Abstract
Purpose
Data on smoking and second cancer risk among cancer survivors are limited. We assessed associations between smoking before first cancer diagnosis and risk of second primary smoking-associated cancers among survivors of lung (stage I), bladder, kidney, and head/neck cancers.
Methods
Data were pooled from 2,552 patients with stage I lung cancer, 6,386 with bladder cancer, 3,179 with kidney cancer, and 2,967 with head/neck cancer from five cohort studies. We assessed the association between prediagnostic smoking and second smoking-associated cancer risk with proportional hazards regression, and compared these estimates to those for first smoking-associated cancers in all cohort participants.
Results
Compared with never smoking, current smoking of ≥ 20 cigarettes per day was associated with increased second smoking-associated cancer risk among survivors of stage I lung (hazard ratio [HR] = 3.26; 95% CI, 0.92 to 11.6), bladder (HR = 3.67; 95% CI, 2.25 to 5.99), head/neck (HR = 4.45; 95% CI, 2.56 to 7.73), and kidney cancers (HR = 5.33; 95% CI, 2.55 to 11.1). These estimates were similar to those for first smoking-associated cancer among all cohort participants (HR = 5.41; 95% CI, 5.23 to 5.61). The 5-year cumulative incidence of second smoking-associated cancers ranged from 3% to 8% in this group of cancer survivors.
Conclusion
Understanding risk factors for second cancers among cancer survivors is crucial. Our data indicate that cigarette smoking before first cancer diagnosis increases second cancer risk among cancer survivors, and elevated cancer risk in these survivors is likely due to increased smoking prevalence. The high 5-year cumulative risks of smoking-associated cancers among current smoking survivors of stage I lung, bladder, kidney, and head/neck cancers highlight the importance of smoking cessation in patients with cancer.
INTRODUCTION
Approximately one in six cancers diagnosed occurs among the 13 million cancer survivors living in the United States1 Although cigarette smoking is a strong, modifiable risk factor for a number of malignancies,2 continued use of tobacco after an initial diagnosis of a smoking-associated cancer is common.3–6 Cancer sites strongly related to smoking and/or alcohol consumption make up more than one third of all second primary malignancies in the United States, and survivors of smoking-associated cancers are at increased risk of developing a second smoking-associated cancer, compared with the general population.7 However, it is unclear whether the elevated risk of second smoking-associated cancers among survivors of first primary smoking-associated cancers is due to higher smoking prevalence among cancer survivors or to increased susceptibility to the effects of cigarette smoking.
Data on the association between smoking and second cancer risk in cancer survivors are limited, largely because registry-based studies lack information on cigarette smoking and other individual-level data, clinical trials generally lack detailed smoking data, and cohort studies have limited numbers of second cancers. Among the few studies to investigate the association between smoking and second primary cancer risk, continued smoking after a lung cancer or head/neck cancer diagnosis has been associated with increased second primary cancer risk.7–15 However, these studies were generally small and did not include survivors of other smoking-related cancers.
In the current study, we pooled data from five large, prospective epidemiologic cohorts to assess the association between prediagnostic (ie, collected on the baseline questionnaire before cancer diagnosis) smoking behaviors and second cancer risk among survivors of bladder, kidney, head/neck, and stage I lung cancers. To assess whether these survivors are more susceptible to the effects of tobacco, we compared these estimates with relative risks for smoking and first smoking-associated cancer risk.
METHODS
Study Population
The study population was derived from five prospective cohort studies: the National Institutes of Health (NIH)-AARP Diet and Health Study; the Agricultural Health Study (AHS); the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study; the Iowa Women's Health Study (IWHS); and the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Details regarding each study population and cohort design as well as a description of the pooling project have been published elsewhere.16–21
Cancers were ascertained either with linkage to population-based cancer registries or by self-report confirmed with medical record review. Individual studies utilized different versions of the International Classification of Diseases for Oncology (ICD-O) to classify cancers; therefore, we coded all cancers according to the SEER Program Incidence Site Recode based on ICD-O-3.22,23 In this analysis, we included individuals with at least 30 days of follow-up, and incident, first primary diagnoses of bladder (ICD-O-3 site: C670-C679), kidney and renal pelvis (C649, C659), head/neck (C000-C009, C019-C0119, C129, C130-C140, C142-C148, C150-C159) and stage I lung cancers (C320-C329), excluding histology codes 9590 to 9989, 9050 to 9055 and 9140. These cancer sites were chosen due to their associations with cigarette smoking, prolonged survival relative to other smoking-associated cancers, and number of cases. The fraction of cases surviving 5 years after diagnosis for each smoking-related cancer site and hazard ratios (HRs) for the association between smoking status and first smoking-associated cancer diagnosis were assessed with data using the pooled cohorts. Cancer sites with 5-year survival less than 50% (eg, pancreas), fewer than 2,000 cases (eg, larynx), or weaker associations (relative risk < 2.0) with cigarette smoking (eg, colorectal) were not included in this analysis. Lung cancers were restricted to stage I tumors, based on SEER summary stage, to allow for sufficient survival.
Our primary analysis focused on the combined end point of second smoking-associated cancers, as defined by the International Agency for Research on Cancer (IARC), including cancers of the oral cavity, oropharynx, nasopharynx, hypopharynx, esophagus (adenocarcinoma/squamous cell carcinoma), stomach, colorectum, liver, pancreas, nasal cavity/paranasal sinuses, larynx, lung, uterine cervix, ovary (mucinous), urinary bladder, kidney (body/pelvis) and ureter, and myeloid leukemia.2 To distinguish multiple reports, recurrence and metastases of the first primary malignancy from a second primary malignancy, we applied the SEER 2007 Multiple Primary and Histology Coding Rules,24 and limited our analyses to non–same-site second primary malignancies.
Prediagnostic smoking parameters and other covariates collected at baseline were harmonized across the cohorts. Herein prediagnostic smoking refers to smoking before first cancer diagnosis. Each cohort had information on smoking status and cigarettes smoked per day. ATBC, AHS, PLCO and IWHS had data on pack-years smoked, and PLCO, IWHS and NIH-AARP had data on years since quitting among former smokers. Individuals without smoking status information at baseline were excluded from the analysis (n = 421).
Statistical Analysis
In separate models among survivors of stage I lung, bladder, kidney, and head/neck cancers, Cox proportional hazards regression was used to assess the association between prediagnostic smoking behaviors and second non–same-site smoking-associated cancer risk. Individuals were followed from their first primary cancer diagnosis to second cancer diagnosis, death or end of cohort follow-up. Same-site second cancers were censored at diagnosis date. All models used age as the underlying time scale, and were adjusted for sex, race (white, nonwhite, missing), education (high school diploma or less, vocational school/some college, college graduate/graduate school, missing), body mass index (< 25, 25-29.9, 30+ kg/m2), cohort, time from baseline to first cancer diagnosis (modeled continuously) and time since diagnosis of primary cancer (modeled as a polynomial spline).25 Due to the relatively low proportion of individuals with missing data, we opted to add an additional category to variables (ie, missing value) as compared with more complicated imputation methods, because such methods would require additional assumptions and are unlikely to significantly change the results.26 Complete cancer stage and alcohol use were not available from PLCO and ATBC, therefore in a sensitivity analysis limited to those cohorts with available data, we in addition adjusted for alcohol and stage, and found similar associations (data not shown). Of note, PLCO did collect stage on lung cancers and ATBC had more complete information for lung cancer stage, therefore we were able to restrict our analysis to stage I lung cancers, despite missing stage information for other cancer sites. Inferences remained the same when those with less than 30 days of follow-up were included (Appendix Table A1, online only).
In our main model, prediagnostic smoking was defined based on both smoking status and number of cigarettes smoked per day at baseline (never-smoker, former smoker < 20 cigarettes per day, former smoker ≥ 20 cigarettes per day, current smoker < 20 cigarettes per day, current smokers ≥ 20 cigarettes per day). This model excluded ATBC, as all participants in this cohort were current smokers at baseline. Additional models examined cigarettes per day among current smokers (all cohorts), pack-years smoked among ever smokers (excluding NIH-AARP), and years since quitting among former smokers (excluding AHS and ATBC). A trend across exposure categories was estimated by treating each categorical exposure as a continuous variable. Interactions by cohort were assessed with a cross-product term in the model.
We carried out several sub-analyses. First, to address the impact that deaths shortly after first primary cancer diagnosis have on our results, we restricted our analysis to 3-year survivors. Next, head/neck cancers were stratified by primary site (oral cavity, oropharynx, other head/neck, and larynx). In addition, stratified by primary cancer site, we assessed the association between smoking status and cigarettes per day with death.
Further, we compared the association between cigarette smoking and second primary smoking-associated cancer risk to the association between cigarette smoking and first primary smoking-associated cancer risk. With data from 754,855 participants from NIH-AARP, AHS, PLCO and IWHS, Cox proportional hazards regression was used to assess the association between smoking behaviors and first primary smoking-associated cancer risk with age as the time scale, adjusting for sex, race, education, body mass index, cohort, and follow-up time.
Finally, we assessed the cumulative incidence of second primary cancers among survivors of each primary cancer site by prediagnostic smoking status, accounting for death as a competing event.27 When assessing the associations between risk factors and subsequent cancer diagnoses in cancer survivors, our analyses must allow for mortality as a competing risk. Cigarette smoking is known to be associated with mortality, and deaths preclude second cancer diagnoses from occurring, thus competing deaths may distort the observed associations between smoking and second cancer risk, likely attenuating the estimates. Following common approaches,28 we estimate the cause-specific29 HRs and the cumulative risks of subsequent cancers.27 These two approaches have the advantage of focusing on observable or estimable quantities, and do not require potentially untestable assumptions about the joint distribution of failure times.29–31 However, caution is still required for interpretation.
RESULTS
In five prospective cohorts, we identified 2,552 individuals with first primary, incident stage I lung cancer, 6,386 with bladder cancer, 3,179 with kidney cancer and 2,967 with head/neck cancer. Eighty second primary smoking-associated cancers occurred in stage I lung cancer survivors, 385 in bladder cancer survivors, 139 in kidney cancer survivors, and 262 in head/neck cancer survivors (distribution of all second cancer diagnoses presented in Appendix Table A2, online only). Table 1 presents baseline characteristics of cancer survivors with and without a second primary smoking-associated cancer diagnosis by first primary site. For each first primary cancer site, survivors who developed a second cancer were more likely to be current smokers at baseline than those that did not. Of note, greater than 50% of all cancer cases occurred in the NIH-AARP cohort, as it was the largest study.
Table 1.
Characteristics of Lung (Stage I), Bladder, Kidney, and Head/Neck Cancer Survivors With and Without Second Smoking-Associated Cancer Diagnoses
| Characteristic | Stage I Lung Cancer* |
Bladder Cancer* |
Kidney Cancer* |
Head/Neck Cancer* |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Second Primary Cancer |
Second Primary Cancer |
No Second Primary Cancer |
Second Primary Cancer |
No Second Primary Cancer |
Second Primary Cancer |
No Second Primary Cancer |
Second Primary Cancer |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Total | 2,472 | 80 | 6,001 | 385 | 3,040 | 139 | 2,705 | 262 | ||||||||
| Age, years | ||||||||||||||||
| < 50 | 10 | 0.4 | 0 | 0 | 39 | 0.7 | 0 | 0 | 58 | 1.9 | 0 | 0 | 54 | 2.0 | 1 | 0.4 |
| 50-54 | 131 | 5.3 | 4 | 5.0 | 408 | 6.8 | 25 | 6.5 | 283 | 9.3 | 8 | 5.8 | 341 | 12.6 | 20 | 7.6 |
| 55-59 | 523 | 21.2 | 19 | 23.8 | 1,094 | 18.2 | 75 | 19.5 | 698 | 23.0 | 33 | 23.7 | 644 | 23.8 | 65 | 24.8 |
| 60-64 | 776 | 31.4 | 22 | 27.5 | 1,737 | 29.0 | 96 | 24.9 | 903 | 29.7 | 45 | 32.4 | 749 | 27.7 | 74 | 28.2 |
| 65-69 | 821 | 33.2 | 28 | 35.0 | 2,258 | 37.6 | 165 | 42.9 | 926 | 30.5 | 44 | 31.7 | 767 | 28.4 | 82 | 31.3 |
| ≥ 70 | 211 | 8.5 | 7 | 8.8 | 465 | 7.8 | 24 | 6.2 | 172 | 5.7 | 9 | 6.5 | 150 | 5.6 | 20 | 7.6 |
| Sex† | ||||||||||||||||
| Male | 1,475 | 59.7 | 56 | 70.0 | 4,973 | 82.9 | 331 | 86.0 | 2,116 | 69.6 | 105 | 75.5 | 2,069 | 76.5 | 220 | 84.0 |
| Female | 997 | 40.3 | 24 | 30.0 | 1,028 | 17.1 | 54 | 14.0 | 924 | 30.4 | 34 | 24.5 | 636 | 23.5 | 42 | 16.0 |
| Race | ||||||||||||||||
| White | 2,317 | 93.7 | 78 | 97.5 | 5,748 | 95.8 | 373 | 96.9 | 2,796 | 92.0 | 132 | 95.0 | 2,540 | 93.9 | 251 | 95.8 |
| Nonwhite | 153 | 6.2 | 2 | 2.5 | 249 | 4.2 | 12 | 3.1 | 244 | 8.0 | 7 | 5.0 | 163 | 6.0 | 11 | 4.2 |
| Missing | 2 | 0.1 | 0 | 0 | 4 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0.1 | 0 | 0 |
| Education‡ | ||||||||||||||||
| ≤ High school diploma | 947 | 38.3 | 26 | 32.5 | 1,904 | 31.7 | 135 | 35.1 | 1,011 | 33.3 | 36 | 25.9 | 899 | 33.2 | 99 | 37.8 |
| Vocational school/some college | 946 | 38.3 | 42 | 52.5 | 2,080 | 34.7 | 155 | 40.3 | 1,063 | 35.0 | 62 | 44.6 | 979 | 36.2 | 98 | 37.4 |
| College graduate/graduate school | 519 | 21.0 | 10 | 12.5 | 1,879 | 31.3 | 83 | 21.6 | 904 | 29.7 | 35 | 25.2 | 782 | 28.9 | 60 | 22.9 |
| Missing | 60 | 2.4 | 2 | 2.5 | 138 | 2.3 | 12 | 3.1 | 62 | 2.0 | 6 | 4.3 | 45 | 1.7 | 5 | 1.9 |
| Smoking status§ | ||||||||||||||||
| Never | 186 | 7.5 | 3 | 3.8 | 1,080 | 18.0 | 28 | 7.3 | 1,029 | 33.9 | 19 | 13.7 | 542 | 20.0 | 18 | 6.9 |
| Former | 1,094 | 44.3 | 25 | 31.3 | 3,323 | 55.4 | 184 | 47.8 | 1,371 | 45.1 | 71 | 51.1 | 1,079 | 39.9 | 100 | 38.2 |
| Current | 1,192 | 48.2 | 52 | 65.0 | 1,598 | 26.6 | 173 | 44.9 | 640 | 21.1 | 49 | 35.3 | 1.084 | 40.1 | 144 | 55.0 |
| BMI category§ | ||||||||||||||||
| < 25 | 1,015 | 41.1 | 32 | 40.0 | 1,717 | 28.6 | 151 | 39.2 | 734 | 24.1 | 39 | 28.1 | 1,001 | 37.0 | 101 | 38.6 |
| 25-29.9 | 969 | 39.2 | 33 | 41.3 | 2,863 | 47.7 | 147 | 38.2 | 1,319 | 43.4 | 58 | 41.7 | 1,110 | 41.0 | 108 | 41.2 |
| ≥ 30.0 | 411 | 16.6 | 13 | 16.3 | 1,262 | 21.0 | 81 | 21.0 | 876 | 28.8 | 35 | 25.2 | 506 | 18.7 | 46 | 17.6 |
| Missing | 77 | 3.1 | 2 | 2.5 | 159 | 2.7 | 6 | 1.6 | 111 | 3.7 | 7 | 5.0 | 88 | 3.3 | 7 | 2.7 |
| Current alcohol use | ||||||||||||||||
| No | 527 | 21.3 | 13 | 16.3 | 1,051 | 17.5 | 62 | 16.1 | 690 | 22.7 | 24 | 17.3 | 570 | 21.1 | 62 | 23.7 |
| Yes | 1,298 | 52.5 | 42 | 52.5 | 3,827 | 63.8 | 268 | 69.6 | 1,783 | 58.7 | 86 | 61.9 | 1,727 | 63.8 | 141 | 53.8 |
| Missing | 647 | 26.2 | 25 | 31.3 | 1,123 | 18.7 | 55 | 14.3 | 667 | 21.9 | 29 | 20.9 | 408 | 15.1 | 59 | 22.5 |
| Cohort§ | ||||||||||||||||
| NIH-AARP | 1,276 | 51.6 | 32 | 40.0 | 3,947 | 65.8 | 227 | 59.0 | 1,864 | 61.3 | 78 | 56.1 | 1.673 | 61.9 | 142 | 54.2 |
| AHS | 82 | 3.3 | 2 | 2.5 | 240 | 4.0 | 6 | 1.6 | 193 | 6.4 | 2 | 1.4 | 160 | 5.9 | 10 | 3.8 |
| ATBC | 320 | 12.9 | 17 | 21.3 | 527 | 8.8 | 83 | 21.6 | 244 | 8.0 | 23 | 16.6 | 331 | 12.2 | 51 | 19.5 |
| IWHS | 166 | 6.7 | 5 | 6.3 | 209 | 3.5 | 16 | 4.2 | 189 | 6.2 | 7 | 5.0 | 149 | 5.5 | 8 | 3.1 |
| PLCO | 628 | 25.4 | 24 | 30.0 | 1,078 | 18.0 | 53 | 13.8 | 550 | 18.1 | 29 | 20.9 | 392 | 14.5 | 51 | 19.5 |
NOTE. Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) study only includes current smokers. Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial did not collect information on alcohol use at baseline. Second smoking-associated cancers included: cancers of the oral cavity, oropharynx, nasopharynx, hypopharynx, esophagus (adenocarcinoma and squamous cell carcinoma), stomach, colorectum, liver, pancreas, nasal cavity and paranasal sinuses, larynx, lung, uterine cervix, ovary (mucinous), urinary bladder, kidney (body and pelvis) and ureter, and myeloid leukemia.
Abbreviations: AHS, Agricultural Health Study; BMI, body mass index IWHS, Iowa Women's Health Study; NIH-AARP, National Institutes of Health–AARP Diet and Health Study.
First primary cancer.
Statistically significant difference between head/neck cancer survivors with and without a second cancer.
Statistically significant difference between bladder and kidney cancer survivors with and without a second cancer.
Statistically significant difference between lung, bladder, kidney and head/neck cancer survivors with and without a second cancer.
The prevalence of current smoking at baseline was higher among individuals that developed stage I lung (41%), bladder (20%), kidney (15%) and head/neck cancers (33%), compared with all cohort participants in NIH-AARP, AHS, PLCO and IWHS (13%). Across all first primary cancer sites, there was a significant trend of increasing risk of second smoking-associated cancers across categories of prediagnostic smoking status and cigarettes smoked per day (Table 2). Compared with never-smokers, current smoking status with ≥ 20 cigarettes per day was associated with an increased risk of second smoking-associated cancer among survivors of stage I lung cancer (HR = 3.26; 95% CI, 0.92 to 11.6), bladder cancer (HR = 3.67; 95% CI, 2.21 to 5.99), head/neck cancer (HR = 4.45; 95% CI, 2.56 to 7.73), and kidney cancer (HR = 5.33; 95% CI, 2.55 to 11.1), with apparently stronger risk estimates in the pooled population restricted to 3-year survivors (Appendix Table A3, online only). Study-specific estimates were generally underpowered and are presented in Appendix Table A4 (online only). No significant interactions were observed between smoking and cohort (all P interactions > 0.4). Associations between cigarette smoking and first smoking-associated cancer risk were similar to associations observed for second smoking-associated cancer risk among cancer survivors. Compared with never-smokers, current smokers who smoked ≥ 20 cigarettes per day had a 5.4-fold increased risk of any first primary smoking-associated cancer. The HRs for current smokers who smoked less than 20 cigarettes per day (HR = 3.72), former smokers who smoked ≥ 20 cigarettes per day (HR = 2.35) and former smokers who smoked less than 20 cigarettes per day (HR = 1.49) were also elevated (Appendix Table A5, online only).
Table 2.
Association Between Smoking Status and Cigarettes Smoked per Day and Risk of a Second Primary Smoking-Associated Cancer Among Survivors of Lung (Stage I), Bladder, Kidney, and Head/Neck Cancers
| Smoking Status | Stage I Lung Cancer |
Bladder Cancer |
Kidney Cancer |
Head/Neck Cancer |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | |||||
| No | Yes | No | Yes | No | Yes | No | Yes | |||||||||
| Never | 186 | 3 | 1.0 | Referent | 1,080 | 28 | 1.0 | Referent | 1,029 | 19 | 1.0 | Referent | 542 | 18 | 1.0 | Referent |
| Former | ||||||||||||||||
| < 20 cig/d | 419 | 6 | 0.90 | 0.22 to 3.68 | 1,523 | 78 | 1.84 | 1.19 to 2.85 | 752 | 27 | 1.90 | 1.05 to 3.47 | 511 | 32 | 1.60 | 0.87 to 2.81 |
| ≥ 20 cig/d* | 674 | 19 | 1.69 | 0.48 to 5.94 | 1,793 | 106 | 2.12 | 1.38 to 3.26 | 617 | 44 | 4.08 | 2.30 to 7.25 | 567 | 66 | 2.97 | 1.74 to 5.07 |
| Current | ||||||||||||||||
| < 20 cig/d | 482 | 18 | 2.58 | 0.74 to 8.98 | 623 | 49 | 2.81 | 1.76 to 4.50 | 243 | 13 | 3.44 | 1.67 to 7.08 | 408 | 42 | 2.89 | 1.64 to 5.07 |
| ≥ 20 cig/d* | 389 | 17 | 3.26 | 0.92 to 11.6 | 448 | 41 | 3.67 | 2.25 to 5.99 | 152 | 13 | 5.33 | 2.55 to 11.1 | 345 | 51 | 4.45 | 2.56 to 7.73 |
| P trend† | .002 | < .001 | < .001 | < .001 | ||||||||||||
NOTE. Adjusted for age, sex, race, education, body mass index, cohort, time from baseline to first cancer, and follow-up time. Excludes Alpha-Tocopherol, Beta-Carotene Cancer Prevention study, as this cohort was limited to current smokers.
Abbreviations: cig/d, cigarettes per day; HR, hazard ratio.
Cigarettes per day was collected as a categorical variable in National Institutes of Health–AARP Diet and Health Study; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; and Agricultural Health Study. The maximum number of cigarettes smoked per day in the Iowa Women's Health Study was as follows: lung (stage I): 60; bladder: 40; kidney: 40; head and neck: 40.
P trends across joint categories of smoking status and intensity categories were estimated by including the categorical exposure variable in the model as a continuous variable.
Among current smokers at baseline, the risk of second primary smoking-associated cancers increased significantly with increasing smoking intensity and among ever smokers at baseline, risk increased significantly with increasing pack-years smoked for survivors of kidney (P trend = .02 and .005, respectively) and head/neck cancers (P trend = .008 and .02, respectively). In contrast, no significant trend in smoking-associated cancer risk was observed across categories of cigarettes per day or pack-years smoked for survivors of stage I lung (P trend = .97 and .58, respectively) and bladder cancers (P trend = .45 and .07, respectively; Figs 1 and 2). Among former smokers at baseline, time since quitting was significantly inversely associated with increased second smoking-associated cancer risk for survivors of bladder (P trend < .001), kidney (P trend = .002) and head/neck cancers (P trend < .001), but not stage I lung cancers (P trend = .99; Fig 3).
Fig 1.
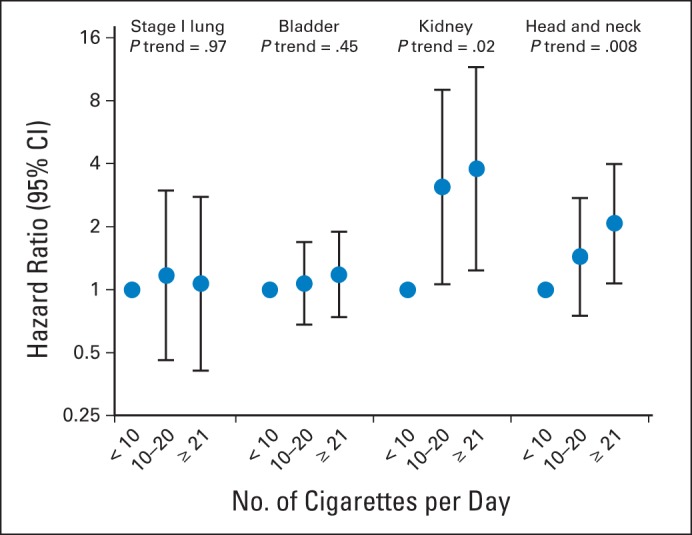
Association between cigarettes smoked per day at baseline and subsequent risk of second smoking-associated cancers among current smokers with stage I lung, bladder, kidney, and head/neck cancers. Points represent odds ratios and lines represent 95% CIs. Cigarettes per day was collected as a categorical variable in the National Institutes of Health–AARP Diet and Health Study, the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial, and the Agricultural Health Study. The maximum number of cigarettes smoked per day among current smokers in the Iowa Women's Health Study was as follows: lung (stage I): 50; bladder: 40; kidney: 25; head and neck: 40, and in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study was as follows: lung (stage I): 60; bladder: 60; kidney: 55; head and neck: 60.
Fig 2.
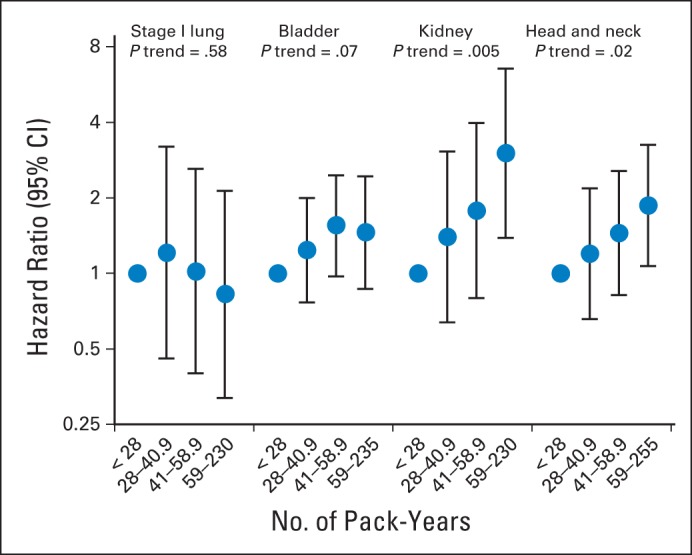
Association between pack-years smoked at baseline and subsequent risk of second smoking-associated cancers among current smokers with stage I lung, bladder, kidney, and head/neck cancers. Points represent odds ratios and lines represent 95% CIs.
Fig 3.

Association between years since smoking cessation at baseline and subsequent risk of second smoking-associated cancers among former smokers with stage I lung, bladder, kidney, and head/neck cancers. Points represent odds ratios and lines represent 95% CIs. Years since smoking cessation was collected as a categorical variable in the National Institutes of Health–AARP Diet and Health Study. The maximum number of years since smoking cessation in the Iowa Women's Health Study was as follows: lung (stage I): 32; bladder: 41; kidney: 36; head and neck: 40, and in the Prostate, Lung, Colorectal and Ovarian Screening Trial was as follows: lung (stage I): 41; bladder: 58; kidney: 53; head and neck: 53.
When anatomic sub-sites within head/neck cancers were examined, the strongest associations between current smokers with ≥ 20 cigarettes per day and second smoking-associated cancer risk were observed among survivors of oral cavity (HR = 6.29; 95% CI, 2.10 to 18.8) and laryngeal cancers (HR = 8.20; 95% CI, 1.89 to 35.6; Appendix Table A6, online only).
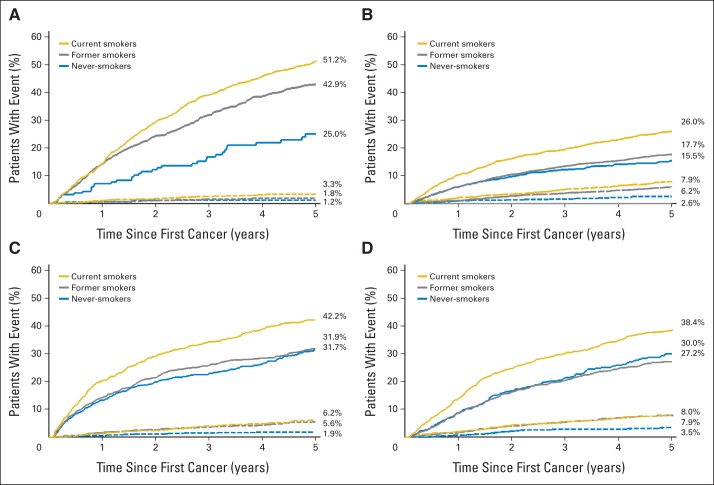
Prediagnostic smoking was significantly associated with mortality in each survivor cohort (Table 3). Compared with never-smokers, current smokers who smoked ≥ 20 cigarettes per day had an increased risk of death among survivors of stage I lung (HR = 3.08; 95% CI, 2.18 to 4.33), bladder (HR = 2.48; 95% CI, 1.99 to 3.09), kidney (HR = 1.57; 95% CI, 1.18 to 2.08), and head/neck cancers (HR = 1.68; 95% CI, 1.34 to 2.10). Appendix Figure A1 (online only) presents the absolute risk of death and second smoking-associated cancers in each survivor cohort by prediagnostic smoking status. These figures emphasize the importance of death as a competing risk in survivors of stage I lung, bladder, kidney and head/neck cancers. For example, the 5-year cumulative incidence of death was higher among baseline current smokers compared with never-smokers among survivors of stage I lung cancer (51.2% v 25.0%), bladder cancer (26.0% v 15.5%), kidney cancer (42.2% v 31.7%) and head/neck cancer (38.4% v 30.0%).
Table 3.
Association Between Smoking Status and Death Among Survivors of First Primary Lung (stage I), Bladder, Kidney, and Head/Neck Cancers
| Smoking Status | Stage I Lung Cancer |
Bladder Cancer |
Kidney Cancer |
Head/Neck Cancer |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | |||||
| No | Yes | No | Yes | No | Yes | No | Yes | |||||||||
| Never | 147 | 42 | 1.00 | Referent | 899 | 209 | 1.00 | Referent | 723 | 325 | 1.00 | Referent | 381 | 179 | 1.00 | Referent |
| Former | ||||||||||||||||
| < 20 cig/d | 261 | 164 | 1.87 | 1.32 to 2.64 | 1,279 | 322 | 1.23 | 1.03 to 1.48 | 534 | 245 | 1.08 | 0.91 to 1.28 | 382 | 161 | 0.95 | 0.77 to 1.19 |
| ≥ 20 cig/d* | 382 | 311 | 2.22 | 1.59 to 3.10 | 1,449 | 450 | 1.57 | 1.31 to 1.87 | 444 | 217 | 1.21 | 1.01 to 1.46 | 419 | 214 | 1.19 | 0.96 to 1.47 |
| Current | ||||||||||||||||
| < 20 cig/d | 261 | 239 | 2.38 | 1.70 to 3.32 | 488 | 184 | 1.71 | 1.40 to 2.10 | 144 | 112 | 1.57 | 1.26 to 1.96 | 243 | 207 | 1.64 | 1.32 to 2.02 |
| ≥ 20 cig/d* | 197 | 209 | 3.08 | 2.18 to 4.33 | 337 | 152 | 2.48 | 1.99 to 3.09 | 103 | 62 | 1.57 | 1.18 to 2.08 | 227 | 169 | 1.68 | 1.34 to 2.10 |
| P trend† | < .001 | < .001 | < .001 | < .001 | ||||||||||||
NOTE. Adjusted for age, sex, race, education, body mass index, cohort, time from baseline to first cancer, and follow-up time. Excludes Alpha-Tocopherol, Beta-Carotene Cancer Prevention study, as this cohort was limited to current smokers.
Abbreviations: cig/d, cigarettes per day; HR, hazard ratio.
Cigarettes per day was collected as a categorical variable in National Institutes of Health–AARP Diet and Health Study; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; and Agricultural Health Study. The maximum number of cigarettes smoked per day in the Iowa Women's Health Study was as follows: lung (stage I): 60; bladder: 40; kidney: 40; head and neck: 40.
P trends across joint categories of smoking status and intensity categories were estimated by including the categorical exposure variable in the model as a continuous variable.
DISCUSSION
In the largest study to date, with observational data for over 15,000 cancer survivors from five prospective cohorts, we showed that cigarette smoking before first cancer diagnosis is associated with subsequent smoking-associated cancer risk among survivors of stage I lung, bladder, kidney, and head/neck cancers. Risks decreased with a greater number of years since smoking cessation in former smoking survivors of bladder, kidney, and head/neck cancers, and were similar to those observed for first primary smoking-associated cancers.
In the United States, 44% of malignancies occurring among survivors of an alcohol or tobacco-related cancer are also alcohol or tobacco-related cancers.7 Prior analyses based on large registries conjectured that the increased rate of second primary smoking-associated cancers following first primary smoking-associated cancers was due to smoking as a shared risk factor; however, those studies were unable to directly address this question. Our results are consistent with previous generally smaller studies among survivors of lung or head/neck cancers.8–12,14,15 In addition, we provide novel data that prediagnostic smoking increases risk of second smoking-associated cancers in survivors of bladder and kidney cancers. Further, we have shown that the strength of the association between smoking and second smoking-associated cancer risk does not exceed that of smoking and first smoking-associated cancer risk. This may indicate that the increased risk of smoking-associated cancers among survivors of smoking-associated cancers is due to increased smoking prevalence in this group of cancer survivors (15% to 41% v 13%), though we could not rule out a role for increased susceptibility to smoking-related damage.
In addition, we have shown that the pooling of large cohort studies with available risk behavioral information is essential to address questions regarding second cancer risk. The main strength of our analysis is the pooling of five large, well-established cohort studies with demographic and behavioral information and extensive follow-up. Studying second cancer risk requires a large number of cancer survivors, and the association between smoking and second cancer risk could not have been addressed in any of these studies alone (Appendix Table A2). However, our approach also had several limitations that reflect general challenges in studying second cancers in existing cohorts, which have been explored by our group in detail previously.21 First, our analysis lacked treatment data, as the participating cohorts do not collect treatment information on cancer cases. Further, despite the over 15,000 cancer survivors included in this study, our power was limited based on a relatively small number of second cancers. In addition, our estimates relied on baseline, prediagnostic assessments of smoking behaviors, as questionnaires in each study were not administered regularly enough to ascertain smoking status at the time of diagnosis with a first cancer, the ideal time point for assessing associations with cancer risk. In addition, among current smokers at baseline, less than 25% had follow-up questionnaires administered after their initial cancer diagnosis, prohibiting the evaluation of change in smoking status after cancer diagnosis and subsequent risk. Further, our reliance on baseline questionnaire data (collected at varying times before first cancer diagnosis) may have resulted in more misclassification of exposure for the assessment of second cancer risk than first cancer risk. As a result, current smokers at baseline who subsequently quit would be included in the estimates for current smokers, potentially attenuating the results. To improve our assessment of smoking and second cancer risk, collaboration of additional cohorts with more frequently administered questionnaires is needed.
When assessing associations between risk factors and subsequent cancer diagnoses in cancer survivors, it is important to consider the impact of mortality. As cigarette smoking is associated with additional diverse causes of mortality, and deaths preclude second cancer diagnoses from occurring, competing deaths may distort the observed associations between smoking and second cancer risk. This is evidenced in our analysis restricted to 3-year survivors, which showed stronger associations between cigarette smoking and second cancer risk, and in our attenuated results for lung cancer survivors, who have the poorest prognosis of the survivor cohorts. Due to the competing risk of death, we have shown the cause-specific HR, which presents the association between smoking and second cancer risk in the presence of competing mortality. To highlight the importance of competing events in this analysis, we have also shown the association between cigarette smoking and death, as well as the absolute risk of mortality and second cancers, taking competing deaths into account. The strong associations between cigarette smoking and death reinforce the need for smoking cessation among cancer survivors.
In addition to increasing the risk of mortality and subsequent malignancies, cigarette smoking among cancer patients also increases surgical complications and toxicity from treatment with chemotherapy and radiation.32,33 The American Society for Clinical Oncology (ASCO) therefore advocates for the integration of tobacco cessation into clinical care.32 However, a recent survey of ASCO members found that only 58% of providers always advise their patients to quit smoking and only 39% usually provide treatment or refer patients for treatment for tobacco dependence.34
As the population of cancer survivors continues to grow, understanding risk factors for second cancers in this population is crucial. Cigarette smoking remains common in cancer survivors, even among those diagnosed with tobacco-related cancer.3–6 Cancer patients may not realize that they are at a higher risk of treatment complications, second primary cancers and death if they continue to smoke. Though more research is needed on smoking and second cancer risks, health care providers should emphasize the importance of smoking cessation to cancer patients.
Acknowledgment
We thank David Campbell and Leslie Carroll (Information Management Services, Calverton, MD) for programming support.
Glossary Terms
- cumulative risk:
a measure of risk of an event (usually disease occurrence) during a specified time period.
- Surveillance, Epidemiology, and End Results (SEER):
a national cancer registry that collects information from all incident malignancies in multiple geographic areas of the United States.
Appendix
Table A1.
Association Between Smoking Status and Cigarettes Smoked per Day and Risk of a Second Primary Smoking-Associated Cancer Among Survivors of Lung (stage I), Bladder, Kidney, and Head/Neck Cancers, Including Follow-Up Time and Event Occurring Within 30 Days of Diagnosis
| Smoking Status | Stage I Lung |
Bladder |
Kidney |
Head/Neck Cancer |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Never | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Former | ||||||||
| < 20 cig/d | 0.56 | 0.17 to 1.88 | 1.94 | 1.25 to 2.99 | 1.95 | 1.11 to 3.42 | 1.63 | 0.91 to 2.91 |
| ≥ 20 cig/d* | 1.09 | 0.39 to 3.05 | 2.22 | 1.45 to 3.40 | 3.86 | 2.24 to 6.65 | 3.08 | 1.81 to 5.25 |
| Current | ||||||||
| < 20 cig/d | 2.05 | 0.75 to 5.89 | 2.12 | 1.96 to 4.95 | 3.45 | 1.75 to 6.78 | 2.90 | 1.65 to 5.09 |
| ≥ 20 cig/d* | 2.08 | 0.73 to 5.59 | 4.10 | 2.54 to 6.62 | 5.01 | 2.49 to 10.1 | 4.84 | 2.80 to 8.36 |
| P trend† | .04 | < .001 | .001 | < .001 | ||||
NOTE. Adjusted for age, sex, race, education, body mass index, cohort, time from baseline to first cancer, and follow-up time. Excludes Alpha-Tocopherol, Beta-Carotene Cancer Prevention study, given that this cohort was limited to current smokers.
Abbreviations: cig/d, cigarettes per day; HR, hazard ratio.
Cigarettes per day was collected as a categorical variable in National Institutes of Health–AARP Diet and Health Study; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; and Agricultural Health Study. The maximum number of cigarettes smoked per day in the Iowa Women's Health Study was as follows: lung (stage I): 60; bladder: 40; kidney: 40; head and neck: 40.
P trends across joint categories of smoking status and intensity categories were estimated by including the categorical exposure variable in the model as a continuous variable.
Table A2.
Second Cancer Types After a Primary Cancer Diagnosis of Stage I Lung Cancer, Bladder Cancer, Kidney Cancer, and Head/Neck Cancer
| Location | Stage I Lung Cancer | Bladder Cancer | Kidney Cancer | Head/Neck Cancer |
|---|---|---|---|---|
| Lip | 1 | 2 | 0 | 0 |
| Tongue | 0 | 2 | 0 | 11 |
| Salivary gland | 1 | 2 | 0 | 4 |
| Floor of mouth | 2 | 2 | 1 | 4 |
| Gum and other mouth | 0 | 1 | 0 | 14 |
| Nasopharynx | 1 | 0 | 0 | 1 |
| Tonsil | 1 | 1 | 0 | 6 |
| Oropharynx | 0 | 1 | 0 | 4 |
| Hypopharynx | 2 | 1 | 0 | 5 |
| Other oral cavity and pharynx | 1 | 0 | 0 | 3 |
| Esophagus | 4 | 7 | 2 | 14 |
| Stomach | 4 | 14 | 6 | 5 |
| Small intestine | 1 | 3 | 2 | 1 |
| Cecum | 8 | 6 | 5 | 7 |
| Appendix | 0 | 0 | 1 | 0 |
| Ascending colon | 2 | 13 | 5 | 3 |
| Hepatic flexure | 0 | 6 | 2 | 0 |
| Transverse colon | 0 | 0 | 2 | 2 |
| Splenic flexure | 2 | 1 | 0 | 1 |
| Descending colon | 0 | 3 | 2 | 0 |
| Sigmoid colon | 2 | 17 | 5 | 9 |
| Large intestine, NOS | 0 | 3 | 1 | 1 |
| Rectosigmoid junction | 0 | 3 | 0 | 4 |
| Rectum | 1 | 17 | 1 | 6 |
| Anus, anal canal, and anorectum | 0 | 0 | 0 | 3 |
| Liver | 3 | 9 | 2 | 7 |
| Intrahepatic bile duct | 0 | 1 | 1 | 0 |
| Gallbladder | 0 | 2 | 1 | 0 |
| Other biliary | 0 | 1 | 0 | 1 |
| Pancreas | 11 | 19 | 9 | 14 |
| Nose, nasal cavity, and middle ear | 0 | 1 | 0 | 0 |
| Peritoneum, omentum, and mesentery | 0 | 0 | 1 | 0 |
| Other digestive organs | 0 | 0 | 0 | 1 |
| Larynx | 7 | 11 | 3 | 5 |
| Lung and bronchus | 74 | 198 | 57 | 153 |
| Trachea, mediastinum, and other respiratory organs | 1 | 0 | 0 | 3 |
| Bones and joints | 0 | 0 | 1 | 0 |
| Soft tissue including heart | 1 | 2 | 2 | 0 |
| Melanoma | 6 | 37 | 15 | 12 |
| Other nonepithelial skin | 0 | 0 | 0 | 1 |
| Breast | 19 | 22 | 24 | 15 |
| Cervix | 0 | 0 | 1 | 1 |
| Corpus uteri | 1 | 3 | 2 | 0 |
| Ovary | 2 | 3 | 0 | 3 |
| Vagina | 1 | 1 | 0 | 0 |
| Vulva | 0 | 1 | 0 | 1 |
| Prostate | 61 | 365 | 101 | 70 |
| Testis | 0 | 1 | 0 | 0 |
| Ureter | 0 | 2 | 0 | 1 |
| Urinary bladder | 10 | 13 | 31 | 23 |
| Kidney and renal pelvis | 14 | 33 | 22 | 10 |
| Other urinary organs | 0 | 3 | 1 | 2 |
| Eye and orbit | 1 | 3 | 2 | 0 |
| Brain | 1 | 3 | 3 | 2 |
| Cranial nerves, other nervous system | 0 | 0 | 1 | 0 |
| Thyroid | 2 | 7 | 7 | 4 |
| Hodgkin nodal | 0 | 1 | 2 | 0 |
| NHL nodal | 11 | 23 | 6 | 8 |
| Extranodal | 5 | 6 | 1 | 2 |
| Myeloma | 1 | 6 | 8 | 3 |
| Chronic lymphocytic leukemia | 3 | 9 | 2 | 3 |
| Acute myeloid leukemia | 2 | 10 | 2 | 4 |
| Chronic myeloid leukemia | 1 | 2 | 1 | 0 |
| Acute monocytic leukemia | 0 | 1 | 0 | 0 |
| Aleukemic, subleukemic NOS | 0 | 0 | 0 | 1 |
| Mesothelioma | 1 | 4 | 1 | 1 |
| Miscellaneous | 4 | 26 | 5 | 8 |
| Invalid | 1 | 5 | 2 | 3 |
NOTE. Bold indicates same-site multiple primaries that were censored in each site-specific analysis.
Abbreviations: NHL, non-Hodgkin lymphoma; NOS, not otherwise specified.
Table A3.
Association Between Smoking Status and Risk of a Second Primary Smoking-Associated Cancer Among 3-Year Survivors Of Lung (stage I), Bladder, Kidney, and Head/Neck Cancers
| Smoking Status | Stage I Lung Cancer |
Bladder Cancer |
Kidney Cancer |
Head/Neck Cancer |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | |||||
| No | Yes | No | Yes | No | Yes | No | Yes | |||||||||
| Never | 101 | 1 | 1.00 | Referent | 606 | 12 | 1.00 | Referent | 516 | 5 | 1.00 | Referent | 305 | 4 | 1.00 | Referent |
| Former | ||||||||||||||||
| < 20 cig/d | 213 | 3 | 1.52 | 0.15 to 15.0 | 826 | 32 | 1.76 | 0.90 to 3.44 | 360 | 10 | 2.32 | 0.77 to 6.97 | 287 | 18 | 3.74 | 1.25 to 11.1 |
| ≥ 20 cig/d* | 309 | 7 | 2.59 | 0.30 to 22.4 | 976 | 43 | 2.05 | 1.06 to 3.95 | 262 | 16 | 5.51 | 1.92 to 15.8 | 290 | 26 | 5.12 | 1.76 to 14.9 |
| Current | ||||||||||||||||
| < 20 cig/d | 247 | 6 | 2.87 | 0.34 to 24.4 | 340 | 25 | 3.11 | 1.55 to 6.25 | 99 | 7 | 5.80 | 1.80 to 18.7 | 209 | 25 | 7.23 | 2.49 to 21.0 |
| ≥ 20 cig/d* | 175 | 6 | 6.02 | 0.67 to 54.0 | 238 | 19 | 3.95 | 1.89 to 8.27 | 64 | 5 | 6.61 | 1.81 to 24.1 | 148 | 23 | 9.25 | 3.15 to 27.2 |
| P trend† | .04 | < .001 | .001 | < .001 | ||||||||||||
NOTE. Adjusted for age, sex, race, education, body mass index, cohort, time from baseline to first cancer, and follow-up time. Excludes Alpha-Tocopherol, Beta-Carotene Cancer Prevention study, given that this cohort was limited to current smokers.
Abbreviations: cig/d, cigarettes per day; HR, hazard ratio.
Cigarettes per day was collected as a categorical variable in National Institutes of Health–AARP Diet and Health Study; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; and Agricultural Health Study. The maximum number of cigarettes smoked per day in the Iowa Women's Health Study was as follows: lung (stage I): 60; bladder: 40; kidney: 40; head and neck: 40.
P trends across joint categories of smoking status and intensity categories were estimated by including the categorical exposure variable in the model as a continuous variable.
Table A4.
Association Between Smoking Status and Risk of a Second Primary Smoking-Associated Cancer Among Survivors of Lung, Bladder, Kidney, and Head/Neck Cancers
| First Primary Cancer | Cohort | Smoking Status | No Second Cancer | Second Cancer | HR | 95% CI |
|---|---|---|---|---|---|---|
| Stage I Lung | NIH-AARP | Never | 77 | 0 | 1.00 | |
| Former | 698 | 16 | > 1,000 | — | ||
| Current | 501 | 16 | > 1,000 | — | ||
| AHS | Never | 19 | 0 | 1.00 | ||
| Former | 29 | 1 | 19.85 | — | ||
| Current | 34 | 1 | 0.23 | — | ||
| IWHS | Never | 29 | 1 | 1.00 | ||
| Former | 42 | 0 | 0.00 | — | ||
| Current | 95 | 4 | 1.46 | 0.08 to 27.54 | ||
| PLCO | Never | 61 | 2 | 1.00 | ||
| Former | 325 | 8 | 0.48 | 0.09 to 2.49 | ||
| Current | 242 | 14 | 1.31 | 0.26 to 6.58 | ||
| Bladder | NIH-AARP | Never | 656 | 14 | 1.00 | |
| Former | 2,529 | 153 | 2.64 | 1.52 to 4.59 | ||
| Current | 762 | 60 | 3.63 | 2.02 to 6.52 | ||
| AHS | Never | 73 | 2 | 1.00 | ||
| Former | 113 | 2 | 0.52 | 0.06 to 4.84 | ||
| Current | 54 | 2 | 0.64 | 0.07 to 6.19 | ||
| IWHS | Never | 101 | 6 | 1.00 | ||
| Former | 52 | 1 | 0.32 | 0.04 to 2.73 | ||
| Current | 56 | 9 | 2.36 | 0.73 to 7.63 | ||
| PLCO | Never | 250 | 6 | 1.00 | ||
| Former | 629 | 28 | 1.77 | 0.72 to 4.36 | ||
| Current | 199 | 19 | 4.53 | 1.74 to 11.8 | ||
| Kidney | NIH-AARP | Never | 579 | 12 | 1.00 | |
| Former | 1,011 | 47 | 2.23 | 1.17 to 4.25 | ||
| Current | 274 | 19 | 3.74 | 1.79 to 7.79 | ||
| AHS | Never | 92 | 0 | 1.00 | ||
| Former | 69 | 2 | — | — | ||
| Current | 32 | 0 | — | — | ||
| IWHS | Never | 126 | 4 | 1.00 | ||
| Former | 39 | 1 | 1.12 | 0.10 to 12.7 | ||
| Current | 24 | 2 | 7.74 | 0.76 to 78.8 | ||
| PLCO | Never | 232 | 3 | 1.00 | ||
| Former | 252 | 21 | 5.45 | 1.56 to 19.05 | ||
| Current | 66 | 5 | 5.77 | 1.29 to 25.85 | ||
| Head/neck | NIH-AARP | Never | 319 | 10 | 1.00 | |
| Former | 814 | 67 | 2.43 | 1.24 to 4.76 | ||
| Current | 540 | 65 | 3.97 | 2.02 to 7.82 | ||
| AHS | Never | 63 | 1 | 1.00 | ||
| Former | 46 | 5 | 1.37 | 0.10 to 18.6 | ||
| Current | 51 | 4 | 1.78 | 0.13 to 25.2 | ||
| IWHS | Never | 82 | 3 | 1.00 | ||
| Former | 30 | 1 | 2.23 | 0.37 to 13.5 | ||
| Current | 37 | 4 | 1.24 | 0.11 to 14.3 | ||
| PLCO | Never | 78 | 4 | 1.00 | ||
| Former | 189 | 27 | 2.39 | 0.81 to 7.05 | ||
| Current | 125 | 20 | 3.66 | 1.18 to 11.3 |
NOTE. Adjusted for age, sex, race, education, body mass index, cohort, time from baseline to first cancer, and follow-up time. Excludes Alpha-Tocopherol, Beta-Carotene Cancer Prevention study, as this cohort was limited to current smokers.
Abbreviations: AHS, Agricultural Health Study; HR, hazard ratio, IWHS, Iowa Women's Health Study; NIH-AARP, National Institutes of Health-AARP Diet and Health Study; PLCO, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial.
Table A5.
Association Between Smoking Status and Risk of a First Primary Smoking-Associated Cancer
| Smoking Status | Cancer Case |
HR | 95% CI | |
|---|---|---|---|---|
| No | Yes | |||
| Never | 307,651 | 11,052 | 1.00 | Referent |
| Former | ||||
| < 20 cig/d | 190,400 | 11,025 | 1.49 | 1.45 to 1.53 |
| ≥ 20 cig/d | 126,965 | 12,175 | 2.35 | 2.29 to 2.42 |
| Current | ||||
| < 20 cig/d | 54,956 | 6,859 | 3.72 | 3.60 to 3.83 |
| ≥ 20 cig/d | 27,797 | 4,957 | 5.41 | 5.22 to 5.60 |
| P trend* | < .001 | |||
NOTE. Adjusted for age, sex, race, education, body mass index, cohort, time from baseline to first cancer, and follow-up time. Excludes Alpha-Tocopherol, Beta-Carotene Cancer Prevention study, as this cohort was limited to current smokers.
Abbreviations: cig/d, cigarettes per day; HR, hazard ratio.
P trends across joint categories of smoking status and intensity categories were estimated by including the categorical exposure variable in the model as a continuous variable.
Table A6.
Association Between Smoking Status and Risk of a Second Primary Smoking-Associated Cancer Among Survivors of Head/Neck Cancer, by Site
| Smoking Status | Oral Cavity |
Oropharynx |
Other Head/Neck |
Larynx |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | Second Cancer |
HR | 95% CI | |||||
| No | Yes | No | Yes | No | Yes | No | Yes | |||||||||
| Never | 198 | 5 | 1.00 | Referent | 123 | 4 | 1.00 | Referent | 149 | 7 | 1.00 | Referent | 72 | 2 | 1.00 | Referent |
| Former | ||||||||||||||||
| < 20 cig/d | 143 | 8 | 1.92 | 0.62 to 5.98 | 108 | 7 | 1.31 | 0.37 to 4.64 | 106 | 8 | 1.84 | 0.62 to 5.46 | 154 | 9 | 1.92 | 0.41 to 8.94 |
| ≥ 20 cig/d* | 122 | 15 | 3.58 | 1.22 to 10.5 | 152 | 11 | 1.29 | 0.40 to 4.16 | 120 | 7 | 1.34 | 0.42 to 4.28 | 173 | 33 | 7.20 | 1.72 to 30.2 |
| Current | ||||||||||||||||
| < 20 cig/d | 81 | 14 | 5.22 | 1.78 to 15.3 | 85 | 6 | 2.81 | 0.74 to 10.6 | 83 | 6 | 1.47 | 0.44 to 4.94 | 159 | 16 | 4.06 | 0.92 to 17.9 |
| ≥ 20 cig/d* | 61 | 12 | 6.29 | 2.10 to 18.8 | 84 | 6 | 2.38 | 0.65 to 8.73 | 58 | 9 | 3.59 | 1.12 to 11.5 | 142 | 24 | 8.20 | 1.89 to 35.6 |
| P trend† | < .001 | .09 | .09 | < .001 | ||||||||||||
NOTE. Adjusted for age, sex, race, education, body mass index, cohort, time from baseline to first cancer, and follow-up time. Excludes Alpha-Tocopherol, Beta-Carotene Cancer Prevention study, as this cohort was limited to current smokers.
Abbreviations: cig/d, cigarettes per day; HR, hazard ratio.
Cigarettes per day was collected as a categorical variable in National Institutes of Health–AARP Diet and Health Study; Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial; and Agricultural Health Study. The maximum number of cigarettes smoked per day in the Iowa Women's Health Study was as follows: lung (stage I): 60; bladder: 40; kidney: 40; head and neck: 40.
P trends across joint categories of smoking status and intensity categories were estimated by including the categorical exposure variable in the model as a continuous variable.
Fig A1.
Five-year cumulative incidence of second smoking-associated malignancies and death among survivors of first primary (A) stage I lung, (B) bladder, (C) kidney, and (D) head/neck cancers. Gold lines represent current smokers, gray lines represent former smokers, and blue lines represent never-smokers. Solid lines indicate 5-year cumulative incidence of death and dashed lines indicate 5-year cumulative incidence of smoking-associated second cancers.
Footnotes
See accompanying article on page 4004
Supported in part by the Intramural program of the National Cancer Institute, National Institutes of Health. The Iowa Women's Health Study was funded by National Cancer Institute Grant No. R01 CA39742.
Presented at the Cohort Consortium Annual Meeting, Rockville, MD, November 18 and 19, 2013.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Meredith S. Shiels, Todd Gibson, Amanda Black, Lindsay M. Morton
Collection and assembly of data: Meredith S. Shiels, Todd Gibson, Demetrius Albanes, Kim Robien, Amanda Black, Lindsay M. Morton
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cigarette Smoking Prior to First Cancer and Risk of Second Smoking-Associated Cancers Among Survivors of Bladder, Kidney, Head and Neck, and Stage I Lung Cancers
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Meredith S. Shiels
No relationship to disclose
Todd Gibson
No relationship to disclose
Joshua Sampson
No relationship to disclose
Demetrius Albanes
No relationship to disclose
Gabriella Andreotti
No relationship to disclose
Laura Beane Freeman
Employment: Procter & Gamble (I)
Leadership: Procter & Gamble (I)
Stock or Other Ownership: Procter & Gamble (I)
Amy Berrington de Gonzalez
No relationship to disclose
Neil Caporaso
No relationship to disclose
Rochelle E. Curtis
No relationship to disclose
Joanne Elena
No relationship to disclose
Neal D. Freedman
No relationship to disclose
Kim Robien
No relationship to disclose
Amanda Black
No relationship to disclose
Lindsay M. Morton
No relationship to disclose
REFERENCES
- 1.Parry C, Kent EE, Mariotto AB, et al. Cancer survivors: A booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Part E: Personal Habits and Indoor Combustions, in (ed 100E) Lyon, France: International Agency for Research on Cancer; 2012. A Review of Human Carcinogens. [PMC free article] [PubMed] [Google Scholar]
- 3.Bellizzi KM, Rowland JH, Jeffery DD, et al. Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. J Clin Oncol. 2005;23:8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 4.Bassett JC, Gore JL, Chi AC, et al. Impact of a bladder cancer diagnosis on smoking behavior. J Clin Oncol. 2012;30:1871–1878. doi: 10.1200/JCO.2011.36.6518. [DOI] [PubMed] [Google Scholar]
- 5.Cooley ME, Sarna L, Kotlerman J, et al. Vol. 66. Amsterdam, Netherlands: Lung Cancer; 2009. Smoking cessation is challenging even for patients recovering from lung cancer surgery with curative intent; pp. 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker MS, Vidrine DJ, Gritz ER, et al. Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2370–2377. doi: 10.1158/1055-9965.EPI-06-0509. [DOI] [PubMed] [Google Scholar]
- 7.Curtis RE, Freedman DM, Ron E, et al. Bethesda, MD: National Cancer Institute; 2006. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. [Google Scholar]
- 8.Richardson GE, Tucker MA, Venzon DJ, et al. Smoking cessation after successful treatment of small-cell lung cancer is associated with fewer smoking-related second primary cancers. Ann Intern Med. 1993;119:383–390. doi: 10.7326/0003-4819-119-5-199309010-00006. [DOI] [PubMed] [Google Scholar]
- 9.Tucker MA, Murray N, Shaw EG, et al. Second primary cancers related to smoking and treatment of small-cell lung cancer: Lung Cancer Working Cadre. J Natl Cancer Inst. 1997;89:1782–1788. doi: 10.1093/jnci/89.23.1782. [DOI] [PubMed] [Google Scholar]
- 10.Kawahara M, Ushijima S, Kamimori T, et al. Second primary tumours in more than 2-year disease-free survivors of small-cell lung cancer in Japan: The role of smoking cessation. Br J Cancer. 1998;78:409–412. doi: 10.1038/bjc.1998.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons A, Daley A, Begh R, et al. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: Systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Do KA, Johnson MM, Lee JJ, et al. Longitudinal study of smoking patterns in relation to the development of smoking-related secondary primary tumors in patients with upper aerodigestive tract malignancies. Cancer. 2004;101:2837–2842. doi: 10.1002/cncr.20714. [DOI] [PubMed] [Google Scholar]
- 13.Gan SJ, Dahlstrom KR, Peck BW, et al. Incidence and pattern of second primary malignancies in patients with index oropharyngeal cancers versus index nonoropharyngeal head and neck cancers. Cancer. 2013;119:2593–2601. doi: 10.1002/cncr.28107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Léon X, del Prado Venegas M, Orús C, et al. Influence of the persistence of tobacco and alcohol use in the appearance of second neoplasm in patients with a head and neck cancer: A case-control study. Cancer Causes Control. 2009;20:645–652. doi: 10.1007/s10552-008-9277-8. [DOI] [PubMed] [Google Scholar]
- 15.Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low-dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. J Natl Cancer Inst. 2006;98:441–450. doi: 10.1093/jnci/djj091. [DOI] [PubMed] [Google Scholar]
- 16.The Alpha-Tocopherol, Beta-Carotene Lung Cancer Prevention Study. Design, methods, participant characteristics, and compliance: The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 17.Alavanja MC, Sandler DP, McMaster SB, et al. The Agricultural Health Study. Environ Health Perspect. 1996;104:362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisgard KM, Folsom AR, Hong CP, et al. Mortality and cancer rates in nonrespondents to a prospective study of older women: 5-year follow-up. Am J Epidemiol. 1994;139:990–1000. doi: 10.1093/oxfordjournals.aje.a116948. [DOI] [PubMed] [Google Scholar]
- 19.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 20.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: The National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154:1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 21.Black A, Gibson TM, Shiels MS, et al. Pooling prospective studies to investigate the etiology of second cancers. Cancer Epidemiol Biomarkers Prev. 2014;23:1598–1608. doi: 10.1158/1055-9965.EPI-14-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surveillance Epidemiology and End Results [SEER] program. Site Recode ICD-O-3 (1/27/2003) Definition, 2013. http://seer.cancer.gov/siterecode/icdo3_d01272003/
- 23.World Health Organization. International Classification of Diseases for Oncology (ed 3) Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 24.Johnson C, Peace S, Adamo P, et al. Bethesda, MD: National Cancer Institute; 2013. Multiple Primary and Histology Coding Rules. [Google Scholar]
- 25.Murphy SA, Sen PK. Time-dependent coefficients in a Cox-type regression model. Stoch Process Their Appl. 1991;39:153–180. [Google Scholar]
- 26.Horton NJ, Kleinman KP. Much ado about nothing: A comparison of missing data methods and software to fit incomplete data regression models. Am Stat. 2007;61:79–90. doi: 10.1198/000313007X172556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;4:103–112. [Google Scholar]
- 28.Bakoyannis G, Touloumi G. Practical methods for competing risks data: A review. Stat Methods Med Res. 2012;21:257–272. doi: 10.1177/0962280210394479. [DOI] [PubMed] [Google Scholar]
- 29.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 30.Gail M. A review and critique of some models used in competing risk analysis. Biometrics. 1975;31:209–222. [PubMed] [Google Scholar]
- 31.Tsiatis A. A nonidentifiability aspect of the problem of competing risks. Proc Natl Acad Sci U S A. 1975:72. doi: 10.1073/pnas.72.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanna N, Mulshine J, Wollins DS, et al. Tobacco cessation and control a decade later: American Society of Clinical Oncology policy statement update. J Clin Oncol. 2013;31:3147–3157. doi: 10.1200/JCO.2013.48.8932. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Department of Health and Human Services. The health consequences of smoking: 50 years of progress—A report of the Surgeon General in. 2014.
- 34.Warren GW, Marshall JR, Cummings KM, et al. Addressing tobacco use in patients with cancer: A survey of American Society of Clinical Oncology members. J Oncol Pract. 2013;9:258–262. doi: 10.1200/JOP.2013.001025. [DOI] [PMC free article] [PubMed] [Google Scholar]