Abstract
Purpose
CALGB 40302 sought to determine whether lapatinib would improve progression-free survival (PFS) among women with hormone receptor–positive metastatic breast cancer treated with fulvestrant.
Patients and Methods
Eligible women had estrogen receptor–positive and/or progesterone receptor–positive tumors, regardless of human epidermal growth factor receptor 2 (HER2) status, and prior aromatase inhibitor treatment. Patients received fulvestrant 500 mg intramuscularly on day 1, followed by 250 mg on days 15 and 28 and every 4 weeks thereafter, and either lapatinib 1,500 mg or placebo daily. The study planned to accrue 324 patients and was powered for a 50% improvement in PFS with lapatinib from 5 to 7.5 months.
Results
At the third planned interim analysis, the futility boundary was crossed, and the data and safety monitoring board recommend study closure, having accrued 295 patients. At the final analysis, there was no difference in PFS (hazard ratio [HR] of placebo to lapatinib, 1.04; 95% CI, 0.82 to 1.33; P = .37); median PFS was 4.7 months for fulvestrant plus lapatinib versus 3.8 months for fulvestrant plus placebo. There was no difference in overall survival (OS) (HR, 0.91; 95% CI, 0.68 to 1.21; P = .25). For HER2-normal tumors, median PFS did not differ by treatment arm (4.1 v 3.8 months). For HER2-positive tumors, lapatinib was associated with longer median PFS (5.9 v 3.3 months), but the differential treatment effect by HER2 status was not significant (P = .53). The most frequent toxicities were diarrhea, fatigue, and rash associated with lapatinib.
Conclusion
Adding lapatinib to fulvestrant does not improve PFS or OS in advanced ER-positive breast cancer and is more toxic.
INTRODUCTION
There are two well-established signal pathways in breast cancer—the estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) pathways—with effective targeted treatment options. Preclinical models strongly support combined targeting of these pathways, but the clinical value of this approach in the setting of metastatic breast cancer remains controversial, in two ways. One relates to the clinical value of simultaneous use of antiestrogen and anti-HER2 treatments in the management of breast cancers that express both ER and HER2. The other centers on whether combined approaches may be of clinical value in tumors that are ER positive but HER2 nonoverexpressing. Laboratory models have suggested that one mechanism of resistance to endocrine therapy may be acquired overexpression or activation of the HER2 pathway.
A variety of antiestrogen agents are available for ER-positive metastatic breast cancer, including selective ER modulators, pure antiestrogens, and aromatase inhibitors (AIs). Fulvestrant is an injectable, pure, steroidal ER antagonist that binds to ER and causes degradation of the receptor complex.1 Fulvestrant has clinical activity in patients previously treated with antiestrogen therapies, including AIs.2,3 It has efficacy comparable to or superior than that seen with AIs in AI-refractory4 and AI-naive metastatic breast cancer.5,6
The mechanisms of resistance to endocrine therapy are not well characterized.7 Preclinical models have suggested important crosstalk between ER and other growth factor signaling pathways, including among others the epidermal growth factor receptor (EGFR) and HER2 pathways.8,9,10 In some models, breast cancer cells developing resistance to endocrine agents acquired overexpression of EGFR and/or HER2 that might account for treatment resistance.11,12 Laboratory evidence has suggested that exposure to EGFR- and HER2-targeting agents can resensitize breast cancers to antiestrogen therapies and restore sensitivity to endocrine treatments.13,14,15,16
Lapatinib is an orally available, reversible, small-molecular tyrosine kinase inhibitor with selectivity for the EGFR and HER2 kinases and biologic activity in cell lines that express EGFR and/or HER2.17,18 Clinical studies indicated that doses up to 1,600 mg per day are reasonably well tolerated; common adverse effects include acneiform rash and diarrhea.19 Lapitinib has modest single-agent activity in refractory HER2-positive breast cancer20,21 and more robust activity as first-line monotherapy treatment.22
The availability of effective, well-tolerated antiestrogen and dual kinase inhibitor therapies allowed us to test the hypothesis that dual pathway targeting of both ER and HER2 signaling would be effective in advanced breast cancer. Therefore, we developed Cancer and Leukemia Group B (CALGB) 40302, in which patients with ER-positive advanced breast cancer were randomly assigned to receive the antiestrogen treatment fulvestrant, administered with or without lapatinib, independent of HER2 expression.
PATIENTS AND METHODS
Patients
The study was open to postmenopausal women with stage III or IV breast cancer considered unamenable to curative therapy. Postmenopausal was defined as: history of bilateral oophorectomy, age ≥ 60 years or age ≥ 45 years with amenorrhea > 12 months, ovarian suppression by gonadotropin-releasing hormone agonist for at least 3 consecutive months before enrollment, or follicle-stimulating hormone levels in the postmenopausal range. Tumors were positive for ER and/or progesterone receptor according to local institution evaluation, with ≥ 1% of cells being positive. Originally, HER2 status eligibility was as follows: HER2 1+, 2+, or 3+ by immunohistochemistry; fluorescent in situ hybridization positive; or serum HER2 extracellular domain ≥ 15 ng. The protocol was subsequently amended to include tumors regardless of HER2 status. Patients with bone-only nonmeasurable disease or with measurable disease by RECIST criteria were eligible. Patients had one or two prior endocrine treatments for at least 3 months without tumor progression in either the adjuvant or metastatic setting, which must have included treatment with a third-generation AI. Patients may have received adjuvant or neoadjuvant chemotherapy and up to one prior chemotherapy regimen for stage IV breast cancer or prior adjuvant trastuzumab and, after protocol amendment, one line of prior chemotherapy with trastuzumab for metastatic cancer. Bisphosphonate therapy initiated before study entry was permitted. Prior therapy with fulvestrant, lapatinib, or EGFR inhibitors such as erlotinib, cetuximab, and gefitinib was prohibited, as was therapeutic anticoagulation. Patients were required to have Eastern Cooperative Oncology Group performance status 0 to 2, without visceral crisis, and baseline absolute neutrophil count ≥ 1000/μL, platelet count ≥ 100,000/μL, creatinine ≤ 2 mg/dL, bilirubin ≤ 2× upper limit of normal, AST and ALT ≤ 3× upper limit of normal (≤ 5× in patients with liver metastases), international normalized ratio ≤ 1.6, and baseline left ventricular ejection fraction (LVEF) within institutional limit of normal. Each participant signed an institutional review board–approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Treatments and Dose Modifications
Patients were randomly assigned at a ratio of 1:1 in double-blind fashion to fulvestrant plus lapatinib or fulvestrant plus placebo, stratified on prior tamoxifen exposure and bone-only metastatic disease. On the basis of pharmacokinetic data emerging at the time of study accrual,23,24 fulvestrant was administered with a loading dose on the following schedule: day 1, 500 mg intramuscularly (IM); days 15 and 28 and every 28 days thereafter, 250 mg IM, without dose modifications. In unrelated clinical trials, this schedule was subsequently found to be superior to monthly dosing at 250 mg IM and equivalent to dosing at 500 mg IM.23,24 Lapatinib was administered orally at 1,500 mg daily. Patients with grade 2 diarrhea or rash resulting from lapatinib had treatment held until resolution of symptoms to at least grade 1 before resuming therapy; those with grade 3 diarrhea or rash could resume lapatinib after similar resolution of symptoms but with dose reduced to 1,000 mg per day. LVEF was redetermined at week 16. Patients free of cardiac symptoms and with LVEF > 50% at 16 weeks required no further cardiac surveillance; those with LVEF < 50% and/or with heart failure symptoms were reassessed every 8 weeks. Patients with heart failure symptoms were removed from study treatment. Patients with grade 1 pneumonitis thought to be the result of lapatinib had treatment held until resolution of symptoms; they could then resume lapatinib at 1,000 mg per day. Patients with pneumonitis grade ≥ 2 were removed from study treatment. Patients were treated until first disease progression regardless of site or until undue therapy-related toxicity. Restaging occurred every 2 months.
Study Design
The primary efficacy end point was progression-free survival (PFS), measured as the interval from study entry until first disease progression or death resulting from any cause, whichever occurred first. Event-free patients were censored at the date of last clinical assessment. Secondary end points were toxicity, objective tumor response, and overall survival (OS). Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria and reported for toxicities that were considered possibly, probably, or definitely treatment related. Objective tumor response was defined as either complete response (CR) or partial response according to RECIST criteria (version 1.0)25 among those with measurable tumors assessable for response. OS was measured as the interval from study entry until death resulting from any cause or last contact.
Superiority of the experimental arm (fulvestrant plus lapatinib) over the control arm (fulvestrant plus placebo) was evaluated using a stratified log-rank test with a one-sided alpha of 0.025. A target enrollment of 324 patients and an anticipated final analysis at 303 events gave 90% power to detect a 50% improvement in median PFS from 5 months in the control arm to 7.5 months in the experimental arm. The study was monitored biannually by a data and safety monitoring board (DSMB) in accordance with National Cancer Institute guidelines. Interim analyses for efficacy were preplanned to start when 65 events (22%) were recorded and allowed early stopping for futility at a one-sided alpha of 0.00526 or superiority using O'Brien-Fleming boundaries.27
Analysis
Efficacy analyses used a modified intent-to-treat approach that included all patients who began protocol therapy. Time-to-event distributions were estimated by the Kaplan-Meier method. The primary analysis of PFS and secondary analysis of OS each used a log-rank test stratified by prior tamoxifen therapy and bone-only disease; hazard ratios (HRs) of placebo to lapatinib and 95% CIs were taken from the corresponding Cox proportional hazards model. Secondary analyses used Cox models to test an interaction between arm and HER2 status on PFS, adjusting for length of disease-free interval (< 2 v ≥ 2 years) and prior chemotherapy for metastatic breast cancer (yes v no) and to evaluate OS under the same models. Logistic regression was used to test main effects and an interaction between arm and HER2 status on response, adjusting for disease-free interval and prior chemotherapy. All other proportions were compared with Fisher's exact tests; CIs around proportions used exact binomial methods. Secondary analyses of PFS and OS are reported with one-sided P values against an alpha of 0.025, whereas other end points were evaluated in two-sided tests with an alpha of 0.05.
Study data were collected and reviewed by the Alliance study data coordinator. Data quality was ensured by review of data by the study chairperson, following group policies. Analyses were performed by Alliance statisticians using SAS software (version 9.2; SAS Institute, Cary, NC). Analyses are based on data available in the CALGB database as of February 14, 2013.
RESULTS
Patient Accrual
The study was activated in September 2006. The first and second interim analyses were reported to the DSMB in June and November 2009 with 74 and 123 events, respectively. At a third planned interim analysis in June 2010 based on 173 PFS events, the observed HR was 0.98 (95% CI, 0.73 to 1.33) and crossed the futility boundary such that the predicted probability of concluding that lapatinib was superior to placebo with continued accrual and follow-up was < 1%. Per the DSMB recommendation, the trial was permanently closed to new accrual on July 14, 2010, with a total of 295 patients, and treatment was unblinded to patients and physicians.
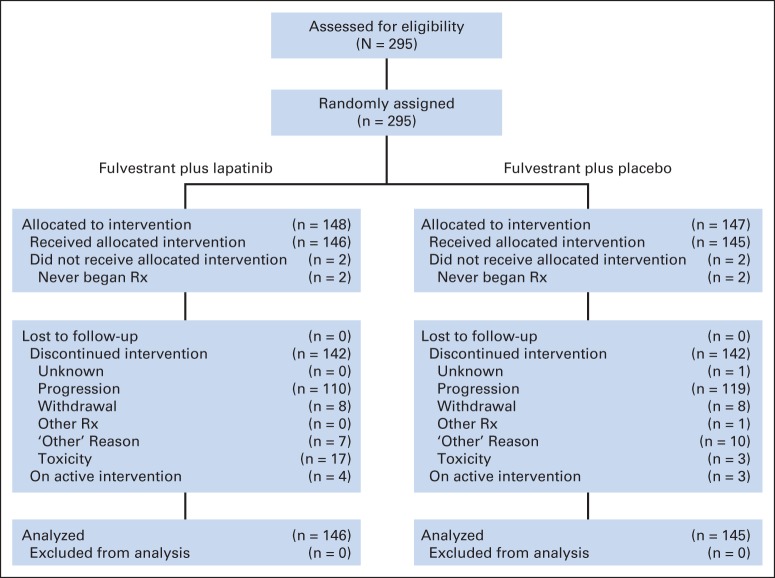
Of the 295 patients enrolled, four patients never began protocol therapy and were excluded from all analyses. The CONSORT diagram (Fig 1) summarizes patient status. At the time of the final analysis, 191 patients had died, 94 had discontinued treatment, and six continued on protocol therapy. Median follow-up for surviving patients was 2.8 years, with a maximum of 5 years.
Fig 1.
CONSORT diagram. Rx, treatment.
Patient demographics and clinicopathologic tumoral characteristics were typical of women with hormone receptor–positive advanced breast cancer being considered for ongoing endocrine therapy and were well balanced between the two treatment arms (Table 1). Nearly all tumors (98%) were ER positive; 70% were progesterone receptor positive; 18% were HER2 positive. More than half of participants (57%) had received prior tamoxifen.
Table 1.
Patient Demographic and Tumor Characteristics
| Characteristic | Lapatinib Arm (n = 146) |
Placebo Arm (n = 145) |
Total (N = 291) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| < 40 | 8 | 5 | 7 | 5 | 15 | 5 |
| 40-49 | 21 | 14 | 17 | 12 | 38 | 13 |
| 50-59 | 49 | 34 | 54 | 37 | 103 | 35 |
| 60-69 | 41 | 28 | 47 | 32 | 88 | 30 |
| ≥ 70 | 27 | 18 | 20 | 14 | 47 | 16 |
| Sex | ||||||
| Female | 146 | 100 | 145 | 100 | 291 | 100 |
| Race | ||||||
| White | 127 | 87 | 132 | 91 | 259 | 89 |
| Black | 11 | 8 | 8 | 6 | 19 | 7 |
| Asian | 4 | 3 | 2 | 1 | 6 | 2 |
| Native American | 1 | 1 | 0 | 0 | 1 | 0 |
| Multiracial | 0 | 0 | 2 | 1 | 2 | 1 |
| Unknown | 3 | 2 | 1 | 1 | 4 | 1 |
| Ethnicity | ||||||
| Hispanic | 7 | 5 | 7 | 5 | 14 | 5 |
| Non-Hispanic | 130 | 89 | 129 | 89 | 259 | 89 |
| Unknown | 9 | 6 | 9 | 6 | 18 | 6 |
| Prior tamoxifen | ||||||
| No | 63 | 43 | 63 | 43 | 126 | 43 |
| Yes | 83 | 57 | 82 | 57 | 165 | 57 |
| Bone disease only | ||||||
| No | 101 | 69 | 102 | 70 | 203 | 70 |
| Yes | 45 | 31 | 43 | 30 | 88 | 30 |
| Performance status | ||||||
| 0 | 86 | 59 | 104 | 72 | 190 | 65 |
| 1 | 57 | 39 | 39 | 27 | 96 | 33 |
| 2 | 3 | 2 | 2 | 1 | 5 | 2 |
| Prior AI therapy | ||||||
| No | 5 | 3 | 3 | 2 | 8 | 3 |
| Yes | 141 | 97 | 140 | 97 | 281 | 97 |
| Unknown | 0 | 0 | 2 | 1 | 2 | < 1 |
| Prior trastuzumab therapy | ||||||
| No | 99 | 68 | 110 | 76 | 209 | 72 |
| Yes | 3 | 2 | 4 | 3 | 7 | 2 |
| Unknown | 44 | 30 | 31 | 21 | 75 | 26 |
| Tumor ER status | ||||||
| Negative | 1 | 1 | 3 | 2 | 4 | 1 |
| Positive | 145 | 99 | 140 | 97 | 285 | 98 |
| Unknown | 0 | 0 | 2 | 1 | 2 | 1 |
| Tumor PgR status | ||||||
| Negative | 38 | 26 | 40 | 28 | 78 | 27 |
| Positive | 106 | 73 | 99 | 68 | 205 | 70 |
| Unknown | 2 | 1 | 6 | 4 | 6 | 2 |
| Tumor HER2 status | ||||||
| Negative (IHC 0, 1+, or 2+ and/or FISH negative) | 122 | 84 | 113 | 78 | 235 | 81 |
| Positive (IHC 3+ and/or FISH positive) | 24 | 16 | 30 | 21 | 54 | 18 |
| Unknown | 0 | 0 | 2 | 1 | 2 | 1 |
| No. of metastatic sites at study entry | ||||||
| 1 | 54 | 37 | 62 | 43 | 116 | 40 |
| 2 | 49 | 34 | 50 | 34 | 99 | 34 |
| 3 | 295 | 20 | 21 | 14 | 50 | 17 |
| 4 or 5 | 14 | 10 | 12 | 8 | 26 | 9 |
| Prior chemotherapy for metastatic breast cancer | ||||||
| No | 120 | 82 | 119 | 82 | 239 | 82 |
| Yes | 24 | 16 | 24 | 17 | 48 | 16 |
| Unknown | 2 | 1 | 2 | 1 | 4 | 1 |
| DFI, years | ||||||
| 0 (de novo) | 42 | 28 | 37 | 26 | 79 | 27 |
| ≤ 1 | 15 | 10 | 12 | 8 | 27 | 9 |
| > 1 to ≤ 2 | 19 | 13 | 17 | 12 | 36 | 13 |
| > 2 | 70 | 48 | 77 | 53 | 147 | 51 |
NOTE. There were no differences between arms.
Abbreviations: AI, aromatase inhibitor; DFI, disease-free interval; ER, estrogen receptor; FISH, fluorescent in situ hybridization; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; PgR, progesterone receptor.
Tolerability
Consistent with the well-described safety profiles for fulvestrant and lapatinib, most patients tolerated treatment reasonably well (Table 2). No grade 4 or 5 adverse events were reported. More patients receiving lapatinib experienced grade 3 adverse events (19% v 5%; P < .001), most commonly acneiform rash, diarrhea, fatigue, and elevations in serum transaminases. Of 285 patients who completed protocol therapy, 20 (7%) ended treatment early because of toxicity, more frequently in the lapatinib arm (12% v 2%; P = .001), resulting in diarrhea, fatigue, and rash. No grade 3 or 4 cardiac toxicity was reported.
Table 2.
AEs
| AE | Grade 3 (severe) |
Grade 4 (life threatening) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Maximum overall | ||||
| Lapatinib | 28 | 19 | 0 | 0 |
| Placebo | 8 | 6 | 0 | 0 |
| Dermatology/skin | ||||
| Rash: acne/acneiform | ||||
| Lapatinib | 4 | 3 | 0 | 0 |
| Placebo | 0 | 0 | 0 | 0 |
| Endocrine | ||||
| Hot flashes/flushes | ||||
| Lapatinib | 1 | 1 | 0 | 0 |
| Placebo | 0 | 0 | 0 | 0 |
| GI | ||||
| Diarrhea | ||||
| Lapatinib | 12 | 8 | 0 | 0 |
| Placebo | 0 | 0 | 0 | 0 |
| Mucositis/stomatitis | ||||
| Lapatinib | 1 | 1 | 0 | 0 |
| Placebo | 0 | 0 | 0 | 0 |
| GI disorders | ||||
| Dyspepsia | ||||
| Lapatinib | 1 | 1 | 0 | 0 |
| Placebo | 0 | 0 | 0 | 0 |
| General disorders and administration site conditions | ||||
| Fatigue | ||||
| Lapatinib | 4 | 3 | 0 | 0 |
| Placebo | 1 | 1 | 0 | 0 |
| Investigations | ||||
| Alanine aminotransferase increased | ||||
| Lapatinib | 2 | 1 | 0 | 0 |
| Placebo | 0 | 0 | 0 | 0 |
| Aspartate aminotransferase increased | ||||
| Lapatinib | 4 | 3 | 0 | 0 |
| Placebo | 1 | 1 | 0 | 0 |
| Blood bilirubin increased | ||||
| Lapatinib | 1 | 1 | 0 | 0 |
| Placebo | 0 | 0 | 0 | 0 |
| Neurology | ||||
| CNS cerebrovascular ischemia | ||||
| Lapatinib | 0 | 0 | 0 | 0 |
| Placebo | 1 | 1 | 0 | 0 |
| Pulmonary/upper respiratory | ||||
| Dyspnea (shortness of breath) | ||||
| Lapatinib | 2 | 1 | 0 | 0 |
| Placebo | 0 | 0 | 0 | 0 |
Abbreviation: AE, adverse event.
PFS and OS
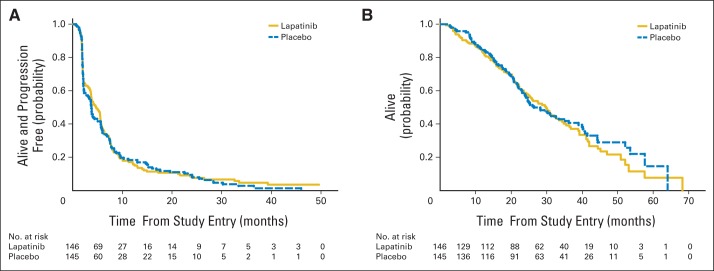
The stratified log-rank test indicated no significant treatment arm effect for either PFS or OS. The HR (placebo to lapatinib) for PFS was 1.04 (95% CI, 0.82 to 1.33; one-sided P = .37). Median PFS was 4.7 months for lapatinib plus fulvestrant and 3.8 months for placebo plus fulvestrant (Fig 2A). The HR for OS was 0.91 (95% CI, 0.68 to 1.21; one-sided P = .25). Median OS was 30 months for the lapatinib plus fulvestrant arm and 26.4 months for the placebo plus fulvestrant arm (Fig 2B).
Fig 2.
(A) Progression-free and (B) overall survival by treatment arm. (A) Hazard ratio (HR), 1.04; 95% CI, 0.82 to 1.33; P = .37. (B) HR, 0.91; 95% CI, 0.68 to 1.21; P = .25.
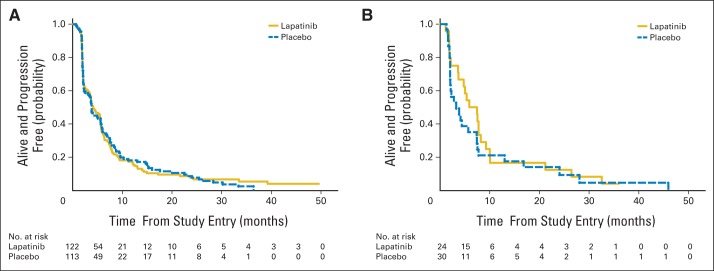
Because of the known effects of lapatinib in HER2-overexpressing breast cancers, an analysis was preplanned to determine whether there was an interaction between treatment arm and tumor expression of HER2. There was no evidence for an interaction between treatment arm and tumor HER2 status regarding PFS (P = .53); therefore, no step-down tests were conducted. For patients with HER2-negative tumors, median PFS was 4.1 months with lapatinib and 3.8 months with placebo (HR, 1.00; 95% CI, 0.76 to 1.30); for those with HER2-positive tumors, median PFS was 5.9 months with lapatinib and 3.3 months with placebo (HR, 1.23; 95% CI, 0.69 to 2.18; Figs 3A and 3B). With the small number of cases of HER2-overexpressing tumors (18%), the study had a conditional power of 0.72 for detecting a clinically relevant difference in this tumor subset.
Fig 3.
Progression-free survival by treatment arm in (A) human epidermal growth factor receptor 2–negative and (B) –positive tumors. (A) Hazard ratio (HR), 1.00; 95% CI, 0.76 to 1.30; interaction-term (A and B combined) P = .53. (B) HR, 1.23; 95% CI, 0.69 to 2.18.
Objective Tumor Response
Seventy percent (n = 203) of patients had measurable disease. Of these, 197 had appropriate documentation that allowed assessment of objective tumor response. The incidence of objective response was 20% (95% CI, 13% to 29%) in the lapatinib arm compared with 9% (95% CI, 5% to 17%) in the placebo arm (P = .048; Table 3). Overall, only four patients achieved CR; progressive disease was the best response for just under half of the patients.
Table 3.
Best Objective Response for Measurable Tumors by Arm
| Response | Lapatinib Arm (n = 101) |
Placebo Arm (n = 102) |
Total (n = 203) |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No. assessable for response | 99 | 100 | 98 | 100 | 197 | 100 |
| CR | 2 | 2 | 2 | 2 | 4 | 2 |
| PR | 18 | 18 | 7 | 7 | 25 | 13 |
| SD | 40 | 40 | 40 | 41 | 80 | 41 |
| PD | 39 | 39 | 49 | 50 | 88 | 45 |
| Objective response (CR plus PR) | 20 | 20 | 9 | 9 | 29 | 15 |
| 95% CI for objective response, % | 13 to 29 | 5 to 17 | 10 to 20 | |||
| Interaction P | .048 | |||||
Abbreviations: CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
There was no interaction between treatment arm and HER2 status for tumor response (P = .53; Table 4). The incidence of objective response for the experimental versus control arm was 13% (95% CI, 5% to 29%) versus 23% (95% CI, 12% to 41%) among patients with HER2-negative disease and 38% (95% CI, 14% to 70%) versus 17% (95% CI, 5% to 45%) for patients with HER2-positive disease, respectively.
Table 4.
Best Objective Response for Measurable Tumors by HER2 Status by Arm
| Response | HER2 Negative |
HER2 Positive |
||||||
|---|---|---|---|---|---|---|---|---|
| Lapatinib (n = 32) |
Placebo (n = 32) |
Lapatinib (n = 8) |
Placebo (n = 13) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| No. assessable for response | 31 | 100 | 30 | 100 | 8 | 100 | 12 | 100 |
| CR | 0 | 0 | 1 | 3 | 1 | 13 | 1 | 8 |
| PR | 4 | 13 | 6 | 20 | 2 | 25 | 1 | 8 |
| SD | 17 | 55 | 8 | 27 | 3 | 38 | 3 | 25 |
| PD | 10 | 32 | 15 | 50 | 2 | 25 | 7 | 58 |
| Objective response (CR plus PR) | 4 | 13 | 7 | 23 | 3 | 38 | 2 | 17 |
| 95% CI for objective response, % | 5 to 29 | 12 to 41 | 14 to 70 | 5 to 45 | ||||
| Interaction P | .53 | |||||||
Abbreviations: CR, complete response; HER2, human epidermal growth factor receptor 2; PD, progressive disease; PR, partial response; SD, stable disease.
DISCUSSION
CALGB 40302 was designed to determine whether concurrent inhibition of the EGFR and HER2 pathways using the dual kinase inhibitor lapatinib, along with antiestrogen treatment with fulvestrant, would improve clinical outcomes for women with ER-positive metastatic breast cancer. Preclinical models had suggested important interactions between these respective growth factor pathways, implying that substantial effects might be seen by simultaneously targeting these pathways. However, in the clinical trial, no evidence for such clinical benefit was observed; patients in each arm had similar times to progression, OS, and tumor response rates regardless of lapatinib exposure. Although generally tolerable, treatment with lapatinib plus fulvestrant was associated with more adverse effects and discontinuation of treatment for toxicity. The aggregate outcomes for fulvestrant treatment in this study (ie, median PFS of approximately 3 to 4 months and response rate of 9% to 16%) are consistent with reports in the literature2,3,4 for women who, like those in our trial, were offered fulvestrant monotherapy after previous treatment with AIs. Planned subset analyses showed no evidence of substantial clinical benefit regardless of tumor HER2 status.
Other clinical trials have explored the possible importance of targeting multiple growth factor pathways as a means for overcoming resistance to endocrine therapy. A randomized trial of the AI anastrozole, administered with or without the EGFR inhibitor gefitinib, suggested that the EGFR blockade concurrent with estrogen deprivation improved PFS.28 This finding was most notable among patients with endocrine-naive tumors, whereas subset studies did not suggest a differential outcome as a function of tumor EGFR or HER2 status. A first-line study of metastatic breast cancer that compared letrozole against letrozole plus lapatinib29 demonstrated improvement in PFS and response rates among patients with ER-positive, HER2-positive tumors, but no benefit was shown among patients with ER-positive, HER2-negative cancers.
It is unclear what may account for the lack of improvement in clinical outcomes with use of the dual kinase inhibitor lapatinib in CALGB 40302. It is possible that any benefit for this approach is narrowly confined to patients with HER2-overexpressing tumors, of which there were few in this trial. To date, treatment of metastatic breast cancer with anti-HER2 agents has been beneficial only when tumors have unequivocal overexpression of HER2. Previous studies have suggested that in ER-positive, HER2-positive breast cancer, the addition of anti-HER2 therapy can modestly improve time to tumor progression beyond that seen with endocrine therapy alone.29,30 Whether this is because of true synergy between these approaches or whether it is simply the result of single-agent activity of the anti-HER2 agent is not clear. The positive findings in the study of letrozole with or without lapatinib29 or in the trial of anastrozole with or without trastuzumab30 were built on more than 200 patients with HER2-positive tumors in each trial. By contrast, CALGB 40302 included only 54 patients with HER2-positive breast cancer. Although our study was not specifically designed for subgroup comparisons, there was nonetheless 72% power to detect treatment differences within the HER2-positive subgroup. Two other important clinical differences—the endocrine agent itself and the extent of prior endocrine therapy—distinguish the populations in CALGB 40302 from those in the other trials of endocrine therapy with or without anti-HER2 agents.
Various models that use multipathway targeting to overcome resistance to endocrine therapies have suggested that the EGFR and HER2 signaling pathways contribute to resistance to antiestrogen treatments. However, those models were built on cell lines with heavy in vitro dependence on exogenous growth factor support and thus may have over-represented the importance of targeting multiple pathways at once. It is also likely that factors associated with clinical resistance to endocrine treatment—the overt worsening of disease despite prior endocrine therapy—differ from the mechanisms observed for in vitro models. Finally, the clinical significance of the dual activity of lapatinib, targeting both EGFR and HER2, is unclear in HER2-negative tumors. Exploratory biomarker studies arising from CALGB 40302 may help delineate which subsets of patients with ER-positive breast cancer might specifically benefit from targeting multiple growth factor pathways at once.
Since the initiation of CALGB 40302, several novel drugs have emerged that are being used in combination with antiestrogen treatments in advanced breast cancer, such as the mammalian target of rapamycin inhibitor everolimus, which extended PFS among patients with tumor progression after treatment with AIs.31 Preliminary data suggest that CDK4/6 inhibitors may add to the efficacy of first-line endocrine therapy.32 These findings underscore both the importance of studies designed in a similar fashion to CALGB 40302 as well as the lack of clinical benefit seen with lapatinib in this setting.
In conclusion, CALGB 40302 demonstrated no evidence that combining the antiestrogen fulvestrant with the dual EGFR/HER2 kinase inhibitor lapatinib leads to clinically significant improvement in outcomes for women with ER-positive metastatic breast. Given the importance of multiple lines of endocrine therapy in the palliation of advanced breast cancer and the opportunities for improving outcomes in the adjuvant setting, other approaches to overcome clinical endocrine resistance are needed.
Acknowledgment
Supported in part by National Cancer Institute Grants No. CA31946 to the Alliance for Clinical Trials in Oncology, No. CA33601 to the Alliance Statistics and Data Center, and No. CA32102 and CA46441 to the Southwest Oncology Group.
Glossary Terms
- epidermal growth factor receptor (EGFR):
a member of a family of receptors (HER2, HER3, HER4 are other members of the family) that binds to the EGF, TGF-α, and other related proteins, leading to the generation of proliferative and survival signals within the cell. EGFR (also known as HER1) also belongs to the larger family of tyrosine kinase receptors and is generally overexpressed in several solid tumors of epithelial origin.
- estrogen receptor (ER):
ligand-activated nuclear proteins, belonging to the class of nuclear receptors, present in many breast cancer cells that are important in the progression of hormone-dependent cancers. After binding, the receptor-ligand complex activates gene transcription. There are two types of estrogen receptors (ERα and ERβ). ERα is one of the most important proteins controlling breast cancer function. ERβ is present in much lower levels in breast cancer, and its function is uncertain. Estrogen receptor status guides therapeutic decisions in breast cancer.
- HER2/neu (human epidermal growth factor receptor 2):
also called ErbB2. HER2/neu belongs to the epidermal growth factor receptor (EGFR) family and is overexpressed in several solid tumors. Like EGFR, it is a tyrosine kinase receptor whose activation leads to proliferative signals within the cells. On activation, the human epidermal growth factor family of receptors are known to form homodimers and heterodimers, each with a distinct signaling activity. Because HER2 is the preferred dimerization partner when heterodimers are formed, it is important for signaling through ligands specific for any members of the family. It is typically overexpressed in several epithelial tumors.
- lapatinib:
a dual tyrosine kinase inhibitor. Lapatinib has been developed as an inhibitor of the tyrosine kinase activities of ErbB1 (EGFR) and ErbB2. Like other tyrosine kinase inhibitors, it competes with ATP binding to the intracellular regions of the receptors that are activated after tyrosine phosphorylation.
Appendix
The following institutions participated in this study: Bay Area Tumor Institute Community Clinical Oncology Program (CCOP), Oakland, CA, Jon M. Grief, MD; Christiana Care Health Services CCOP, Wilmington, DE, Stephen Grubbs, MD (supported by National Cancer Institute [NCI] Grant No. CA45418); Dana-Farber Cancer Institute, Boston, MA, Harold J. Burstein, MD, PhD (supported by NCI Grant No. CA32291); Duke University Medical Center, Durham, NC, Jeffrey Crawford, MD (supported by NCI Grant No. CA47577); Cancer Centers of the Carolinas, Greenville, SC, Jeffrey K. Giguere, MD (supported by NCI Grant No. CA29165); Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY, Jeffrey Kirshner, MD (supported by NCI Grant No. CA45389); Illinois Oncology Research Association, Peoria, IL, John W. Kugler, MD (supported by NCI Grant No. CA35113); Georgetown University Medical Center, Washington, DC, Bruce Cheson, MD (supported by NCI Grant No. CA77597); Mount Sinai Medical Center, Miami, FL, Michael A. Schwartz, MD (supported by NCI Grant No. CA45564); New Hampshire Oncology-Hematology, Concord, NH, Douglas J. Weckstein, MD; Northern Indiana Cancer Research Consortium CCOP, South Bend, IN, Rafat Ansari, MD (supported by NCI Grant No. CA86726); Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, MD (supported by NCI Grant No. CA59518); Sibley Memorial Hospital, Washington, DC, Frederick Barr, MD; Southeast Cancer Control Consortium CCOP, Goldsboro, NC, James N. Atkins, MD (supported by NCI Grant No. CA45808); State University of New York Upstate Medical University, Syracuse, NY, Stephen L. Graziano, MD (supported by NCI Grant No. CA21060); University of California at San Diego, San Diego, CA, Barbara A. Parker, MD (supported by NCI Grant No. CA11789); University of Chicago, Chicago, IL, Hedy L. Kindler, MD (supported by NCI Grant No. CA41287); University of Illinois Minority-Based CCOP, Chicago, IL, David J. Peace, MD (supported by NCI Grant No. CA74811); University of Iowa, Iowa City, IA, Daniel A. Vaena, MD (supported by NCI Grant No. CA47642); University of Maryland Greenebaum Cancer Center, Baltimore, MD, Martin Edelman, MD (supported by NCI Grant No. CA31983); University of Minnesota, Minneapolis, MN, Bruce A. Peterson, MD (supported by NCI Grant No. CA16450); University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, MD (supported by NCI Grant No. CA47559); University of Vermont, Burlington, VT, Steven M. Grunberg, MD (supported by NCI Grant No. CA77406); Wake Forest University School of Medicine, Winston-Salem, NC, David D. Hurd, MD (supported by NCI Grant No. CA03927); Washington University School of Medicine, St Louis, MO, Nancy Bartlett, MD (supported by NCI Grant No. CA77440); and Yale University, New Haven, CT, Lyndsay N. Harris, MD (supported by NCI Grant No. CA16359).
Footnotes
Clinical trial information: NCT00390455.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Harold J. Burstein, Constance T. Cirrincione, William T. Barry, Helen K. Chew, Kimberly L. Blackwell, Eric P. Winer, Clifford A. Hudis
Provision of study materials or patients: Harold J. Burstein, Helen K. Chew, Sara M. Tolaney, Diana E. Lake, Cynthia Ma, Kimberly L. Blackwell, Eric P. Winer, Clifford A. Hudis
Collection and assembly of data: Harold J. Burstein, Constance T. Cirrincione, William T. Barry, Sara M. Tolaney, Eric P. Winer, Clifford A. Hudis
Data analysis and interpretation: Harold J. Burstein, Constance T. Cirrincione, William T. Barry, Sara M. Tolaney, Diana E. Lake, Cynthia Ma, Kimberly L. Blackwell, Eric P. Winer, Clifford A. Hudis
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Endocrine Therapy With or Without Inhibition of Epidermal Growth Factor Receptor and Human Epidermal Growth Factor Receptor 2: A Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Fulvestrant With or Without Lapatinib for Postmenopausal Women With Hormone Receptor-Positive Advanced Breast Cancer—CALGB 40302 (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Harold J. Burstein
No relationship to disclose
Constance T. Cirrincione
No relationship to disclose
William T. Barry
No relationship to disclose
Helen K. Chew
No relationship to disclose
Sara M. Tolaney
Research Funding: Genentech
Diana E. Lake
Consulting or Advisory Role: Genentech
Honoraria: Genentech
Cynthia Ma
Consulting or Advisory Role: Novartis
Research Funding: Novartis (Inst), Puma (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Novartis
Kimberly L. Blackwell
Consulting or Advisory Role: Celgene, Novartis, Genentech/Roche
Research Funding: Celgene, Genentech, Pfizer, Novartis
Eric P. Winer
Consulting or Advisory Role: Genentech/Roche
Research Funding: Genentech/Roche (Inst)
Travel, Accommodations, Expenses: Genentech/Roche, Novartis
Clifford A. Hudis
Other Relationship: Breast Cancer Research Foundation
REFERENCES
- 1.Schiavon G, Smith IE. Endocrine therapy for advanced/metastatic breast cancer. Hematol Oncol Clin North Am. 2013;27:715–736. doi: 10.1016/j.hoc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Perey L, Paridaens R, Hawle H, et al. Clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer and primary or acquired resistance to aromatase inhibitors: Final results of phase II Swiss Group for Clinical Cancer Res Trial (SAKK 21/00) Ann Oncol. 2007;18:64–69. doi: 10.1093/annonc/mdl341. [DOI] [PubMed] [Google Scholar]
- 3.Ingle JN, Suman VJ, Rowland KM, et al. Fulvestrant in women with advanced breast cancer after progression on prior aromatase inhibitor therapy: North Central Cancer Treatment Group Trial N0032. J Clin Oncol. 2006;24:1052–1056. doi: 10.1200/JCO.2005.04.1053. [DOI] [PubMed] [Google Scholar]
- 4.Chia S, Gradishar WJ, Mauriac L, et al. Double-blind, randomized placebo-controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor–positive advanced breast cancer: Results from EFECT. J Clin Oncol. 2008;26:1664–1670. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 5.Robertson JF, Osborne CK, Howell A, et al. Fulvestrant versus anastrozole for the treatment of advanced breast carcinoma in postmenopausal women: A prospective combined analysis. Cancer. 2003;98:229–238. doi: 10.1002/cncr.11468. [DOI] [PubMed] [Google Scholar]
- 6.Roberston JF, Llombart-Cussac A, Rolski J, et al. Activity of fulvestrant 500 mg versus anastrozole 1 mg as first-line treatment for advanced breast cancer: Results from the FIRST study. J Clin Oncol. 2009;27:4530–4535. doi: 10.1200/JCO.2008.21.1136. [DOI] [PubMed] [Google Scholar]
- 7.Johnson SR. New strategies in estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:1979–1987. doi: 10.1158/1078-0432.CCR-09-1823. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez MC, Detre S, Johnston S, et al. Molecular changes in tamoxifen-resistant breast cancer: Relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23:2469–2476. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 9.Osborne CK, Shou J, Massarweh S, et al. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res. 2005;11:865s–870s. [PubMed] [Google Scholar]
- 10.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: Increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 11.Knowlden JM, Hutcheson IR, Jones HE, et al. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology. 2003;144:1032–1044. doi: 10.1210/en.2002-220620. [DOI] [PubMed] [Google Scholar]
- 12.Martin LA, Farmer I, Johnston SR, et al. Enhanced estrogen receptor (ER) α, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem. 2003;278:30458–30468. doi: 10.1074/jbc.M305226200. [DOI] [PubMed] [Google Scholar]
- 13.Leary AF, Drury S, Detre S, et al. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin Cancer Res. 2010;16:1486–1497. doi: 10.1158/1078-0432.CCR-09-1764. [DOI] [PubMed] [Google Scholar]
- 14.Chu I, Blackwell K, Chen S, et al. The dual ErbB1/ErbB2 inhibitor, lapatinib (GW572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 2005;65:18–25. [PubMed] [Google Scholar]
- 15.Bayliss J, Hilger A, Vishnu P, et al. Reversal of the estrogen receptor negative phenotype in breast cancer and restoration of antiestrogen response. Clin Cancer Res. 2007;13:7029–7036. doi: 10.1158/1078-0432.CCR-07-0587. [DOI] [PubMed] [Google Scholar]
- 16.Sabnis G, Schayowitz A, Goloubeva O, et al. Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res. 2009;69:1416–1428. doi: 10.1158/0008-5472.CAN-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rusnak DW, Affleck K, Cockerill SG, et al. The characterization of novel, dual ErbB-2/EGFR, tyrosine kinase inhibitors: Potential therapy for cancer. Cancer Res. 2001;61:7196–7203. [PubMed] [Google Scholar]
- 18.Spector NL, Xia W, Burris H, 3rd, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005;23:2502–2512. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 19.Burris HA, 3rd, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 20.Blackwell KL, Pegram MD, Tan-Chiu E, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20:1026–1031. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 21.Burstein HJ, Storniolo AM, Franco S, et al. A phase II study of lapatinib monotherapy in chemotherapy refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol. 2008;19:1068–1074. doi: 10.1093/annonc/mdm601. [DOI] [PubMed] [Google Scholar]
- 22.Gomez HL, Doval DC, Chavez MA, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 23.McCormack P, Sapunar F. Pharmacokinetic profile of the fulvestrant loading dose regimen in postmenopausal women with hormone receptor-positive advanced breast cancer. Clin Breast Cancer. 2008;8:347–351. doi: 10.3816/CBC.2008.n.040. [DOI] [PubMed] [Google Scholar]
- 24.Pritchard KI, Rolski J, Papai Z, et al. Results of a phase II study comparing three dosing regimens of fulvestrant in postmenopausal women with advanced breast cancer (FINDER2) Breast Cancer Res Treat. 2010;123:453–461. doi: 10.1007/s10549-010-1022-9. [DOI] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Freidlin B, Korn EL. A comment on futility monitoring. Control Clin Trials. 2002;23:355–366. doi: 10.1016/s0197-2456(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 28.Cristofanilli M, Valero V, Mangalik A, et al. Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010;16:1904–1914. doi: 10.1158/1078-0432.CCR-09-2282. [DOI] [PubMed] [Google Scholar]
- 29.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor–positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 30.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2–positive, hormone receptor–positive metastatic breast cancer: Results from the randomized phase III TAnDEM study. J Clin Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 31.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finn RS, Crown JP, Lang I, et al. Final results of a randomized phase II study of PD 0332991, a cyclin-dependent kinase (CDK)-4/6 inhibitor, in combination with letrozole vs letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (PALOMA-1; TRIO-18). Presented at the 2014 Annual Meeting of the American Association of Cancer Research; April 5-9, 2014; San Diego, CA. (abstr CT101) [Google Scholar]