Abstract
We have established a cohort of 64 Natural Viral Suppressors (NVS) (similar to Elite Controllers/Elite Suppressors), 30 of which have chronic HCV. We investigated T cell phenotypic changes in association with HCV infection. NVS without HCV and normal controls had similar T cell phenotypes. However, NVS with HCV had lower naïve cell proportions (CD4 and CD8) compared to NVS without HCV (p=.0008 and p=.02) or normal controls (p=.0163 and p=.017). These results and previous reported data suggest that HCV co-infection increases immune activation and T cell disturbances. Any associated T cell functional changes or potential clinical consequences need further study.
The Natural Viral Suppressors (NVS) cohort is comprised of HIV-infected patients with the ability to suppress HIV-1 to undetectable levels in the absence of therapy [1-4]. We have established a cohort of 64 NVS patients, of which 46.9% have chronic HCV infection (positive PCR for HCV). We previously reported that within this NVS cohort, patients with HCV infection had lower CD4 counts, CD4 count percentages, CD4/CD8 ratio, and higher levels of immune activation than NVS without HCV infection [4]. In this study, we further investigated whether there are specific phenotypic immunological deficits associated with HCV infection in this cohort.
The NVS cohort and controls (HIV/HCV negative individuals, HCV monoinfected patients, and non-NVS HIV and HIV/HCV coinfected) have been described in detail elsewhere [1-4]. Briefly, after informed consent was obtained, NVS patients have demonstrated viral loads <400 copies/ml for a 2 year time period without antiretroviral therapy. Flow cytomtery was performed by using FACSCalibur flow (BD Biosciences, San Jose, CA). PBMCs were stained with either CD4 (APC) or CD8 (APC), and CD3 (PerCP), CD45RA (FITC), and CCR7 (PE) and optimized according to the experiment. Data was analyzed by using FlowJo software (Ashland, OR). For data with normal distribution Student’s t test was performed; otherwise, the Mann-Whitney test was used. All p values were two-tailed and considered significant if < .05.
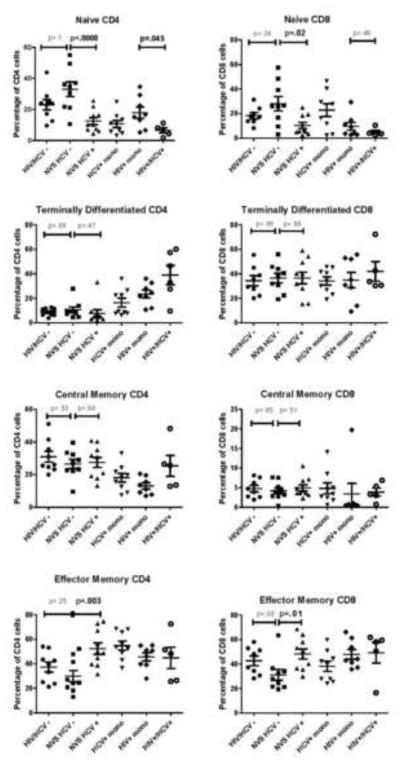
T cell subsets were measured in the NVS with HCV infection (n=10), NVS without HCV infection (n=9), HIV−/HCV− controls (n=8), HCV+ monoinfected (n=9), HIV+ monoinfected (n=8), and HIV+/HCV+ coinfected patients (n=5) (Figure 1). NVS without HCV had a trend toward greater CD4 and CD8 cell naïve cell proportions than the normal controls who are HCV and HIV negative (p=.1 and p=.24, respectively). In contrast, NVS with HCV had lower naïve cell proportions (CD4 and CD8) compared to NVS without HCV (p=.0008 and p=.02, respectively) or normal controls (p=.0163 and p=.017, respectively). NVS with HCV also had higher effector memory cell proportions (CD4 and CD8) compared to NVS without HCV (p=.003 and p=.01, respectively). Within the NVS, there was no significant difference in central memory or terminally differentiated cell compartments in relation to HCV infection. Interestingly, the naïve CD4 cell population was also lower in our HIV/HCV controls compared to HIV monoinfected controls (p=.045).
Figure 1.
T cell subsets in the NVS and controls. PBMCs were divided into 2 groups and stained with either CD3 (PerCP), CD4 (APC), CD45RA (FITC), and CCR7 (PE); or CD3 (PerCP), CD8 (APC), CD45RA (FITC), and CCR7 (PE). After gating on the lymphocyte population (Forward Scatter vs. Side Scatter), either the CD3+CD4+ or CD3+CD8+ were analyzed for subsets based on CCR7 and CD45RA. Naïve Cells=CD45+CCR7+; Central Memory Cells=CD45-CCR7+; Effector Memory Cells=CD45-CCR7−; Terminally Differentiated Cells=CD45+CCR7−. mono= mono-infected
The clinical consequences of altered T cell subsets, specifically reduction in the proportion of naïve T cells, are not well characterized in the context of HCV infection. We evaluated clinical outcomes in the NVS group based on HCV infection. Compared to NVS without HCV, NVS with HCV have higher prevalence rates of cancer (23.3% vs. 8.8%), death (16.7% vs. 5.9%), and coronary artery disease (6.7% vs. 0%) after HIV-1 diagnosis. Of note, none of the deaths or cancers were directly attributable to HCV. The number of events was too small to carry out meaningful statistical analysis.
In this study, we demonstrate further immunological deficits as well as possible differences in clinical outcome based on the presence or absence of HCV coinfection. Proportions of CD4 and CD8 naïve cells were similar in NVS without HCV and normal controls, suggesting preservation of these subsets in NVS without HCV. NVS with HCV had lower naïve cell proportions (CD4 and CD8) compared to NVS without HCV or normal controls, suggesting a loss of this subset in association with dual HIV/HCV infection. Reduced number of naïve T cells are a hallmark of HIV infection [5-7], however, there is only one report of HCV causing a reduction of naïve T cell populations [8]. In this study we also note lower CD4 naive cell proportion in HIV/HCV controls compared to HIV monoinfected controls. Higher levels of apoptosis of naïve CD4 and CD8 cells in HIV/HCV infection, as reported by Nunez et al [9], could explain our findings of immune activation, lower CD4 cells, and lower naïve cells.
Taken together, the most likely explanation for the above data is that HCV affects the course of HIV infection in the NVS. HCV has not been reported to cause a decreased CD4 count or increased immune activation in HCV mono-infected individuals [10]. However, we hypothesize that in the setting of HIV infection, HCV co-infection can increase immune activation, and cause T cell disturbances (decreased CD4 count, decreased naïve T cells, increased effector T cells). This interaction between HIV and HCV as it pertains to T cell dynamics has still not been consistently demonstrated before. Recently, HIV/HCV coinfected patients have been shown to have increased levels of CD8 activation compared to HIV monoinfected patients [11-13], with HCV treatment leading to a decrease in immune activation [11].
Age and duration of HIV infection need to be considered in interpreting our data. NVS are both aging (median age 53) and have carried HIV infection for decades (median year of HIV diagnosis 1995), although these were equally matched in the NVS HCV positive and negative groups. Aging is known to affect the normal immune system [14-16], and thus it is entirely plausible that the presence of 2 agents known to cause immune changes/deficits could lead to worse immunologic and clinical outcomes. One reason this may have not been noted before is that the median year of HIV diagnosis of the NVS predates the HAART era, and these defects may not be seen in HAART-infected cohorts for several more years.
In conclusion, NVS patients with chronic HCV infection have lower proportions of naïve CD4 and CD8 cells, and elevated effector memory CD4 and CD8 cells. Lower proportion of naïve CD4 cells were noted in the HIV/HCV controls (non-NVS) when compared to the HIV monoinfected controls. There appears to be higher rates of clinical complications (CAD, cancer, and death) in the NVS with chronic HCV infection compared to those without HCV infection. The data suggests that in the NVS cohort (elite controllers) HCV coinfection detrimentally alters the natural history of HIV infection. Further studies will be needed to evaluate potential functional deficits associated with HCV coinfection, and if HCV treatment leads to lower immune activation levels and rise in CD4 counts in these patients.
Acknowledgments
We would like to thank members of the NVS cohort, as well as Becky Boyce and Tisha Trinh, the study coordinators.
Funding: M.M.S. supported by award number 5K23AI084580-03
Footnotes
Conflict of Interest: M.M.S., R.T., R.P. and R.R.R. report no conflict of interests.
References
- 1.Sajadi MM, Constantine NT, Mann DL, Charurat M, Dadzan E, Kadlecik P, Redfield RR. Epidemiologic characteristics and natural history of HIV-1 natural viral suppressors. J Acquir Immune Defic Syndr. 2009;50:403–408. doi: 10.1097/QAI.0b013e3181945f1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sajadi MM, Heredia A, Le N, Constantine NT, Redfield RR. HIV-1 natural viral suppressors: control of viral replication in the absence of therapy. AIDS. 2007;21:517–519. doi: 10.1097/QAD.0b013e328013d9eb. [DOI] [PubMed] [Google Scholar]
- 3.Sajadi MM, Pulijala R, Redfield RR, Talwani R. Chronic immune activation and decreased CD4 counts associated with Hepatitis C Infection in HIV-1 Natural Viral Suppressors. AIDS. 2012 doi: 10.1097/QAD.0b013e328357f5d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sajadi MM, Shakeri N, Talwani R, Redfield RR. Hepatitis C infection in HIV-1 natural viral suppressors. AIDS. 2010;24:1689–1695. doi: 10.1097/QAD.0b013e32833a2a32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rickabaugh TM, Kilpatrick RD, Hultin LE, Hultin PM, Hausner MA, Sugar CA, et al. The dual impact of HIV-1 infection and aging on naive CD4 T-cells: additive and distinct patterns of impairment. PLoS One. 6:e16459. doi: 10.1371/journal.pone.0016459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabin RL, Roederer M, Maldonado Y, Petru A, Herzenberg LA. Altered representation of naive and memory CD8 T cell subsets in HIV-infected children. J Clin Invest. 1995;95:2054–2060. doi: 10.1172/JCI117891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yonkers NL, Sieg S, Rodriguez B, Anthony DD. Reduced naive CD4 T cell numbers and impaired induction of CD27 in response to T cell receptor stimulation reflect a state of immune activation in chronic hepatitis C virus infection. J Infect Dis. 2011;203:635–645. doi: 10.1093/infdis/jiq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunez M, Soriano V, Lopez M, Ballesteros C, Cascajero A, Gonzalez-Lahoz J, Benito JM. Coinfection with hepatitis C virus increases lymphocyte apoptosis in HIV-infected patients. Clin Infect Dis. 2006;43:1209–1212. doi: 10.1086/508355. [DOI] [PubMed] [Google Scholar]
- 10.Cacoub P, Musset L, Hausfater P, Ghillani P, Fabiani FL, Charlotte F, et al. No evidence for abnormal immune activation in peripheral blood T cells in patients with hepatitis C virus (HCV) infection with or without cryoglobulinaemia. Multivirc Group. Clin Exp Immunol. 1998;113:48–54. doi: 10.1046/j.1365-2249.1998.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez VD, Falconer K, Blom KG, Reichard O, Morn B, Laursen AL, et al. High levels of chronic immune activation in the T-cell compartments of patients coinfected with hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by alpha interferon and ribavirin treatment. J Virol. 2009;83:11407–11411. doi: 10.1128/JVI.01211-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs A, Al-Harthi L, Christensen S, Mack W, Cohen M, Landay A. CD8(+) T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis. 2008;197:1402–1407. doi: 10.1086/587696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacs A, Karim R, Mack WJ, Xu J, Chen Z, Operskalski E, et al. Activation of CD8 T cells predicts progression of HIV infection in women coinfected with hepatitis C virus. J Infect Dis. 2010;201:823–834. doi: 10.1086/650997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J Immunol. 1998;160:1627–1637. [PubMed] [Google Scholar]
- 15.Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, et al. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–1507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]