Abstract
Purpose
To determine whether the addition of advanced-practice nurse (APN) telephone counseling to a printed survivorship care plan (SCP) significantly increases the proportion of at-risk survivors who complete cardiomyopathy screening.
Patients and Methods
Survivors age ≥ 25 years participating in the Childhood Cancer Survivor Study who received cardiotoxic therapy and reported no history of cardiomyopathy screening in the previous 5 years were eligible for enrollment. The 472 participants (mean age, 40.1 years; range, 25.0 to 59.0; 53.3% women) were randomly assigned to either standard care, consisting of an SCP summarizing cancer treatment and cardiac health screening recommendations (n = 234), or standard care plus two APN telephone counseling sessions (n = 238). The primary outcome—completion of cardiomyopathy screening within 1 year—was validated by medical records and compared between the two arms using adjusted relative risks (RRs) with 95% CIs.
Results
Participants in the standard and APN counseling groups were not statistically different by demographic or clinical characteristics. At the time of 1-year follow-up, 107 (52.2%) of 205 survivors in the APN group completed screening compared with 46 (22.3%) of 206 survivors in the non-APN group (P < .001). With adjustment for sex, age (< 30 v ≥ 30 years), and Children's Oncology Group–recommended screening frequency group (annual, 2 years, or 5 years), survivors in the APN group were > 2× more likely than those in the control group to complete the recommended cardiomyopathy screening (RR, 2.31; 95% CI, 1.74 to 3.07).
Conclusion
The addition of telephone counseling to an SCP with cardiac health screening recommendations increases cardiomyopathy screening in at-risk survivors.
INTRODUCTION
Adults treated with anthracycline chemotherapy and/or chest irradiation for pediatric malignancies are at increased risk for a spectrum of cardiovascular diseases including cardiomyopathy, valve dysfunction, atherosclerotic vascular disease, and dysrhythmia.1 Among the common cancer-related toxicities, cardiomyopathy has been studied the most extensively in pediatric cancer survivors. Anthracycline chemotherapy and chest-directed radiation therapy involving cardiac structures predispose to cardiomyopathy in a dose-related fashion.2–4 An estimated 5% of anthracycline-exposed survivors develop heart failure within 15 years after treatment, while still relatively young.5 The incidence of heart failure approaches 10% among those treated with higher cumulative anthracycline doses in the range of 250 to 600 mg/m2 and exceeds 30% for doses > 600 mg/m2.2–4 Chest-directed radiation therapy, especially at doses exceeding 35 Gy or at lower doses to treatment fields involving large volumes of the heart, is also associated with an increased risk of cardiomyopathy.6,7 As is typical for many pediatric malignancies, combination therapy with an anthracycline and chest-directed radiation therapy results in a higher risk of adverse cardiac outcomes compared with that observed after treatment with single cardiotoxic modality.1 Importantly, survivors have a 15-fold increased risk of developing heart failure8 and seven-fold higher risk of premature cardiovascular death9 compared with population controls.
Cardiomyopathy exhibits a progressive course with a variable period of asymptomatic cardiac dysfunction that results in heart failure in a significant minority.2,4,7,10,11 In as many as 57% of survivors, cardiac injury remains asymptomatic until exacerbated by physiologic stressors such as infection, pregnancy, or organ dysfunction associated with common comorbid health conditions.12,13 With contemporary treatment approaches that limit exposure to cardiotoxic antineoplastic modalities, cardiomyopathy and heart failure typically manifest most often during adulthood, long after the survivor has been discharged from pediatric cancer care.2,3,7,12 Because most childhood cancer survivors at risk for cardiomyopathy are asymptomatic, and the latency to clinically symptomatic cardiac dysfunction is delayed after exposure, proactive surveillance provides opportunities for early detection and intervention that may preserve cardiovascular function. All current pediatric oncology survivorship guidelines recommend baseline and periodic cardiac imaging, typically echocardiography, to monitor left ventricular systolic function of at-risk survivors treated with cardiotoxic modalities.14–17 Despite these recommendations, adherence to cardiomyopathy screening remains suboptimal. In the Childhood Cancer Survivor Study (CCSS), only 511 (28.2%) of 1,810 childhood cancer survivors designated to be at high risk for cardiomyopathy (treatment with ≥ 300 mg/m2 of anthracycline or any anthracycline dose plus chest irradiation) reported undergoing screening during the previous 24 months.18 We conducted a randomized controlled trial—Evaluation of Cardiovascular Health Outcomes Among Survivors (ECHOS)—to determine whether the addition of tailored telephone counseling delivered by advanced-practice nurses (APNs) to the receipt of a mailed personalized survivorship care plan (SCP) would increase the proportion of at-risk survivors who completed cardiomyopathy screening.
PATIENTS AND METHODS
Study Design and Participants
Participants were recruited for this institutional review board–approved study from the CCSS, a 26-institution retrospective cohort study currently observing > 12,000 long-term survivors of childhood cancer diagnosed between 1970 and 1986. Since enrollment in 1994 to 1998, participants have been surveyed periodically to track important health outcomes, health care use patterns, and health behaviors and practices (Fig 1). The CCSS cohort methodology and study design have been previously described in detail.9,19,20
Fig 1.
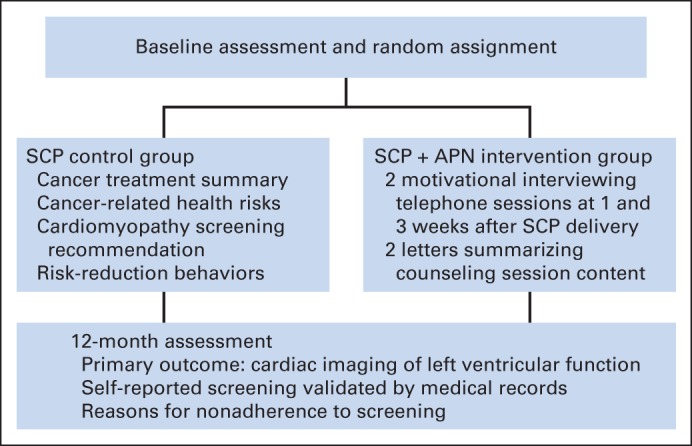
Evaluation of Cardiovascular Health Outcomes Among Survivors study design. APN, advanced-practice nurse; SCP, survivorship care plan.
Survivors were eligible to participate in ECHOS if they were age ≥ 25 years, had received anthracyclines and/or chest-directed radiation therapy involving cardiac structures, had received no cardiomyopathy screening during the past 5 years, were not actively participating in a long-term follow-up program that provided risk-based health screening, and had a history of providing direct (nonsurrogate) responses to CCSS surveys. In addition, for logistical reasons, survivors living outside North America and those without telephone access were excluded from participation. Participants were categorized by Children's Oncology Group (COG) cardiomyopathy risk group as high, intermediate, or low risk, for whom the frequency of cardiomyopathy screening is recommended every year, 2 years, and 5 years, respectively.17
Randomization and Study Interventions
After receipt of informed consent, participants were assigned to study arms using a computerized, randomly permuted block method; they were stratified by age (< 30 v ≥ 30 years), sex, and COG-recommended screening frequency group (1, 2, or 5 years). After a baseline assessment, members of the standard-care group were mailed a personalized SCP outlining their specific cancer treatments and health risks and providing tailored recommendations for cardiomyopathy screening from the COG guidelines (version 3.0).17 The packet also included a laminated card summarizing treatment exposures, future health risks, and recommendations for follow-up that could be given to a primary care provider. After baseline assessment, survivors in the APN intervention arm were mailed the same personalized SCP and laminated card as described for participants in the standard-care arm. These survivors also received two telephone counseling sessions from an APN 1 and 3 weeks after receiving the individualized SCP. After each call, the survivor was sent a follow-up letter summarizing the conversation. The counseling sessions were tailored to address individual barriers to completion of cardiomyopathy screening. Factors addressed in tailoring of APN counseling to overcome barriers to screening included health knowledge deficits (eg, cancer treatment history, cardiomyopathy risk associated with cancer treatment, health screening tests recommended for cardiomyopathy, benefits of early detection of cardiomyopathy), health perceptions (eg, risk of cardiomyopathy to future health, importance of cardiomyopathy screening based on cancer treatment, fear/anxiety related to undergoing cardiomyopathy screening, fear/anxiety about what screening tests will show), and health care access (eg, insurance access, insurance coverage of screening, identification of primary care practitioner, communication with primary care practitioner and insurance company, identification of screening facilities).
Assessment of Study Outcomes
One year after completion of the intervention (ie, receipt of personalized SCP for standard-care group and last APN telephone call for intervention group), a follow-up questionnaire was distributed to assess self-reported adherence to cardiomyopathy screening and reasons for nonadherence. Among those self-reporting having undergone cardiomyopathy screening, medical records were requested to validate screening participation and results.
Statistical Analysis
t, χ2, and Fisher's exact tests were used to compare categorical and continuous characteristics in the two groups at baseline. The proportions of survivors completing cardiomyopathy screening within 1 year of intervention were compared between the groups using relative risks (RRs) based on a generalized linear model with a log link and Poisson working model with robust SEs. The model was adjusted for stratification factors: sex, age (< 30 v ≥ 30 years), and COG-recommended screening frequency group (1, 2, or 5 years). Additional post hoc analyses were carried out to evaluate whether any subgroups of survivors seemed to benefit more than others from the intervention. For these analyses, each of the following factors was included, along with an interaction term with the study arm, in the model just described: sex, age at study (< 30 v ≥ 30 years), household income (< $20,000 v ≥ $20,000), education (< college graduation v ≥ college graduation), race (white non-Hispanic v other), and having health insurance. All analyses were based on intent to treat, including all randomly assigned patients with end point evaluated, and were performed using SAS statistical software (version 9.3; SAS Institute, Cary, NC). The sample size of 411 survivors with 1-year follow-up available provides > 80% power to detect a two-fold difference in the primary outcome of cardiomyopathy screening, based on two-sided tests with type I error of 5% (Appendix, online only).
RESULTS
Among 1,257 survivors meeting eligibility criteria, introductory study packets were mailed to 1,256 CCSS participants living in the United States or Canada (Fig 2). After initial contact for study participation, 158 were determined to be ineligible because of history of recent cardiomyopathy screening (n = 139), death (n = 15), relocation outside of the United States or Canada (n = 2), lack of telephone or e-mail access (n = 1), and cognitive or medical condition requiring surrogate response to survey (n = 1). Among the remaining 1,098 eligible CCSS participants, only 245 survivors actively declined participation; recruitment of the remaining 344 was not pursued when accrual was met to provide sufficient statistical power for planned study analyses. In total, 509 study participants were enrolled, of whom 472 were randomly assigned to the standard-care SCP-only control or APN-plus-SCP intervention groups. After enrollment and random assignment, three additional survivors were discovered to be ineligible because of recent cardiomyopathy screening; 34 others withdrew consent for participation. Survivors randomly assigned to the standard-care SCP-only control (n = 234) and APN-plus-SCP intervention groups (n = 238) did not differ by baseline demographic or clinical characteristics (Table 1). Compared with survivors who were enrolled and randomly assigned, eligible survivors who did not participate were more likely to be men and < 5 years of age at cancer diagnosis, have lower educational attainment and household income, have received cranial irradiation, report health status as fair to poor, have a lower prevalence of grade 3 or 4 chronic health conditions, and have a shorter interval between their last survey completion and study participation (Appendix Table A1, online only).
Fig 2.
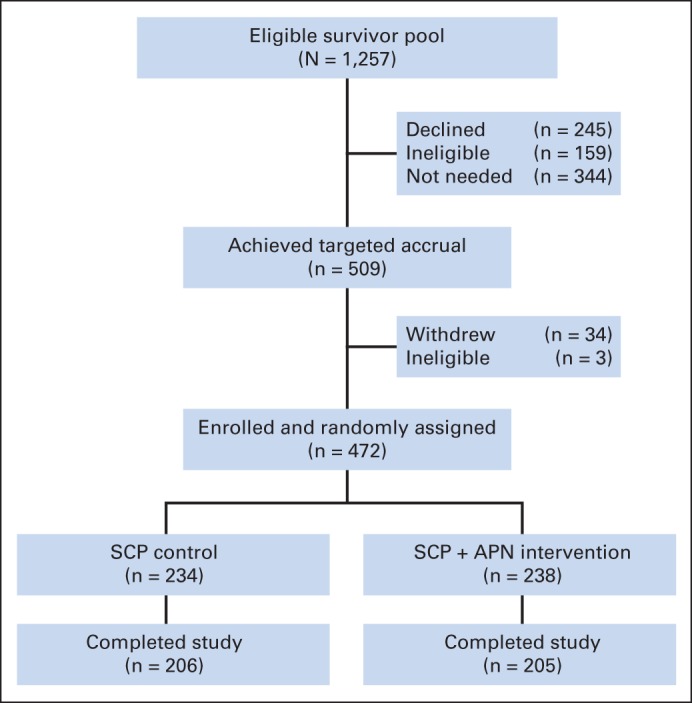
CONSORT diagram showing participant distribution in the Evaluation of Cardiovascular Health Outcomes Among Survivors study. APN, advanced-practice nurse; SCP, survivorship care plan.
Table 1.
Baseline Demographic and Clinical Characteristics of At-Risk Adult Survivors of Childhood Cancer Assigned to Standard-Care Control or APN Intervention Group
| Characteristic | SCP (n = 234) |
SCP Plus APN (n = 238) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Recommended COG screening frequency† | .40 | ||||
| Every 2 years | 51 | 21.8 | 53 | 22.3 | |
| Every 5 years | 43 | 18.4 | 33 | 13.9 | |
| Every year | 140 | 59.8 | 152 | 63.9 | |
| Sex† | .59 | ||||
| Female | 122 | 52.1 | 130 | 54.6 | |
| Male | 112 | 47.9 | 108 | 45.4 | |
| Age at random assignment, years† | .94 | ||||
| ≥ 30 | 202 | 86.3 | 206 | 86.6 | |
| < 30 | 32 | 13.7 | 32 | 13.4 | |
| Race | .89 | ||||
| White non-Hispanic | 208 | 88.9 | 210 | 88.2 | |
| Black | 3 | 1.3 | 3 | 1.3 | |
| Other | 21 | 9.0 | 25 | 10.5 | |
| Unknown | 2 | 0.9 | 0 | 0.0 | |
| Education level | .86 | ||||
| ≤ High school graduate | 25 | 10.7 | 21 | 8.8 | |
| Post–high school training/some college | 65 | 27.8 | 70 | 29.4 | |
| College graduate | 92 | 39.3 | 90 | 37.8 | |
| Postgraduate | 52 | 22.2 | 57 | 23.9 | |
| Household income | .56 | ||||
| < $20,000 | 16 | 6.8 | 20 | 8.4 | |
| $20,000 to $60,000 | 74 | 31.6 | 65 | 27.3 | |
| ≥ $60,000 | 138 | 59.0 | 144 | 60.5 | |
| Unknown | 6 | 2.6 | 9 | 3.8 | |
| Health insurance | .71 | ||||
| Yes or Canadian | 210 | 89.7 | 215 | 90.3 | |
| No | 22 | 9.4 | 20 | 8.4 | |
| Unknown | 2 | 0.9 | 3 | 1.3 | |
| Diagnosis | .08 | ||||
| Bone cancer | 39 | 16.7 | 44 | 18.5 | |
| CNS tumor | 1 | 0.4 | 0 | 0.0 | |
| Hodgkin lymphoma | 43 | 18.4 | 37 | 15.5 | |
| Kidney (Wilms) | 27 | 11.5 | 11 | 4.6 | |
| Leukemia | 77 | 32.9 | 81 | 34.0 | |
| Non-Hodgkin lymphoma | 20 | 8.5 | 28 | 11.8 | |
| Neuroblastoma | 11 | 4.7 | 10 | 4.2 | |
| Soft tissue sarcoma | 16 | 6.8 | 27 | 11.3 | |
| Age at cancer diagnosis, years | .86 | ||||
| 0-4 | 65 | 27.8 | 60 | 25.2 | |
| 5-9 | 47 | 20.1 | 52 | 21.8 | |
| 10-14 | 57 | 24.4 | 63 | 26.5 | |
| 15-20 | 65 | 27.8 | 63 | 26.5 | |
| Years since diagnosis | .37 | ||||
| ≤ 28 | 104 | 44.4 | 96 | 40.3 | |
| > 28 | 130 | 55.6 | 142 | 59.7 | |
| Years since last survey | .79 | ||||
| 1 | 8 | 3.4 | 12 | 5.0 | |
| 2 | 100 | 42.7 | 97 | 40.8 | |
| 3 | 118 | 50.4 | 119 | 50.0 | |
| 4 | 8 | 3.4 | 10 | 4.2 | |
| Chemotherapy | .38 | ||||
| Yes | 211 | 90.2 | 220 | 92.4 | |
| No | 23 | 9.8 | 18 | 7.6 | |
| Radiation therapy | .58 | ||||
| Yes | 157 | 67.1 | 166 | 69.7 | |
| No | 76 | 32.5 | 72 | 30.3 | |
| Unknown | 1 | 0.4 | 0 | 0.0 | |
| Both chemotherapy and radiation therapy | .30 | ||||
| Yes | 134 | 57.3 | 148 | 62.2 | |
| No | 99 | 42.3 | 90 | 37.8 | |
| Unknown | 1 | 0.4 | 0 | 0.0 | |
| Chest irradiation | .66 | ||||
| Yes | 65 | 27.8 | 63 | 26.5 | |
| No | 163 | 69.7 | 173 | 72.7 | |
| Unknown | 6 | 2.6 | 2 | 0.8 | |
| Brain irradiation | .10 | ||||
| Yes | 48 | 20.5 | 65 | 27.3 | |
| No | 180 | 76.9 | 171 | 71.8 | |
| Unknown | 6 | 2.6 | 2 | 0.8 | |
| Alkylating agent | .25 | ||||
| Yes | 164 | 70.1 | 178 | 74.8 | |
| No | 70 | 29.9 | 60 | 25.2 | |
| Anthracycline | .35 | ||||
| Yes | 189 | 80.8 | 200 | 84.0 | |
| No | 45 | 19.2 | 38 | 16.0 | |
| Surgery | .99 | ||||
| Yes | 189 | 80.8 | 193 | 81.1 | |
| No | 44 | 18.8 | 45 | 18.9 | |
| Unknown | 1 | 0.4 | 0 | 0.0 | |
| Amputation | .68 | ||||
| Yes | 19 | 8.1 | 22 | 9.2 | |
| No | 214 | 91.5 | 216 | 90.8 | |
| Unknown | 1 | 0.4 | 0 | 0.0 | |
| Completed cardiomyopathy screening form | .54 | ||||
| Yes | 206 | 88.0 | 205 | 86.1 | |
| No | 28 | 12.0 | 33 | 13.9 | |
| Grade 1 to 4 chronic condition at any time | .35 | ||||
| No | 44 | 18.8 | 37 | 15.5 | |
| Yes | 190 | 81.2 | 201 | 84.5 | |
| Grade 1 to 4 chronic condition at any time | .74 | ||||
| No | 150 | 64.1 | 156 | 65.5 | |
| Yes | 84 | 35.9 | 82 | 34.5 | |
| ≥ Two grade 3 to 4 chronic conditions at any time | .52 | ||||
| No | 209 | 89.3 | 208 | 87.4 | |
| Yes | 25 | 10.7 | 30 | 12.6 | |
| Health status | .86 | ||||
| Excellent/very good/good | 219 | 93.6 | 219 | 92.0 | |
| Fair/poor | 15 | 6.4 | 17 | 7.1 | |
| Unknown | 0 | 0.0 | 2 | 0.8 | |
Abbreviations: APN, advanced-practice nurse; COG, Children's Oncology Group; SCP, survivorship care plan.
P value based on χ2 comparison among participants with known value of covariate. Fisher's exact test used when cell count < 5.
Stratification factors for randomization.
Screening Outcomes
At the 1-year follow-up, 411 of 472 randomly assigned survivors completed the follow-up survey. Among these, 107 (52.2%) of 205 survivors in the APN group were confirmed to have completed cardiomyopathy screening compared with 46 (22.3%) of 206 in the standard-care SCP-only control group (P < .001). With adjustment for sex, age, and COG cardiomyopathy risk group assignment, survivors in the APN group were > 2× more likely than the control group to have the recommended cardiomyopathy screening (adjusted RR, 2.31; 95% CI, 1.74 to 3.07; unadjusted RR, 2.34; 95% CI, 1.76 to 3.11). In additional analyses, no factors were identified that modified the effect of the intervention on completion of screening.
Among the 258 participants without confirmed cardiomyopathy screening, 26 had screening limited to electrocardiography, and self-report of screening in six could not be validated by medical record review. One or more reasons were endorsed for lack of screening in 224 of the remaining 226, including: lack of time (n = 62), screening not perceived to be important (n = 43), concerns about insurance coverage of testing (n = 43), could not afford or did not have insurance (n = 41), physician did not recommend or order screening (n = 35), forgot about need for screening (n = 21), and other reasons (n = 23). Compared with survivors assigned to standard care, survivors in the APN counseling group were more likely to identify concerns about insurance coverage of testing as a reason for not completing cardiomyopathy screening (12.8% v. 29.4%; P = .002; Table 2). However, survivors assigned to standard care were more likely to relate lack of physician recommendation as a reason for not completing cardiomyopathy screening when compared with those in the APN counseling group (19.9% v. 8.2%; P = .02). Other reasons provided for nonadherence to cardiomyopathy screening did not differ significantly by group assignment.
Table 2.
Comparisons of Reasons for No Screening Between Control and Intervention Arms Among Those Without Confirmed Cardiomyopathy Screening
| Reason | SCP (n = 141) |
SCP Plus APN (n = 85) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Did not think important/did not understand why needed | .07 | ||||
| No | 109 | 77.3 | 74 | 87.1 | |
| Yes | 32 | 22.7 | 11 | 12.9 | |
| Too busy/did not have time | .15 | ||||
| No | 107 | 75.9 | 57 | 67.1 | |
| Yes | 34 | 24.1 | 28 | 32.9 | |
| Could not afford test/had no insurance | .10 | ||||
| No | 120 | 85.1 | 65 | 76.5 | |
| Yes | 21 | 14.9 | 20 | 23.5 | |
| Concerns about insurance coverage or payment | .002 | ||||
| No | 123 | 87.2 | 60 | 70.6 | |
| Yes | 18 | 12.8 | 25 | 29.4 | |
| Physician did not recommend/order | .02 | ||||
| No | 113 | 80.1 | 78 | 91.8 | |
| Yes | 28 | 19.9 | 7 | 8.2 | |
| Forgot/have not done it/did not think about it | .07 | ||||
| No | 124 | 87.9 | 81 | 95.3 | |
| Yes | 17 | 12.1 | 4 | 4.7 | |
| Other | .71 | ||||
| No | 136 | 96.5 | 83 | 97.6 | |
| Yes | 5 | 3.5 | 2 | 2.4 | |
| Not undergoing medical follow-up/do not like medical procedures | 1.00 | ||||
| No | 138 | 97.9 | 84 | 98.8 | |
| Yes | 3 | 2.1 | 1 | 1.2 | |
| Had previous testing | .63 | ||||
| No | 139 | 98.6 | 83 | 97.6 | |
| Yes | 2 | 1.4 | 2 | 2.4 | |
| Plan to have screening in future | 1.00 | ||||
| No | 138 | 97.9 | 84 | 98.8 | |
| Yes | 3 | 2.1 | 1 | 1.2 | |
Abbreviations: APN, advanced-practice nurse; SCP, survivorship care plan.
P value based on χ2 comparison among participants with known value of covariate. Fisher's exact test used when cell count < 5.
Echocardiography Outcomes
Results from 80 (52.2%) of 153 patients confirmed to have undergone echocardiography (24 of 46 in standard-care control group and 56 of 107 in APN counseling group), for whom medical records were received, showed ≥ one cardiac abnormality requiring ongoing monitoring. Screening detected previously undiagnosed cardiomyopathy (defined as ejection fraction < 50%) in eight participants; three additional participants showed global biventricular hypokinesis in the presence of a normal ejection fraction. Six participants demonstrated impaired left ventricular relaxation consistent with diastolic dysfunction. Three participants had elevated tricuspid regurgitant velocity suggesting pulmonary hypertension, and two showed concentric left ventricular hypertrophy consistent with prolonged systemic hypertension. In addition, screening identified insufficiency involving 89 heart valves described as mild (n = 81) to moderate (n = 8) in severity. Heart valve dysfunction affected the mitral (n = 36), tricuspid (n = 33), aortic (n = 16), and pulmonic valves (n = 4); one other participant had abnormal mitral valve calcification with preserved function. Additional abnormalities detected by screening (one patient case each) included aortic root dilation, atrial enlargement, pericardial effusion, and pleural effusion.
DISCUSSION
Despite a well-established increased risk for cardiac mortality, adherence to recommended cardiomyopathy screening is low among adults treated with anthracycline chemotherapy and chest irradiation for pediatric malignancies.18 The progressive nature of cardiac injury in at-risk survivors suggests that screening may enhance opportunities for early detection and intervention to preserve heart function. In a pilot study evaluating the utility of a brief SCP detailing cancer treatment, general cardiac risk, and cardiomyopathy screening recommendations among adults at-risk for cardiomyopathy, 20% of participants reported completing screening during the study.21 Building on this study, we undertook the randomized, controlled ECHOS trial to evaluate the value added by delivery of tailored APN telephone counseling to a personalized SCP in motivating adherence to screening among at-risk childhood cancer survivors. Study findings demonstrated that tailored APN counseling addressing personal obstacles to screening increased adherence to screening by > two-fold compared with that achieved with the distribution of a personalized SCP detailing cancer treatment history and cardiomyopathy screening recommendations. Moreover, screening detected cardiomyopathy in 10% and other abnormalities consistent with evolving cardiac dysfunction (eg, global hypokinesis, diastolic dysfunction) in another 11% of participants who had screening validated by medical records. Our findings concur with a recent report identifying a high prevalence of undiagnosed cardiac dysfunction in adult survivors of childhood cancer after risk-based screening.22 Among St Jude Lifetime Cohort participants (median age, 32 years; median time from diagnosis, 25 years) exposed to cardiotoxic therapies, the prevalence of cardiac abnormalities was 56.4% (95% CI, 53.5% to 59.2%), with almost half first detected as a result of risk-based screening.22 These data underscore the importance of promotion of ongoing surveillance as this population ages.
Evaluation of reasons endorsed by survivors for lack of completion of cardiomyopathy screening highlight the significance of addressing personal and health care system barriers affecting participation in screening. Lack of time and lack of understanding about the need for cardiomyopathy screening emerged as common personal obstacles to completing the screening. The proportion of survivors relating these concerns did not differ by intervention group assignment, which suggests that efforts should be enhanced in assuring that survivors fully understand cancer treatment–related health risks and the potential benefits of prioritizing medical follow-up. Our results also suggest that these barriers could be exacerbated by lack of awareness of providers regarding the health risks associated with treatment for childhood cancer, with resulting failure to recommend or order screening. Knowledge deficits regarding cardiomyopathy risk and screening recommendations have been observed among both pediatric oncology and primary care providers, which may preclude their ability to recommend and advocate for appropriate health services for long-term survivors.23–25 Surveillance guidelines and treatment summaries have been identified by these groups as the most useful resources for caring for childhood cancer survivors.23–25 Despite the provision of these items as part of the intervention, primary care practitioners did not uniformly order cardiomyopathy screening or considered other screening tests (eg, ECG) as sufficient for evaluation of ventricular systolic function. This variance may result from failure of survivors to share these documents with primary care practitioners, lack of understanding about the natural history of cancer treatment–related cardiomyopathy and screening appropriate for evaluation of subclinical left ventricular systolic dysfunction, or a difference in opinion regarding potential benefits and harms or risks of recommended screening. Collectively, results emphasize the need for regular communication between pediatric oncology and primary care providers during care transitions to facilitate understanding about the unique and emerging health risks associated with treatment for childhood cancer and health screening recommended for at-risk groups.26
Screening costs and insurance coverage of screening also presented barriers to survivors' access to screening. Although the proportion of survivors relating that lack of insurance or funds to pay for screening was similar in both groups, more survivors in the APN group implicated concerns about insurance coverage as the reason for not completing cardiomyopathy screening. The cause for this difference is not clear, because counseling delivered to survivors assigned to this group specifically provided resources (eg, form letters to insurance companies emphasizing cardiomyopathy risk and COG cardiomyopathy surveillance recommendations) and strategies (eg, enlisting advocacy of primary care practitioner for coverage) to overcome this barrier. In our experience, communication with primary care practitioners and insurance carriers, who may not be aware that cancer treatment predisposes survivors with earlier-onset or accelerated progression of adverse health conditions commonly associated with aging,8,12,27 improves access to and coverage of screening evaluations. Even with these interventions, insured survivors may choose to forgo screening because of prohibitive out-of-pocket medical expenses related to high deductibles and copays.28,29 Despite concerns about their future health, uninsured survivors have also been noted to minimize their need for health care because of unaffordable health care costs.29 Education of survivors about health care legislation that can be leveraged to facilitate their access to insurance, as well as advocacy for inclusion of coverage and reimbursement of cancer treatment late effects screening as a mandated essential health benefit, may reduce these disparities.30,31
The results of our study should be considered within the context of its limitations. Characteristics of randomly assigned participants demonstrate a substantial proportion with high socioeconomic status based on education level, household income, and insurance access, which may not be representative of the overall childhood cancer population. Because of their long-standing participation in the CCSS study, study participants had been educated about their cancer-related health risks through biannual newsletters. Their greater awareness may limit the generalizability of our findings. However, we specifically targeted only those survivors who reported that they had not undergone cardiomyopathy screening during the previous 5 years. Therefore, these results should apply to all survivors who are resistant to or neglectful of their need for such screening. Also, although our assessment of whether our intervention was more efficacious within particular subgroups of survivors did not reveal evidence of such effects, our study was not powered to detect interactions of this type, so it is possible that some differences could become evident in larger groups of survivors.
In summary, a distance-delivered (via mail and telephone) intervention that included two brief telephone counseling sessions conducted by an APN significantly increased the likelihood of cardiomyopathy screening among at-risk survivors of childhood cancer. This method of intervention provides pediatric cancer follow-up centers with a long reach to their survivor population that can be adapted to support other types of health-protective screening in other at-risk survivor populations. Future efforts will assess the value of interventions that take advantage of electronic and mobile health applications, which may similarly facilitate survivors' interaction with the appropriate health care providers.
Appendix
Sample Size Calculation
In the original protocol, the sample size was larger by 87 participants per arm because of an a priori lower-than-observed hypothesized rate of the primary end point in the survivorship care plan (SCP; standard care) arm. Accrual was slow for the study, so power was reevaluated assuming 230 participants per arm. On the basis of the observed rate of 20% in the SCP arm, this showed that we had at least 80% power to detect a relative risk of 1.6 for two-sided tests with type I error of 5%. This was presented to the study data safety monitory board, which approved the decision to close the study at a lower-than-planned accrual. The effective sample size of 411 survivors with 1-year follow-up available provides more than 80% power to detect a two-fold difference in the primary outcome of cardiomyopathy screening, based on two-sided tests with type I error of 5%. Type I error was not affected by this modification, because the power re-evaluation was based only on the response rate in the control arm, not in the comparison between study arms.
Table A1.
Characteristics of Source Population of At-Risk Adult Survivors of Childhood Cancer Eligible for Study Recruitment Who Were Not Enrolled Versus Those Enrolled and Randomly Assigned
| Characteristic | Eligible But Not Enrolled |
Enrolled and Randomly Assigned |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Recommended COG screening frequency | .18 | ||||
| Every 2 years | 142 | 24.1 | 104 | 22.0 | |
| Every 5 years | 72 | 12.2 | 76 | 16.1 | |
| Every year | 375 | 63.7 | 292 | 61.9 | |
| Sex | .005 | ||||
| Female | 263 | 44.7 | 252 | 53.4 | |
| Male | 326 | 55.3 | 220 | 46.6 | |
| Race | .20 | ||||
| White non-Hispanic | 502 | 85.2 | 418 | 88.6 | |
| Black | 13 | 2.2 | 6 | 1.3 | |
| Other | 73 | 12.4 | 46 | 9.7 | |
| Unknown | 1 | 0.2 | 2 | 0.4 | |
| Education (2007 survey) | < .001 | ||||
| ≤ High school graduate | 89 | 15.1 | 36 | 7.6 | |
| Post–high school training/some college | 192 | 32.6 | 140 | 29.7 | |
| College graduate | 219 | 37.2 | 189 | 40.0 | |
| Postgraduate | 89 | 15.1 | 107 | 22.7 | |
| Household income (2007 survey) | < .001 | ||||
| < $20,000 | 56 | 9.5 | 39 | 8.3 | |
| $20,000 to $60,000 | 202 | 34.3 | 121 | 25.6 | |
| ≥ $60,000 | 275 | 46.7 | 287 | 60.8 | |
| Unknown | 56 | 9.5 | 25 | 5.3 | |
| Health insurance (2007 survey) | .35 | ||||
| Yes or Canadian | 512 | 86.9 | 421 | 89.2 | |
| No | 73 | 12.4 | 50 | 10.6 | |
| Unknown | 4 | 0.7 | 1 | 0.2 | |
| Diagnosis | .19 | ||||
| Bone cancer | 67 | 11.4 | 83 | 17.6 | |
| CNS tumor | 1 | 0.2 | 1 | 0.2 | |
| Hodgkin lymphoma | 93 | 15.8 | 80 | 16.9 | |
| Kidney (Wilms) | 55 | 9.3 | 38 | 8.1 | |
| Leukemia | 236 | 40.1 | 158 | 33.5 | |
| Non-Hodgkin lymphoma | 65 | 11.0 | 48 | 10.2 | |
| Neuroblastoma | 24 | 4.1 | 21 | 4.4 | |
| Soft tissue sarcoma | 48 | 8.1 | 43 | 9.1 | |
| Age at cancer diagnosis, years | .049 | ||||
| 0-4 | 193 | 32.8 | 125 | 26.5 | |
| 5-9 | 130 | 22.1 | 99 | 21.0 | |
| 10-14 | 142 | 24.1 | 120 | 25.4 | |
| 15-20 | 124 | 21.1 | 128 | 27.1 | |
| Years since diagnosis | .31 | ||||
| ≤ 28 | 283 | 48.0 | 212 | 44.9 | |
| > 28 | 306 | 52.0 | 260 | 55.1 | |
| Years since last survey | < .001 | ||||
| 1 | 116 | 19.7 | 60 | 12.7 | |
| 2 | 360 | 61.1 | 279 | 59.1 | |
| 3 | 113 | 19.2 | 133 | 28.2 | |
| Age at random assignment, years | .28 | ||||
| ≥ 30 | 495 | 84.0 | 408 | 86.4 | |
| < 30 | 94 | 16.0 | 64 | 13.6 | |
| Chemotherapy | .55 | ||||
| Yes | 542 | 92.0 | 431 | 91.3 | |
| No | 45 | 7.6 | 41 | 8.7 | |
| Unknown | 2 | 0.3 | 0 | 0.0 | |
| Radiation therapy | .31 | ||||
| Yes | 420 | 71.3 | 323 | 68.4 | |
| No | 168 | 28.5 | 148 | 31.4 | |
| Unknown | 1 | 0.2 | 1 | 0.2 | |
| Both chemotherapy and radiation therapy | .21 | ||||
| Yes | 373 | 63.3 | 282 | 59.7 | |
| No | 213 | 36.2 | 189 | 40.0 | |
| Unknown | 3 | 0.5 | 1 | 0.2 | |
| Chest irradiation | .55 | ||||
| Yes | 169 | 28.7 | 128 | 27.1 | |
| No | 408 | 69.3 | 336 | 71.2 | |
| Unknown | 12 | 2.0 | 8 | 1.7 | |
| Brain irradiation | .032 | ||||
| Yes | 175 | 29.7 | 113 | 23.9 | |
| No | 402 | 68.3 | 351 | 74.4 | |
| Unknown | 12 | 2.0 | 8 | 1.7 | |
| Alkylating agent | .70 | ||||
| Yes | 419 | 71.1 | 342 | 72.5 | |
| No | 168 | 28.5 | 130 | 27.5 | |
| Unknown | 2 | 0.3 | 0 | 0.0 | |
| Anthracycline | .63 | ||||
| Yes | 477 | 81.0 | 389 | 82.4 | |
| No | 110 | 18.7 | 83 | 17.6 | |
| Unknown | 2 | 0.3 | 0 | 0.0 | |
| Surgery | .13 | ||||
| Yes | 450 | 76.4 | 382 | 80.9 | |
| No | 132 | 22.4 | 89 | 18.9 | |
| Unknown | 7 | 1.2 | 1 | 0.2 | |
| Amputation | .50 | ||||
| Yes | 44 | 7.5 | 41 | 8.7 | |
| No | 538 | 91.3 | 430 | 91.1 | |
| Unknown | 7 | 1.2 | 1 | 0.2 | |
| Grade 1 to 4 chronic condition at any time | .18 | ||||
| No | 120 | 20.4 | 81 | 17.2 | |
| Yes | 469 | 79.6 | 391 | 82.8 | |
| Grade 3 to 4 chronic condition at any time | .24 | ||||
| No | 402 | 68.3 | 306 | 64.8 | |
| Yes | 187 | 31.7 | 166 | 35.2 | |
| ≥ Two grade 3 to 4 chronic conditions at any time | .015 | ||||
| No | 546 | 92.7 | 417 | 88.3 | |
| Yes | 43 | 7.3 | 55 | 11.7 | |
| Health status (2007 survey) | .005 | ||||
| Excellent/very good/good | 523 | 88.8 | 445 | 94.3 | |
| Fair/poor | 52 | 8.8 | 21 | 4.4 | |
| Unknown | 14 | 2.4 | 6 | 1.3 | |
Abbreviation: COG, Children's Oncology Group.
P value based on χ2 comparison among participants with known value of covariate. Fisher's exact test used when cell count < 5.
Footnotes
See accompanying editorial on page 3923
Supported by National Institutes of Health Grants R01 NR011322 (M.M.H., C.L.C.), CA55727, and CA21765 and by the American Lebanese Syrian Associated Charities.
Clinical trial information: NCT01003574.
Agencies providing support had no role in study design; collection, analysis, or interpretation of data; writing of the report; or decision to submit the report for publication.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Melissa M. Hudson, Wendy Leisenring, Kevin C. Oeffinger, Leslie L. Robison, Cheryl L. Cox
Financial support: Melissa M. Hudson, Leslie L. Robison, Cheryl L. Cox
Administrative support: Melissa M. Hudson, Brenda D. Steen, Susan Ogg, Linda Barnes, Leslie L. Robison, Cheryl L. Cox
Provision of study materials or patients: Melissa M. Hudson, Kevin C. Oeffinger, Leslie L. Robison, Cheryl L. Cox
Collection and assembly of data: Melissa M. Hudson, Wendy Leisenring, Kayla K. Stratton, Brenda D. Steen, Susan Ogg, Linda Barnes, Leslie L. Robison, Cheryl L. Cox
Data analysis and interpretation: Melissa M. Hudson, Wendy Leisenring, Kayla K. Stratton, Nina Tinner, Kevin C. Oeffinger, Leslie L. Robison, Cheryl L. Cox
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Increasing Cardiomyopathy Screening in At-Risk Adult Survivors of Pediatric Malignancies: A Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Melissa M. Hudson
No relationship to disclose
Wendy Leisenring
Research Funding: Merck
Kayla K. Stratton
No relationship to disclose
Nina Tinner
No relationship to disclose
Brenda D. Steen
No relationship to disclose
Susan Ogg
No relationship to disclose
Linda Barnes
No relationship to disclose
Kevin C. Oeffinger
No relationship to disclose
Leslie L. Robison
No relationship to disclose
Cheryl L. Cox
No relationship to disclose
REFERENCES
- 1.Lipshultz SE, Adams MJ, Colan SD, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions—A scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 2.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pein F, Sakiroglu O, Dahan M, et al. Cardiac abnormalities 15 years and more after adriamycin therapy in 229 childhood survivors of a solid tumour at the Institut Gustave Roussy. Br J Cancer. 2004;91:37–44. doi: 10.1038/sj.bjc.6601904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Dalen EC, van der Pal HJ, Kok WE, et al. Clinical heart failure in a cohort of children treated with anthracyclines: A long-term follow-up study. Eur J Cancer. 2006;42:3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Kremer LC, van Dalen EC, Offringa M, et al. Frequency and risk factors of anthracycline-induced clinical heart failure in children: A systematic review. Ann Oncol. 2002;13:503–512. doi: 10.1093/annonc/mdf118. [DOI] [PubMed] [Google Scholar]
- 6.van der Pal HJ, van Dalen EC, Kremer LC, et al. Risk of morbidity and mortality from cardiovascular disease following radiotherapy for childhood cancer: A systematic review. Cancer Treat Rev. 2005;31:173–185. doi: 10.1016/j.ctrv.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 7.van der Pal HJ, van Dalen EC, van Delden E, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 8.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 9.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute–supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanco JG, Sun CL, Landier W, et al. Anthracycline-related cardiomyopathy after childhood cancer: Role of polymorphisms in carbonyl reductase genes—A report from the Children's Oncology Group. J Clin Oncol. 2012;30:1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kremer LC, van der Pal HJ, Offringa M, et al. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: A systematic review. Ann Oncol. 2002;13:819–829. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 14.Scottish Intercollegiate Guidelines Network. Long term follow up of survivors of childhood cancer: A national clinical guideline. http://www.sign.ac.uk/guidelines/fulltext/132/index.html.
- 15.United Kingdom Children's Cancer Study Group Late Effects Group. Therapy based long term follow up practice statement. http://www.cclg.org.uk/dynamic_files/LTFU-full.pdf.
- 16.Dutch Childhood Oncology Group. Richtlijn follow-up na kinderkanker meer dan 5 jaar na diagnose. https://www.skion.nl/workspace/uploads/richtlijn_follow-up_na_kinderkanker_deel_1.pdf.
- 17.Children's Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers (version 3.0) http://www.survivorshipguidelines.org/
- 18.Nathan PC, Greenberg ML, Ness KK, et al. Medical care in long-term survivors of childhood cancer: A report from the childhood cancer survivor study. J Clin Oncol. 2008;26:4401–4409. doi: 10.1200/JCO.2008.16.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: Experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 21.Oeffinger KC, Hudson MM, Mertens AC, et al. Increasing rates of breast cancer and cardiac surveillance among high-risk survivors of childhood Hodgkin lymphoma following a mailed, one-page survivorship care plan. Pediatr Blood Cancer. 2011;56:818–824. doi: 10.1002/pbc.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudson MM, Ness KK, Gurney JG, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309:2371–2381. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson TO, Hlubocky FJ, Wroblewski KE, et al. Physician preferences and knowledge gaps regarding the care of childhood cancer survivors: A mailed survey of pediatric oncologists. J Clin Oncol. 2010;28:878–883. doi: 10.1200/JCO.2009.25.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathan PC, Daugherty CK, Wroblewski KE, et al. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv. 2013;7:275–282. doi: 10.1007/s11764-013-0271-0. [DOI] [PubMed] [Google Scholar]
- 25.Suh E, Daugherty CK, Wroblewski K, et al. General internists' preferences and knowledge about the care of adult survivors of childhood cancer: A cross-sectional survey. Ann Intern Med. 2014;160:11–17. doi: 10.7326/M13-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117–5124. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- 27.Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: A report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31:4496–4503. doi: 10.1200/JCO.2013.52.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirchhoff AC, Lyles CR, Fluchel M, et al. Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer. 2012;118:5964–5972. doi: 10.1002/cncr.27537. [DOI] [PubMed] [Google Scholar]
- 29.Park ER, Kirchhoff AC, Zallen JP, et al. Childhood Cancer Survivor Study participants' perceptions and knowledge of health insurance coverage: Implications for the Affordable Care Act. J Cancer Surviv. 2012;6:251–259. doi: 10.1007/s11764-012-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller EL, Park ER, Davis MM. What the affordable care act means for survivors of pediatric cancer. J Clin Oncol. 2014;32:615–617. doi: 10.1200/JCO.2013.52.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warner EL, Park ER, Stroup A, et al. Childhood cancer survivors' familiarity with and opinions of the Patient Protection and Affordable Care Act. J Oncol Pract. 2013;9:246–250. doi: 10.1200/JOP.2013.000919. [DOI] [PMC free article] [PubMed] [Google Scholar]


