OFS, ovarian function suppression.
Linear mixed effect models analysis showed similar results after adjusting for age, race, hormone receptor status, tumor size, and surgical procedure (Appendix Table A4, online only). The lognormal survival model analysis was conducted as a sensitivity analysis, and the results showed no significant changes compared with the previous results after considering the possible informative censoring as a result of death (data not shown).
PROs were examined by OFS type among patients on the tamoxifen plus OFS arm in the report. Patients with surgically induced menopause had slightly lower scores over all PRO end points at year 3, although this was statistically significant only for the FACT-B subscale and Sexual Activity Questionnaire (Appendix Table A5, online only). Given the small numbers as well as small overall differences in scores, results should be interpreted with caution.
DISCUSSION
E-3193 is a randomized phase III trial comparing tamoxifen alone with tamoxifen plus OFS for premenopausal women with early-stage breast cancer. The trial closed to accrual before meeting the enrollment goal for survival end points. DFS and OS were excellent overall; because of this and the early termination of accrual, the DFS and OS comparisons are underpowered. However, valuable data about toxicity, menopausal symptom burden, sexual function, and HRQoL were obtained. Accrual met the protocol's prespecified target goal for the PRO end points. PRO results suggest that adding ovarian suppression to tamoxifen leads to increased toxicity, more menopausal symptom burden, and lowered sexual function, all ultimately translating into lower HRQoL. The difference in HRQoL scores between the two arms is statistically significant and clinical meaningful by year 3.32 However, the negative impact of OFS diminished over time, likely because increasing numbers of women receiving tamoxifen alone reached menopause (median age at study entry was 45 years). Outcomes for the tamoxifen alone arm in particular trended upward and were significantly better at year 3 than at baseline, consistent with other studies of HRQoL in women receiving endocrine therapy.33
Limited data are available comparing OFS in addition to or in place of standard adjuvant endocrine therapy for premenopausal breast cancer (ie, tamoxifen). PRO data are similarly lacking. As shown in Table 4,34–40 studies have mainly examined OFS compared with or combined with cytotoxic chemotherapy. Trials examining tamoxifen alone versus tamoxifen plus OFS have used short durations of OFS (2 years). Our results suggest that durations of less than 3 years fail to capture the adverse effect peak and the HRQoL nadir from OFS.34,35 Because inclusion criteria varied widely from trial to trial, it is difficult to quantify the impact of chemotherapy-induced ovarian failure in women randomly assigned to tamoxifen alone after adjuvant chemotherapy. Women receiving tamoxifen who had chemotherapy-induced ovarian failure would have symptoms mimicking tamoxifen plus OFS.
Table 4.
Sample Published Studies in Premenopausal Breast Cancer Containing an OFS Arm
| Trial Name | Trial Acronym or Study Group | Reference | Population | Key Inclusion Criteria |
Chemotherapy Permitted | Treatment Arms | Outcomes Measured | ||
|---|---|---|---|---|---|---|---|---|---|
| Menopausal Status | Stage | Receptor and Node Status | |||||||
| Tamoxifen Versus Anastrozole, Alone or in Combination With Zoledronic Acid | ABCSG-12 | Gnant et al11,36 | 1,803 | Pre | I-II | Hormone receptor positive, < 10 positive nodes | Yes | Tamoxifen + goserelin for 3 years v aromatase inhibitor + goserelin for 3 years (also randomized to zoledronic acid or not) | DFS, OS, toxicity |
| ZEBRA | Jonat et al37 | 1,640 | Pre or peri | II | Node positive, age ≤ 50 years | Yes | CMF v goserelin for 2 years | DFS, OS, toxicity, QOL34 | |
| Phase III Randomized Comparison of Adjuvant Therapies in Premenopausal Women with Resected Node-Positive Hormone Receptor–Positive Adenocarcinoma of the Breast: CAF (CTX/DOX/5-FU) v CAF Followed by ZDX v CAF Followed by ZDX/TMX | E5188/INT 0101 | Davidson et al7 | Pre | Node positive, hormone receptor positive | Yes | After CAF, randomized to no treatment v goserelin for 5 years v goserelin + tamoxifen for 5 years | TTR, DFS, OS, toxicity | ||
| Scottish Cancer Trials Breast Group | Thomson et al38 | 332 | Pre | Node positive | Yes | CMF v ablation (surgical or radiation) | Recurrence, survival | ||
| Randomized Study Comparing CMF and Goserelin + Tamoxifen in Premenopausal Receptor-Positive Patients | ABCSG-5 | Jakesz et al5 | 1,034 assessable | Pre | I-II | Yes | CMF v tamoxifen for 5 years + goserelin for 3 years | RFS, OS, toxicity | |
| Adjuvant Chemotherapy, Goserelin, or Their Sequential Combination for Pre/Perimenopausal Patients with Node-negative Breast Cancer | IBCSG Trial VIII | International Breast Cancer Study Group et al6 | 1,111 | Pre or peri | III | Randomized, hormone receptor positive, node negative, T1 | Yes | CMF v CMF + goserelin for 18 months v goserelin for 2 years v no treatment (no treatment arm was discontinued with only 42 patients enrolled) | DFS, OS, toxicity |
| GABG IV-A | von Minckwitz et al13 | 771 | Pre | Node negative, hormone receptor positive | Yes | CMF v goserelin for 2 years | EFS, OS, toxicity | ||
| GABG IV-B | Kaufmann et al16 | 776 | Pre | Initially only hormone receptor negative, but amended to include hormone receptor positive, node positive | Yes | Hormone receptor–negative patients treated with chemotherapy, randomized to goserelin for 2 years v no further treatment | EFS, OS, toxicity | ||
| Zoladex in Premenopausal Patients | ZIPP | Hackshaw et al17 | 2,076 | Pre | I-II | Age < 50 years | Yes | Randomized after primary therapy to no treatment v goserelin for 2 years v tamoxifen for 2 years v goserelin for 2 years + tamoxifen | EFS, OS, QOL35 |
| Takeda Adjuvant Breast Cancer Study with Leuprorelin Acetate | TABLE | Schmid et al14,15 | 589 assessable | Pre or peri | II-IIIa | Assessable, node positive, estrogen receptor positive | Yes | CMF v leuprorelin acetate for 2 years | RFS, OS |
| ABC OAS Trial | Adjuvant Breast Cancer Trials Collaborative Group20 | 2,144 | Pre or peri | T1-3aN0-1 | Yes | Tamoxifen for 5 years v tamoxifen + OFS for 5 years | RFS, OS | ||
| Fédération Nationale des Centres de Lutte contre le Cancer | Arriagada18 | 926 | Pre | Node positive or grade 2 to 3 | Yes | Chemotherapy v chemotherapy + OFS (irradiation or surgical ablation or triptorelin for 3 years) | DFS, OS | ||
| Trial 02 | Italian Breast Cancer Adjuvant Study Group | Boccardo19 | 244 | Pre or peri | Estrogen receptor positive | Yes | CMF v OFS (irradiation or surgical ablation v goserelin for 2 years) + tamoxifen for 5 years | DFS, OS, toxicity | |
| MAM-1 | GOCSI | De Placido et al39 | 466 | Pre | Node positive | Yes | Goserelin + tamoxifen for 2 years v observation | DFS, toxicity | |
| Trial 89B | DBCG | Ejlertsen et al40 | 762 | Pre | High risk (node positive or > 5 cm primary) | Yes | Pelvic irradiation v CMF | DFS, OS | |
| Tamoxifen and Ovarian Ablation in Treating Patients With Node-Negative, Receptor-Positive Breast Cancer | E-3193, INT-0142 | 345 | Pre | Hormone receptor positive, node negative | No | Tamoxifen for 5 years v tamoxifen + OFS for 5 years | DFS, OS, PROs, toxicity | ||
NOTE. If chemotherapy was used, the type was not specified by the protocol.
Abbreviations: ABCSG, Austrian Breast and Colorectal Cancer Study Group; CAF, cyclophosphamide + doxorubicin + fluorouracil; CMF, cyclophosphamide + methotrexate + fluorouracil; CTX, chemotherapy; DBCG, Danish Breast Cancer Cooperative Group; DOX, doxorubicin; DFS, disease-free survival; EFS, event-free survival; FU, fluorouracil; GABG, German Adjuvant Breast Cancer Study Group; GOCSI, Gruppo Oncologico Centro-Sud-Isole; OAB, Ovarian Ablation or Suppression; OFS, ovarian function suppression; OS, overall survival; peri, perimenopausal; pre, premenopausal; PRO, patient-reported outcome; QOL, quality of life; RFS, recurrence-free or relapse-free survival; TMX, tamoxifen; TTR, time to recurrence; ZDX, Zoladex; ZEBRA, Zoladex Early Breast Cancer Research Association.
SOFT will provide valuable insights into the survival benefit from OFS as well as its impact on PROs such as hot flashes and loss of sexual interest. Particular strengths of E-3193 (when viewed in light of SOFT) include the maturity of our data. Furthermore, our patients were not exposed to chemotherapy; thus, the added symptom burden seen is solely a result of OFS. This is a unique feature of our data that will not be recapitulated by SOFT (although SOFT eligibility requirements carefully define the required residual menstrual function.)
One limitation of our study was the early termination of the trial because of poor accrual (although this applies only to the efficacy end points because the sample size needed for the PRO end points was achieved). In addition, participants were not blinded to assigned treatment arm, which could have influenced their perception of adverse effects. However, practical considerations make it unlikely that patients and treating physicians could have been blinded to continued menstrual function. Another limitation is the absence of data on other outcomes potentially impacted by OFS such as bone health, vaginal atrophy, or cognitive function. Finally, as is typical for PRO end points, multiple comparison adjustment was made only for the primary PRO end points, although multiple measures were administered at multiple time points in the study.
In conclusion, this study provides unique insight into the detrimental impact of OFS beyond that seen with tamoxifen alone. The impact of OFS on PROs is not complicated by the prior use of chemotherapy and its effects on ovarian function, unlike in many other studies. Questions generated by this research include exploring shorter durations of OFS, which might result in less symptom burden for patients. Given these data and in the absence of definitive data for improvement in DFS or OS with OFS, the Cancer Care Ontario guidelines endorsed by American Society of Clinical Oncology are appropriately cautious about adding OFS to tamoxifen.12 These data provide valuable information for oncologists and patients who are debating whether to add OFS to tamoxifen.
Acknowledgment
Presented at the 39th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31-June 3, 2003. The authors thank the participants, research staff, and local site investigators of the adjuvant trial included in this analysis.
Glossary Terms
- estrogen receptor (ER):
ligand-activated nuclear proteins, belonging to the class of nuclear receptors, present in many breast cancer cells that are important in the progression of hormone-dependent cancers. After binding, the receptor-ligand complex activates gene transcription. There are two types of estrogen receptors (ERα and ERβ). ERα is one of the most important proteins controlling breast cancer function. ERβ is present in much lower levels in breast cancer, and its function is uncertain. Estrogen receptor status guides therapeutic decisions in breast cancer.
- health-related quality of life (HRQoL):
a broad multidimensional concept that usually includes self-reported measures of physical and mental health.
Appendix
Table A1.
HRs and 95% CIs by Type of OFS Among Eligible Patients
| Outcome and Treatment | No. of Patients | HR | 95% CI | P |
|---|---|---|---|---|
| DFS, unadjusted | ||||
| Tamoxifen v tamoxifen + LHRH analog | 228 | 1.44 | 0.59 to 3.53 | .421 |
| Tamoxifen v tamoxifen + oophorectomy | 239 | 0.93 | 0.46 to 1.91 | .850 |
| Tamoxifen v tamoxifen + radiation | 189 | 1.06 | 0.32-3.54 | .918 |
| DFS, adjusted | ||||
| Tamoxifen v tamoxifen + LHRH analog | 228 | 1.47 | 0.59 to 3.65 | .408 |
| Tamoxifen v tamoxifen + oophorectomy | 239 | 0.98 | 0.47 to 2.03 | .955 |
| Tamoxifen v tamoxifen + radiation | 189 | 0.93 | 0.27 to 3.22 | .905 |
| OS, unadjusted | ||||
| Tamoxifen v tamoxifen + LHRH analog | 228 | 1.14 | 0.37 to 3.49 | .822 |
| Tamoxifen v tamoxifen + oophorectomy | 239 | 1.40 | 0.46 to 4.28 | .559 |
| Tamoxifen v tamoxifen + radiation | 189 | 0.57 | 0.16 to 2.01 | .385 |
| OS, adjusted | ||||
| Tamoxifen v tamoxifen + LHRH analog | 228 | 1.19 | 0.38 to 3.78 | .763 |
| Tamoxifen v tamoxifen + oophorectomy | 239 | 1.38 | 0.44 to 4.32 | .576 |
| Tamoxifen v tamoxifen + radiation | 189 | 0.48 | 0.12 to 1.82 | .277 |
NOTE. Tamoxifen + ovarian function suppression (OFS) is the reference group for all comparisons. A hazard ratio (HR) > 1 indicates improved outcome for tamoxifen + OFS (ie, higher risk for tamoxifen). Variables included in the adjusted models were age (≤ 40, 41-50, ≥ 51 years), tumor size (< 1, 1-2, > 2 cm), hormone receptor (progesterone receptor (PgR) positive or PgR negative/PgR unknown), and most extensive surgery (breast-sparing procedure or mastectomy). Race and menopausal status were not included because of lack of variability in the data (315 patients were white; 336 patients were premenopausal).
Abbreviations: DFS, disease-free survival; LHRH, luteinizing hormone-releasing hormone; OS, overall survival.
Table A2.
Estimated HR for Treatment Within Race Among Eligible Patients
| Outcome | Race | No. of Patients | HR | 95% CI | P |
|---|---|---|---|---|---|
| DFS | White | 308 | 1.18 | 0.63 to 2.21 | .599 |
| Other | 29 | 1.02 | 0.13 to 7.96 | .984 | |
| OS | White | 308 | 0.98 | 0.42 to 2.32 | .969 |
| Other* | — |
NOTE. Tamoxifen + ovarian function suppression is the reference group for all comparisons. A hazard ratio (HR) > 1 indicates improved outcome for tamoxifen + ovarian function suppression (ie, higher risk for tamoxifen alone arm). Adjusted for age (≤ 40, 41-50, ≥ 51 years), tumor size (< 1, 1-2, > 2 cm), hormone receptor (progesterone receptor (PgR) positive or PgR negative/PgR unknown), and most extensive surgery (breast-sparing procedure or mastectomy).
Abbreviations: DFS, disease-free survival; OS, overall survival.
Not computable (zero deaths in this group).
Table A3.
PRO Score Changes Compared With Baseline by Treatment Arm
| PRO/Baseline Time Point | Tamoxifen Alone |
Tamoxifen + OFS |
P (t test) | ||||
|---|---|---|---|---|---|---|---|
| No. of Participants | Mean | SD | No. of Participants | Mean | SD | ||
| FACT-B | |||||||
| Month 6 | 94 | −0.09 | 14.82 | 84 | −0.02 | 14.40 | .97 |
| Year 1 | 88 | 0.43 | 14.56 | 86 | −1.27 | 15.36 | .45 |
| Year 2 | 70 | 0.82 | 15.03 | 72 | 0.64 | 14.02 | .94 |
| Year 3 | 69 | 4.06 | 14.72 | 68 | 0.00 | 18.38 | .16 |
| Year 4 | 66 | 1.90 | 15.71 | 66 | 0.05 | 17.61 | .53 |
| Year 5 | 64 | 1.88 | 16.05 | 52 | 5.30 | 15.36 | .25 |
| FACT-G | |||||||
| Month 6 | 144 | 1.20 | 11.74 | 137 | −0.12 | 11.87 | .35 |
| Year 1 | 132 | 1.96 | 12.00 | 135 | −1.04 | 11.40 | .038 |
| Year 2 | 114 | 2.56 | 12.71 | 123 | 0.73 | 12.62 | .27 |
| Year 3 | 101 | 5.51 | 11.69 | 111 | 1.15 | 14.19 | .016 |
| Year 4 | 103 | 3.49 | 13.69 | 103 | 1.13 | 13.06 | .21 |
| Year 5 | 95 | 3.94 | 13.35 | 89 | 2.44 | 13.05 | .44 |
| Breast subscale | |||||||
| Month 6 | 95 | −0.45 | 4.87 | 85 | −0.25 | 5.19 | .80 |
| Year 1 | 89 | −0.36 | 5.27 | 86 | −0.79 | 5.94 | .61 |
| Year 2 | 70 | −0.75 | 4.54 | 72 | −1.24 | 5.58 | .57 |
| Year 3 | 69 | −0.14 | 5.01 | 68 | −0.88 | 6.69 | .47 |
| Year 4 | 66 | −0.80 | 4.70 | 66 | −0.99 | 5.86 | .83 |
| Year 5 | 66 | −0.97 | 5.95 | 53 | 1.47 | 6.36 | .033 |
| Menopausal symptoms | |||||||
| Month 6 | 126 | −5.24 | 8.19 | 123 | −8.90 | 9.05 | .001 |
| Year 1 | 119 | −4.80 | 8.20 | 129 | −9.72 | 9.03 | < .001 |
| Year 2 | 105 | −5.60 | 8.70 | 115 | −9.57 | 9.69 | .002 |
| Year 3 | 89 | −4.13 | 9.47 | 107 | −9.23 | 9.40 | < .001 |
| Year 4 | 95 | −5.71 | 9.32 | 99 | −9.00 | 9.46 | .016 |
| Year 5 | 90 | −4.46 | 9.56 | 84 | −7.71 | 10.20 | .031 |
| Sexual function | |||||||
| Month 6 | 99 | −1.67 | 3.81 | 105 | −2.99 | 4.03 | .017 |
| Year 1 | 99 | −1.92 | 3.95 | 113 | −3.29 | 4.29 | .017 |
| Year 2 | 87 | −2.23 | 4.62 | 99 | −4.47 | 5.07 | .002 |
| Year 3 | 73 | −2.22 | 4.33 | 95 | −4.73 | 4.91 | .001 |
| Year 4 | 73 | −3.04 | 4.72 | 79 | −4.78 | 5.55 | .041 |
| Year 5 | 72 | −3.06 | 4.90 | 69 | −4.86 | 5.09 | .034 |
Abbreviation: FACT-B, Functional Assessment of Cancer Therapy-Breast; FACT-G, FACT-General; PRO, patient-reported outcome; SD, standard deviation.
Table A4.
Multivariable Linear Mixed Effect Model Analysis for PRO End Points
| End Point | Variable | Coefficient | 95% CI | P |
|---|---|---|---|---|
| FACT-G | Treatment (tamoxifen + OFS v tamoxifen alone) | −0.54 | −3.61 to 2.54 | .733 |
| Time points (v baseline) | ||||
| Month 6 | 0.99 | −0.63 to 2.62 | .232 | |
| Year 1 | 2.05 | 0.32 to 3.78 | .020 | |
| Year 2 | 2.61 | 0.70 to 4.53 | .008 | |
| Year 3 | 5.05 | 2.92 to 7.18 | < .001 | |
| Year 4 | 3.13 | 0.84 to 5.43 | .007 | |
| Year 5 | 3.38 | 0.83 to 5.93 | .009 | |
| Treatment-by-time interaction | ||||
| Tamoxifen + OFS at month 6 | −1.33 | −3.65 to 0.99 | .262 | |
| Tamoxifen + OFS at year 1 | −2.97 | −5.42 to −0.53 | .017 | |
| Tamoxifen + OFS at year 2 | −2.49 | −5.17 to 0.19 | .069 | |
| Tamoxifen + OFS at year 3 | −5.06 | −8.03 to −2.09 | .001 | |
| Tamoxifen + OFS at year 4 | −2.73 | −5.97 to 0.51 | .098 | |
| Tamoxifen + OFS at year 5 | −3.48 | −7.10 to 0.14 | .059 | |
| Breast subscale | Treatment (tamoxifen + OFS v tamoxifen alone) | −0.95 | −2.30 to 0.41 | .171 |
| Time points (v baseline) | ||||
| Month 6 | −0.39 | −1.25 to 0.48 | .381 | |
| Year 1 | −0.39 | −1.28 to 0.50 | .389 | |
| Year 2 | −0.78 | −1.74 to 0.18 | .113 | |
| Year 3 | −0.57 | −1.59 to 0.45 | .276 | |
| Year 4 | −1.14 | −2.21 to −0.06 | .038 | |
| Year 5 | −0.60 | −1.73 to 0.54 | .306 | |
| Treatment-by-time interaction | ||||
| Tamoxifen + OFS at month 6 | 0.35 | −0.89 to 1.59 | .579 | |
| Tamoxifen + OFS at year 1 | −0.54 | −1.81 to 0.72 | .400 | |
| Tamoxifen + OFS at year 2 | −0.41 | −1.77 to 0.95 | .556 | |
| Tamoxifen + OFS at year 3 | −0.40 | −1.84 to 1.04 | .590 | |
| Tamoxifen + OFS at year 4 | −0.25 | −1.78 to 1.27 | .743 | |
| Tamoxifen + OFS at year 5 | −0.54 | −2.18 to 1.10 | .518 | |
| Menopausal symptom | Treatment (tamoxifen + OFS v tamoxifen alone) | 1.04 | −0.83 to 2.90 | .275 |
| Time points (v baseline) | ||||
| Month 6 | −5.22 | −6.50 to −3.94 | < .001 | |
| Year 1 | −5.00 | −6.34 to −3.66 | < .001 | |
| Year 2 | −5.35 | −6.80 to −3.89 | < .001 | |
| Year 3 | −4.40 | −6.01 to −2.78 | < .001 | |
| Year 4 | −5.52 | −7.22 to −3.83 | < .001 | |
| Year 5 | −4.61 | −6.48 to −2.75 | < .001 | |
| Treatment-by-time interaction | ||||
| Tamoxifen + OFS at month 6 | −3.58 | −5.40 to −1.76 | < .001 | |
| Tamoxifen + OFS at year 1 | −4.80 | −6.67 to −2.93 | < .001 | |
| Tamoxifen + OFS at year 2 | −4.35 | −6.39 to −2.32 | < .001 | |
| Tamoxifen + OFS at year 3 | −4.66 | −6.89 to −2.44 | < .001 | |
| Tamoxifen + OFS at year 4 | −3.50 | −5.89 to −1.11 | .004 | |
| Tamoxifen + OFS at year 5 | −3.91 | −6.56 to −1.27 | .004 | |
| Sexual function | Treatment (tamoxifen + OFS v tamoxifen alone) | −0.16 | −1.12 to 0.79 | .739 |
| Time points (v baseline) | ||||
| Month 6 | −1.44 | −2.16 to −0.72 | < .001 | |
| Year 1 | −1.88 | −2.63 to −1.13 | < .001 | |
| Year 2 | −2.17 | −2.99 to −1.36 | < .001 | |
| Year 3 | −2.32 | −3.23 to −1.41 | < .001 | |
| Year 4 | −2.75 | −3.71 to −1.78 | < .001 | |
| Year 5 | −3.22 | −4.28 to −2.16 | < .001 | |
| Treatment-by-time interaction | ||||
| Tamoxifen + OFS at month 6 | −1.45 | −2.45 to −0.44 | .005 | |
| Tamoxifen + OFS at year 1 | −1.52 | −2.56 to −0.49 | .004 | |
| Tamoxifen + OFS at year 2 | −2.01 | −3.14 to −0.88 | < .001 | |
| Tamoxifen + OFS at year 3 | −2.24 | −3.48 to −1.00 | < .001 | |
| Tamoxifen + OFS at year 4 | −1.99 | −3.35 to −0.64 | .004 | |
| Tamoxifen + OFS at Year 5 | −2.12 | −3.61 to −0.63 | .005 |
NOTE. Adjusted covariates included age, race, hormone receptor status, tumor size, and surgical procedure. The estimated coefficients for these adjusted covariates and the estimated random effects parameters are not shown in the table. The coefficient for variable “treatment: tamoxifen + OFS v tamoxifen alone” showed the treatment difference in the PRO end point at baseline. The coefficient for variable “Time points (v baseline)” showed the difference in PRO end point between a follow-up time point and the baseline visit on tamoxifen. The sum of the coefficients for “Time point” and “Treatment-by-time interaction” showed the difference in PRO end points between a follow-up time point and the baseline visit on tamoxifen + OFS. The sum of the coefficients for “Treatment” and “Treatment-by-time interaction” showed the difference in PRO end points between treatment arms at a follow-up time point.
Abbreviations: FACT-G, Functional Assessment of Cancer Therapy-General; OFS, ovarian function suppression; PRO, patient-reported outcome.
Table A5.
PRO Scores by OFS Type in Patients in the Tamoxifen + OFS Arm
| PRO/Time Point | LHRH Agonist |
Oophorectomy |
Radiation |
P (ANOVA test) | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| FACT-B | |||||||
| Baseline | 109.30 | 20.95 | 112.90 | 17.74 | 108.20 | 15.15 | .60 |
| Month 6 | 113.10 | 18.94 | 111.50 | 18.28 | 113.90 | 17.02 | .85 |
| Year 1 | 111.70 | 20.16 | 110.90 | 18.43 | 112.20 | 15.89 | .95 |
| Year 2 | 107.10 | 20.41 | 112.30 | 17.85 | 111.80 | 13.83 | .38 |
| Year 3 | 116.60 | 18.83 | 107.60 | 16.14 | 116.60 | 18.67 | .040 |
| Year 4 | 115.10 | 22.15 | 111.30 | 17.80 | 114.60 | 19.29 | .68 |
| Year 5 | 117.50 | 15.15 | 116.00 | 14.35 | 116.50 | 18.34 | .92 |
| FACT-G | |||||||
| Baseline | 86.65 | 14.82 | 86.61 | 12.63 | 83.23 | 11.93 | .55 |
| Month 6 | 87.10 | 14.15 | 85.89 | 14.35 | 86.15 | 16.96 | .91 |
| Year 1 | 86.60 | 16.12 | 86.31 | 14.05 | 87.06 | 13.09 | .98 |
| Year 2 | 83.87 | 15.09 | 87.44 | 14.22 | 88.13 | 11.61 | .39 |
| Year 3 | 91.05 | 14.71 | 84.82 | 13.03 | 89.13 | 15.19 | .10 |
| Year 4 | 89.39 | 16.35 | 87.62 | 14.22 | 89.31 | 15.21 | .84 |
| Year 5 | 91.90 | 12.37 | 88.58 | 12.15 | 90.43 | 14.55 | .54 |
| Breast subscale | |||||||
| Baseline | 25.89 | 5.81 | 25.20 | 5.43 | 24.44 | 6.13 | .71 |
| Month 6 | 25.94 | 6.11 | 25.30 | 5.44 | 25.22 | 3.89 | .81 |
| Year 1 | 24.91 | 5.31 | 24.55 | 6.01 | 25.17 | 5.61 | .90 |
| Year 2 | 23.24 | 6.16 | 24.43 | 5.97 | 23.46 | 4.29 | .60 |
| Year 3 | 25.45 | 5.21 | 23.49 | 5.79 | 27.36 | 4.18 | .040 |
| Year 4 | 25.56 | 6.57 | 24.29 | 5.48 | 25.33 | 5.10 | .60 |
| Year 5 | 25.90 | 5.27 | 26.09 | 5.65 | 25.54 | 5.90 | .95 |
| PEPI | |||||||
| Baseline | 48.28 | 7.74 | 49.28 | 8.03 | 49.66 | 7.35 | .70 |
| Month 6 | 42.37 | 8.40 | 38.97 | 8.17 | 38.48 | 9.65 | .09 |
| Year 1 | 41.17 | 7.75 | 37.86 | 8.90 | 40.41 | 7.48 | .10 |
| Year 2 | 39.50 | 8.95 | 38.34 | 6.51 | 36.57 | 6.84 | .44 |
| Year 3 | 42.25 | 7.04 | 38.25 | 8.53 | 39.88 | 9.02 | .07 |
| Year 4 | 41.43 | 8.40 | 40.05 | 7.88 | 40.29 | 7.43 | .74 |
| Year 5 | 42.08 | 8.22 | 41.17 | 7.76 | 42.92 | 6.05 | .74 |
| SAQ | |||||||
| Baseline | 16.49 | 3.23 | 15.90 | 3.74 | 15.74 | 4.25 | .61 |
| Month 6 | 14.42 | 4.35 | 12.19 | 3.88 | 13.53 | 4.68 | .041 |
| Year 1 | 14.12 | 3.87 | 11.45 | 4.78 | 13.27 | 4.91 | .012 |
| Year 2 | 13.13 | 5.07 | 11.16 | 4.33 | 10.90 | 2.82 | .09 |
| Year 3 | 13.11 | 3.95 | 10.14 | 4.82 | 10.85 | 5.24 | .014 |
| Year 4 | 12.27 | 4.18 | 10.85 | 5.04 | 10.69 | 4.44 | .43 |
| Year 5 | 12.56 | 3.97 | 10.68 | 4.88 | 11.11 | 3.33 | .25 |
Abbreviation: ANOVA, analysis of variance; FACT-B, Functional Assessment of Cancer Therapy-Breast; FACT-G, FACT-General; LHRH, luteinizing hormone-releasing hormone; OFS, ovarian function suppression; PEPI, Postmenopausal Estrogen/Progestin Intervention (checklist); PRO, patient-reported outcome; SAQ, Sexual Activity Questionnaire; SD, standard deviation.
See accompanying editorial on page 3920
Support information appears at the end of this article.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Support
Supported in part by Public Health Service Grants No. CA23318, CA66636, CA21115, CA21076, CA16116, CA17145, CA14958, CA32102, and CA25224 from the National Cancer Institute, National Institutes of Health (NIH), Department of Health and Human Services, and by Grant No. UL1TR000427 from the Clinical and Translational Science Award program through the NIH National Center for Advancing Translational Sciences (A.J.T.).
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Molin Wang, John H. Fetting, David Cella, Silvana Martino, James N. Ingle, Joseph A. Sparano, Lawrence J. Solin, William C. Wood, Nicholas J. Robert
Administrative support: Joseph A. Sparano
Provision of study materials or patients: Joseph A. Sparano, William C. Wood, Nicholas J. Robert
Collection and assembly of data: Molin Wang, John H. Fetting, David Cella, Silvana Martino, James N. Ingle, Joseph A. Sparano, Lawrence J. Solin, Nicholas J. Robert
Data analysis and interpretation: Amye J. Tevaarwerk, Molin Wang, Fengmin Zhao, David Cella, Lynne I. Wagner, William C. Wood, Nicholas J. Robert
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Nunn T. On cancer of the breast. London, United Kingdom: J. & A, Churchill; 1882. p. 71. [Google Scholar]
- 2.Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: Suggestions for a new method of treatment, with illustrative cases. Lancet. 1896;148:162–165. [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson A. Analysis of cases in which oophorectomy was performed for inoperable carcinoma of the breast. Br Med J. 1902;2:1538–1541. doi: 10.1136/bmj.2.2184.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.[No authors listed] Adjuvant ovarian ablation versus CMF chemotherapy in premenopausal women with pathological stage II breast carcinoma: The Scottish trial—Scottish Cancer Trials Breast Group and ICRF Breast Unit, Guy's Hospital, London. Lancet. 1993;341:1293–1298. [PubMed] [Google Scholar]
- 5.Jakesz R, Hausmaninger H, Kubista E, et al. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: Evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer—Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol. 2002;20:4621–4627. doi: 10.1200/JCO.2002.09.112. [DOI] [PubMed] [Google Scholar]
- 6.International Breast Cancer Study Group (IBCSG) Castiglione-Gertsch M, O'Neill A, et al. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: A randomized trial. J Natl Cancer Inst. 2003;95:1833–1846. doi: 10.1093/jnci/djg119. [DOI] [PubMed] [Google Scholar]
- 7.Davidson NE, O'Neill AM, Vukov AM, et al. Chemoendocrine therapy for premenopausal women with axillary lymph node-positive, steroid hormone receptor-positive breast cancer: Results from INT 0101 (E5188) J Clin Oncol. 2005;23:5973–5982. doi: 10.1200/JCO.2005.05.551. [DOI] [PubMed] [Google Scholar]
- 8.LHRH-Agonists in Early Breast Cancer Overview Group1. Cuzick J, Ambroisine L, et al. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: A meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007;369:1711–1723. doi: 10.1016/S0140-6736(07)60778-8. [DOI] [PubMed] [Google Scholar]
- 9.Roché H, Kerbrat P, Bonneterre J, et al. Complete hormonal blockade versus epirubicin-based chemotherapy in premenopausal, one to three node-positive, and hormone-receptor positive, early breast cancer patients: 7-year follow-up results of French Adjuvant Study Group 06 randomised trial. Ann Oncol. 2006;17:1221–1227. doi: 10.1093/annonc/mdl107. [DOI] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 11.Gnant M, Mlineritsch B, Stoeger H, et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 12.Griggs JJ, Somerfield MR, Anderson H, et al. American Society of Clinical Oncology endorsement of the Cancer Care Ontario practice guideline on adjuvant ovarian ablation in the treatment of premenopausal women with early-stage invasive breast cancer. J Clin Oncol. 2011;29:3939–3942. doi: 10.1200/JCO.2011.36.4950. [DOI] [PubMed] [Google Scholar]
- 13.von Minckwitz G, Graf E, Geberth M, et al. CMF versus goserelin as adjuvant therapy for node-negative, hormone-receptor-positive breast cancer in premenopausal patients: A randomised trial (GABG trial IV-A-93) Eur J Cancer. 2006;42:1780–1788. doi: 10.1016/j.ejca.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Schmid P, Untch M, Wallwiener D, et al. Cyclophosphamide, methotrexate and fluorouracil (CMF) versus hormonal ablation with leuprorelin acetate as adjuvant treatment of node-positive, premenopausal breast cancer patients: Preliminary results of the TABLE-study (Takeda Adjuvant Breast cancer study with Leuprorelin Acetate) Anticancer Res. 2002;22:2325–2332. [PubMed] [Google Scholar]
- 15.Schmid P, Untch M, Kossé V, et al. Leuprorelin acetate every-3-months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: The TABLE study. J Clin Oncol. 2007;25:2509–2515. doi: 10.1200/JCO.2006.08.8534. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann M, Graf E, Jonat W, et al. A randomised trial of goserelin versus control after adjuvant, risk-adapted chemotherapy in premenopausal patients with primary breast cancer: GABG-IV B-93. Eur J Cancer. 2007;43:2351–2358. doi: 10.1016/j.ejca.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Hackshaw A, Baum M, Fornander T, et al. Long-term effectiveness of adjuvant goserelin in premenopausal women with early breast cancer. J Natl Cancer Inst. 2009;101:341–349. doi: 10.1093/jnci/djn498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arriagada R, Lê MG, Spielmann M, et al. Randomized trial of adjuvant ovarian suppression in 926 premenopausal patients with early breast cancer treated with adjuvant chemotherapy. Ann Oncol. 2005;16:389–396. doi: 10.1093/annonc/mdi085. [DOI] [PubMed] [Google Scholar]
- 19.Boccardo F, Rubagotti A, Amoroso D, et al. Cyclophosphamide, methotrexate, and fluorouracil versus tamoxifen plus ovarian suppression as adjuvant treatment of estrogen receptor-positive pre-/perimenopausal breast cancer patients: Results of the Italian Breast Cancer Adjuvant Study Group 02 randomized trial. J Clin Oncol. 2000;18:2718–2727. doi: 10.1200/JCO.2000.18.14.2718. [DOI] [PubMed] [Google Scholar]
- 20.Adjuvant Breast Cancer Trials Collaborative Group. Ovarian ablation or suppression in premenopausal early breast cancer: Results from the international adjuvant breast cancer ovarian ablation or suppression randomized trial. J Natl Cancer Inst. 2007;99:516–525. doi: 10.1093/jnci/djk109. [DOI] [PubMed] [Google Scholar]
- 21.Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–118. doi: 10.1056/NEJMoa1404037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes LL, Gray RJ, Solin LJ, et al. Efficacy of radiotherapy for ovarian ablation: Results of a breast intergroup study. Cancer. 2004;101:969–972. doi: 10.1002/cncr.20481. [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute, National Institutes of Health, Department of Health and Human Services. Bethesda, MD: National Cancer Institute; 1982. Common Toxicity Criteria Manual, version 1.0. [Google Scholar]
- 24.Moos RH, Leiderman DB. Toward a menstrual cycle symptom typology. J Psychosom Res. 1978;22:31–40. doi: 10.1016/0022-3999(78)90088-0. [DOI] [PubMed] [Google Scholar]
- 25.Thirlaway K, Fallowfield L, Cuzick J. The Sexual Activity Questionnaire: A measure of women's sexual functioning. Qual Life Res. 1996;5:81–90. doi: 10.1007/BF00435972. [DOI] [PubMed] [Google Scholar]
- 26.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 27.Agresti A. Categorical Data Analysis. Hoboken, NJ: Wiley; 1958. [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 29.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 30.Schluchter MD. Methods for the analysis of informatively censored longitudinal data. Stat Med. 1992;11:1861–1870. doi: 10.1002/sim.4780111408. [DOI] [PubMed] [Google Scholar]
- 31.Efron B. Bootstrap methods: Another look at the jackknife. Ann Stat. 1979;7:1–26. [Google Scholar]
- 32.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57:898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Cella D, Fallowfield L, Barker P, et al. Quality of life of postmenopausal women in the ATAC (“Arimidex”, tamoxifen, alone or in combination) trial after completion of 5 years' adjuvant treatment for early breast cancer. Breast Cancer Res Treat. 2006;100:273–284. doi: 10.1007/s10549-006-9260-6. [DOI] [PubMed] [Google Scholar]
- 34.de Haes H, Olschewski M, Kaufmann M, et al. Quality of life in goserelin-treated versus cyclophosphamide + methotrexate + fluorouracil-treated premenopausal and perimenopausal patients with node-positive, early breast cancer: The Zoladex Early Breast Cancer Research Association Trialists Group. J Clin Oncol. 2003;21:4510–4516. doi: 10.1200/JCO.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 35.Berglund G, Nystedt M, Bolund C, et al. Effect of endocrine treatment on sexuality in premenopausal breast cancer patients: A prospective randomized study. J Clin Oncol. 2001;19:2788–2796. doi: 10.1200/JCO.2001.19.11.2788. [DOI] [PubMed] [Google Scholar]
- 36.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 37.Jonat W, Kaufmann M, Sauerbrei W, et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: The Zoladex Early Breast Cancer Research Association Study. J Clin Oncol. 2002;20:4628–4635. doi: 10.1200/JCO.2002.05.042. [DOI] [PubMed] [Google Scholar]
- 38.Thomson CS, Twelves CJ, Mallon EA, et al. Adjuvant ovarian ablation vs CMF chemotherapy in premenopausal breast cancer patients: Trial update and impact of immunohistochemical assessment of ER status. Breast. 2002;11:419–429. doi: 10.1054/brst.2002.0451. [DOI] [PubMed] [Google Scholar]
- 39.De Placido S, De Laurentiis M, De Lena M, et al. A randomised factorial trial of sequential doxorubicin and CMF vs CMF and chemotherapy alone vs chemotherapy followed by goserelin plus tamoxifen as adjuvant treatment of node-positive breast cancer. Br J Cancer. 2005;92:467–474. doi: 10.1038/sj.bjc.6602355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ejlertsen B, Mouridsen HT, Jensen MB, et al. Similar efficacy for ovarian ablation compared with cyclophosphamide, methotrexate, and fluorouracil: From a randomized comparison of premenopausal patients with node-positive, hormone receptor-positive breast cancer. J Clin Oncol. 2006;24:4956–4962. doi: 10.1200/JCO.2005.05.1235. [DOI] [PubMed] [Google Scholar]