Abstract
The transcription factor ThPOK promotes CD4+ T cell differentiation in the thymus. Here, using a mouse strain that allows post-thymic gene deletion, we show that ThPOK maintains CD4+ T lineage integrity and couples effector differentiation to environmental cues after antigenic stimulation. ThPOK preserved the integrity and amplitude of effector responses, and was required for proper TH1 and TH2 differentiation in vivo by restraining the expression and function of the transcriptional regulator of cytotoxic T cell differentiation, Runx3. The transcription factor LRF contributed in a redundant manner with ThPOK to prevent the trans-differentiation of mature CD4+ T cells into CD8+ T cells. As such, the ThPOK-LRF transcriptional module was essential for CD4+ T cell integrity and responses.
CD4+ T cells typically recognize peptide antigens presented by the class II major histocompatibility complex (MHC class II) molecules, and upon activation differentiate into subtypes of effector helper T cells (TH) expressing specific transcription factors and cytokines1,2. Such effector T cell fates include TH1, which express interferon γ (IFN-γ) and the transcription factors T-bet and Runx3, TH2, which express interleukin 4 (IL-4) IL-13 and the transcription factor GATA3, or TH17, which express IL-17 and the transcription factor RORγt. In contrast, MHC class I-restricted T cells express CD8 and typically differentiate into cytotoxic effectors after antigen stimulation3.
CD4+ and CD8+ T cells differentiate in the thymus from CD4+CD8+ (double positive, DP) precursors that lack functional potential4. Commitment to either lineage involves the mutually exclusive expression of two transcription factors, ThPOK and Runx35–8, so that CD4+ T cells express ThPOK but no Runx3, whereas the opposite is true of CD8+ T cells9. Current views propose that these factors participate in a dual negative regulatory loop, in which ThPOK represses the genes encoding Runx3 and CD8 subunits (CD8α and CD8β), and Runx3, redundantly with the related molecule Runx1, represses those encoding ThPOK and CD410–13. Such a model accounts for the observation that intrathymic or germline disruption of ThPOK unleashes Runx3 expression and ‘redirects’ MHC class II-restricted thymocytes into CD8+ T cells7.
In addition to controlling CD4 and CD8 expression, ThPOK contributes to thymocyte functional differentiation. Even though effector differentiation is manifest only upon antigen activation, mature thymocytes are functionally ‘pre-programmed’ as helper or cytotoxic and express genes specific of either fate9. Redundantly with the related transcription factor LRF14, ThPOK is required in the thymus for helper ‘pre-programming’, as ThPOK and LRF-deficient MHC II-restricted thymocytes fail to express CD40L, a CD4+-lineage specific molecule involved in multiple aspects of CD4+ T cell function15, and to give rise to functional TH cells16.
Although ThPOK remains highly expressed in peripheral CD4+ T cells7,10–12, little is known about its role in these cells, whether before (naïve T cells) or after (T effector cells) antigen contact. Because TH1 effector cells co-express ThPOK and Runx3, it remains unclear whether post thymic ThPOK represses Runx3. While ThPOK disruption increases the expression of the cytotoxic enzyme Granzyme B and that of IFN-γ in CD4+ T effector cells10, the biological impact of ThPOK disruption remains to be determined. It has been proposed that ThPOK expression in intestinal CD4+ T cells promotes gut inflammation by sustaining TH17 responses17, but whether ThPOK affects CD4+ T effector cell differentiation in vivo remains unknown.
In this study, we used a mouse strain expressing the Cre recombinase in post-thymic T cells to inactivate ThPOK in naïve CD4+ T cells, prior to activation and effector differentiation. We show that post-thymic ThPOK restrains the expression of Runx3 in resting and activated CD4+ T cells and is needed for TH2, but not for TH17, effector responses. In addition, even though Runx3 promotes expression of the TH1 cytokine IFN-γ18,19, ThPOK was required for TH1 differentiation and prevented the diversion of TH1 CD4+ cells to a cytotoxic gene expression program. Last, we demonstrate that ThPOK and LRF redundantly prevented the trans-differentiation of CD4+ into CD8+ T cells. These findings demonstrate that ThPOK is essential to preserve the functional diversity of CD4+ T cells and the proper matching of CD4+ effector responses to the cytokine environment conditioning effector differentiation.
Results
Post-thymic Thpok inactivation in resting CD4+ T cell
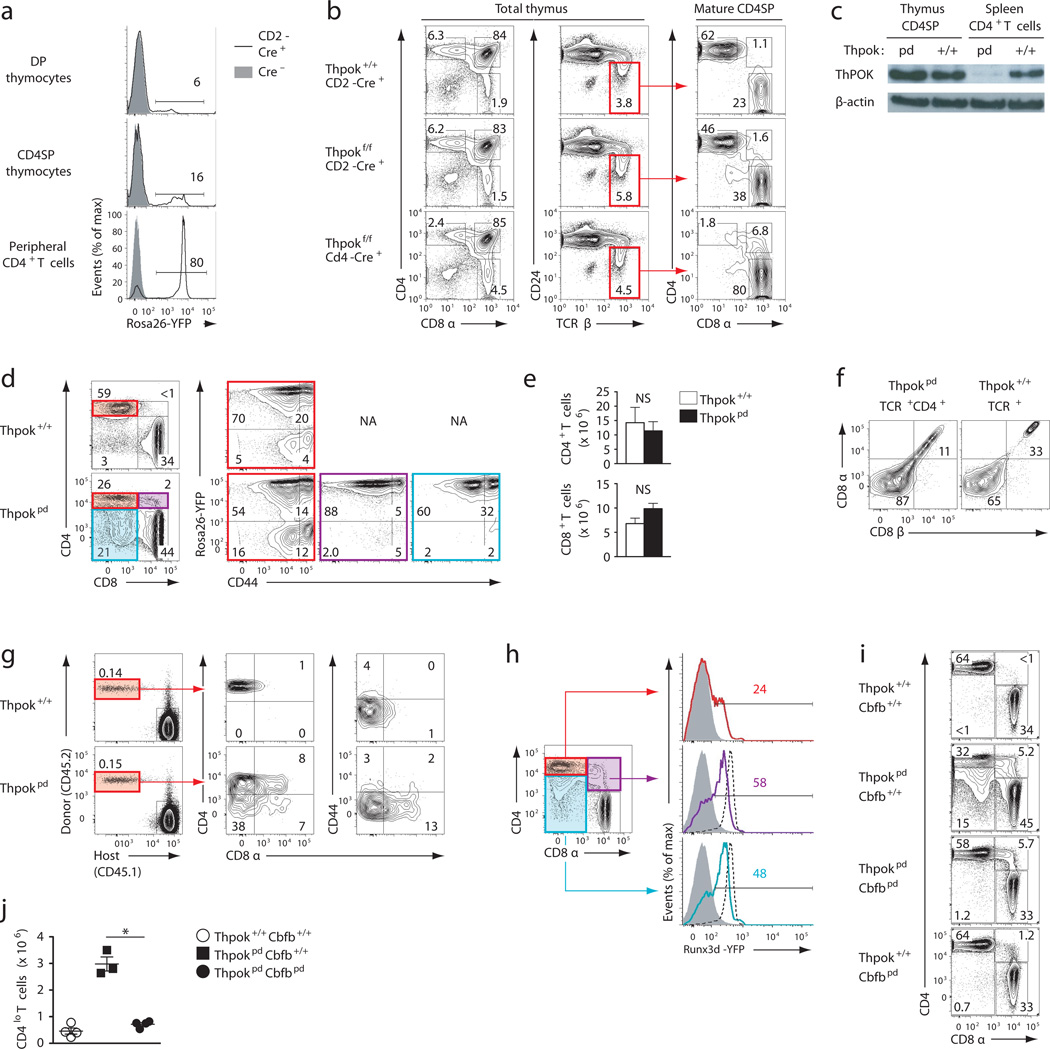
To evaluate the post-thymic functions of ThPOK, we conditionally disrupted Zbtb7b (the gene encoding ThPOK, thereafter called Thpok) using a Cre recombinase transgene driven by the human CD2 promoter. Contrary to other CD2-driven transgenes, this construct is not active in most DP and CD4SP thymocytes, but is upregulated after thymic development; thus, it ensures efficient deletion in peripheral CD4+ T cells, as shown with a Rosa26YFP reporter for Cre activity (Fig. 1a). Deletion was also efficient in CD8+ T cells, and was already substantial in CD8SP thymocytes (Supplementary Fig. 1a), consistent with CD8SP thymocytes taking longer than CD4SP cells to develop from DP thymocytes20,21. Consistent with this expression pattern, and unlike thymic (germline or Cd4-Cre-mediated) Thpok disruption7,11,12,22, Thpokfl/fl CD2-Cre mice (hereafter called Thpokpd for ‘peripheral deleter’) had large numbers of CD4SP thymocytes, which expressed a normal amount of ThPOK protein (Fig. 1b,c and Supplementary Fig. 1b). Furthermore, and contrasting with thymic deletion, Thpokpd mice had CD4+CD8− naïve T cells, most of which expressed Rosa26YFP (Fig. 1d and Supplementary Fig. 1c) and little or no ThPOK (Fig. 1c). Thus, this experimental system is suitable to study post-thymic functions of ThPOK. Of note, deletion was incomplete in Foxp3-expressing regulatory T cells1 (Treg)(Supplementary Fig. 1d), whether in the thymus or in the spleen, making this system poorly suited to study ThPOK function in Treg cells.
Figure 1. Post thymic ThPOK maintains CD4+-lineage integrity.
(a) Histogram overlays of Rosa26YFP expression in thymocytes or spleen T cells from Rosa26YFP mice carrying (plain line) or not (grey-filled) the CD2-Cre transgene. (b) Contours plots of CD4 versus CD8 expression on total (left) and TCRhiCD24lo thymocytes (right, defined in middle column) from 6–8 week old Thpokpd, Thpok+/+ and Thpokf/f Cd4-cre mice. (c) Immunoblot analysis of ThPOK protein expression in sorted CD4SP thymocytes or spleen CD4+CD8− T cells from Thpok+/+ or Thpokpd mice. Expression of β-actin serves as a loading control. (d) Contour plots of CD4 versus CD8 expression on spleen T cells (left) define subsets analyzed for CD44 versus Rosa26YFP expression (right three columns), in Thpokpd or wild-type littermate mice. (e) CD4+ (including CD4lo and pDP) and CD4−CD8+ spleen T cell numbers (mean ± SEM, ×106) from 6–8 week-old Thpokpd (n=7) and Thpok+/+ littermates (n=6). No significant difference by Student’s t-test. (f) Contour plots of CD8α versus CD8β expression on indicated spleen T cell subsets. (g) (left) Contour plots on gated T cells (TCRβ+B220−) distinguish donor (CD45.2+) from host (CD45.1+) subsets 14 days after transfer of naive CD4+ T cells from Thpokpd or Thpok+/+ CD45.2+ mice. Center and right plots show donor cell expression of CD8 vs. CD4 or CD44. (h) CD4 vs. CD8 expression on gated CD44lo TCRβ+ spleen cells from Runx3d-YFP Thpokpd mice define subsets analyzed for Runx3d-YFP expression (left). Histogram plots overlay YFP signal in each defined population from Thpokpd Runx3dYFP mice (colored lines) over their CD4−CD8+ counterparts (dashed), and Thpok+/+ CD4+ splenocytes (solid grey). Cre-negative cells expressing the reporter were excluded from analysis. (i) CD4–CD8 expression plots on lymph node T cells of indicated genotype. (j) Absolute numbers of CD4lo spleen T cells in mice analyzed in (i). Each symbol represents one mouse. (*: P<0.0002). (a–i) All panels are representative of three independent experiments except for (b) (2 experiments) and (g) (2 experiments, each with 4 mice of each genotype).
Thpokpd mice had fewer CD4+CD8− T cells than wild-type mice (Supplementary Fig. 1c). However, Thpokpd mice had unusual CD4loCD8− (hereafter CD4lo) and CD4+CD8+ (peripheral DP, hereafter pDP) cells, which expressed Rosa26YFP and were predominantly naive (CD44lo; Fig. 1d); as a result, the total number of CD4+ T cells (including CD4lo and pDP) was similar in Thpokpd and wild-type mice (Fig. 1e and Supplementary Fig. 1c). Whereas preselection DP thymocytes are TCRlo, pDP cells were TCRhi (data not shown); pDP cells expressed both CD8α and CD8β subunits (Fig. 1f), unlike the small subset of CD4+CD8α+β− cells normally found among gut intraepithelial lymphocytes (IELs)23. Upon adoptive transfer into lympho-replete recipients, purified Thpokpd CD4+ T cells gave rise to pDP and CD4lo peripheral T cells (Fig. 1g). The transferred cells remained naïve (CD44lo), confirming that changes in CD4 or CD8 expression occurred without proliferation. Of note, unlike thymic Thpok disruption, very few transferred CD4+ T cells became CD4−CD8+. Thus, post-thymic ThPOK is needed for the proper control of CD4 and CD8 coreceptor gene expression in naïve MHC class II-restricted T cells.
ThPOK represses Runx3 in thymocytes, so that MHC II-signaled thymocytes that are ThPOK deficient up-regulate Runx3 to a level characteristic of MHC I-restricted CD8SP thymocytes12. To examine if ThPOK represses Runx3 in peripheral T cells, we generated Thpokpd mice carrying a Runx3dYFP allele that reports Runx3 expression12. Contrary to Thpok+/+ CD4+ T cells, about half of naïve Thpokpd CD4lo and pDP cells expressed Runx3 (Fig. 1h); thus, post-thymic ThPOK restrains expression of Runx3 in naïve CD4+ T cells. However, most Thpokpd CD4+CD8− T cells failed to express Runx3dYFP, and immunoblot analyses only found low expression of Runx3 protein in these cells (Supplementary Fig. 1e). Thus, unlike in thymocytes, ThPOK is not required for Runx3 repression in mature CD4+ T cells.
To evaluate the impact of Runx3 de-repression, we generated Thpokpd Cbfbpd mice, which inactivate ThPOK and the obligatory Runx cofactor Cbfβ post-thymically. We targeted Cbfβ, rather than Runx3, because of the overlapping activities of Runx1 and Runx3 in thymocytes and T cells24. Post-thymic disruption of Cbfβ, whether alone or in addition to that of ThPOK, had little impact on thymic development (Supplementary Fig. 1f,g). However, Thpokpd Cbfbpd mice had no peripheral CD4lo T cells (Fig. 1i,j), suggesting that Runx3 up-regulation mediates Cd4 repression in Thpokpd cells. Of note, there were pDP cells in Thpokpd Cbfbpd mice; however, the presence of these cells did not imply that Runx activity was dispensable for CD8 re-expression, because Cbfβ inactivation may impair Cd4 silencing in CD8+ T cells24. We conclude from these experiments that post-thymic ThPOK protects CD4+ T lineage integrity, at least in part by restraining Runx3 expression.
Conserved TH17 potential of Thpok-deficient cells
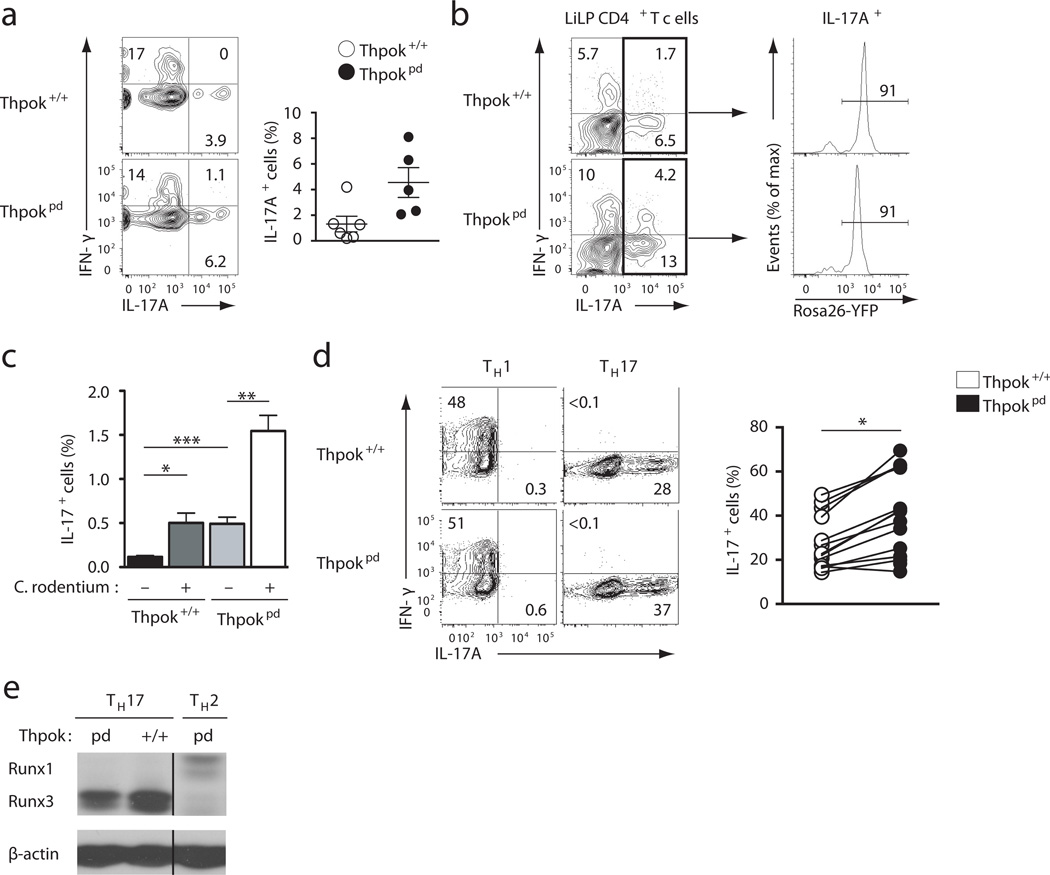
Having shown that ThPOK preserves the differentiation of resting CD4+ T cells, we examined its functions during T cell effector differentiation. Because it was recently reported that ThPOK was important for TH17 differentiation through restraining Runx3 expression17, we assessed TH17 responses in the large intestine lamina propria (liLP) and draining (mesenteric) lymph nodes of mice. Both at steady state or after infection with Citrobacter rodentium, an enterobacterium that generates a strong colonic TH17 response25, Thpokpd mice had a higher frequency of TH17 cells than wild-type mice (Fig. 2a,b,c). Thpokpd IL-17+ T cells expressed Rosa26YFP, indicating that TH17 responses did not result from the outgrowth of cells having escaped deletion (Fig. 2b and data not shown). Thpokpd IL-17+ T cells did not express CD8 (data not shown) and the fraction of IL-17+ cells that also made IFN-γ was similar in Thpokpd and Thpok+/+ mice (Fig. 2b). Accordingly, the course of Citrobacter rodentium infection was similar in wild type and Thpokpd mice (Supplementary Fig. 2a,b). Consistent with these results, IL-17 expression was conserved in Thpokpd T effector cells generated in vitro in TH17 polarizing conditions. Although the frequency of IL-17+ T cells was modestly increased by ThPOK disruption (Fig. 2d), there was no effect on IL-17 cytokine production assessed by ELISA (Supplementary Fig. 2c), and little or no change in Runx3, IFN-γ or granzyme B expression (Fig. 2d,e and S2d). Altogether, these experiments support the conclusion that TH17 differentiation of naïve CD4+ T cells does not require ThPOK.
Figure 2. ThPOK is not needed for TH17 differentiation.
(a, b) Contour plots of IFN-γ versus ı IL-17A expression on large intestine lamina propria (liLP) CD4+ TCRβ+ cells from Thpok+/+ and Thpokpd mice. Data is from unmanipulated mice (a, scatter plot on the right summarizes data on 5 mice of each genotype analyzed in 2 experiments), or 11 days after infection with C. rodentium (b, 2 independent experiments, 6 mice of each genotype per experiment). In (b), histograms show YFP expression in Il-17+ CD4+ T cells. (c) Bar graph shows percentages (mean ± SEM) of IL 17-producing cells in MLN of mice analyzed in (b)(*: p<0.05, **: p<0.01 ***: p<0.001 per Student’s t-test). (d) Contour plots (left) show IFN-γ versus IL-17A expression on TH1 (left) or TH17 (right) effector cells derived from naïve CD4+CD8− T cells after 5 day culture. The graph on the right summarizes 10 such experiments; each symbol represents a distinct culture. Percentages (mean ± SEM) of IL-17 producing cells were 28 ± 3.4 (Thpok+/+) and 39.7 ± 4.9 (Thpokpd). Significance (*: P<10−4) was determined by two-tailed paired t-test. (e) Immunoblot analysis of Runx protein expression in TH17 effector cells generated as in (d); Thpok+/+ TH2 effector cells are shown as a negative control for Runx3. β-actin expression controls for lane loading. Representative of 3 independent experiments.
Thpok protects TH2 responses by repressing Runx3
We previously showed that the MHC II-restricted CD8+ T cells generated following thymic Thpok disruption fail to undergo TH2 differentiation, whether in vitro or in vivo16. In contrast, naïve Thpokpd cells made IL-4 when activated in vitro in TH2 polarizing conditions (Fig. 3a). To elucidate the role of ThPOK expression during TH2 responses in vivo, we immunized mice with inactivated Schistosoma mansoni eggs26. While this typical TH2 stimulus induces CD4+CD8− T cells expressing IL-4 or the TH2 regulator GATA327 in wild-type mice, there was almost no T cells expressing GATA3 or IL-4 in Thpokpd mice (Fig. 3b). These results suggest ThPOK is important for TH2 differentiation in vivo,
Figure 3. ThPOK ‘protects’ TH2 responses.
(a) Contour plots of IFN-γ versus IL-4 expression on effector cells derived from naïve Thpokpd or Thpok+/+ CD4+ T cells after 5-day culture under TH2 conditions. (b) Graphs indicate percentage (mean ± SEM) of IL 4- or GATA3-expressing cells among spleen CD44hi CD4+ T cells from Thpok+/+ or Thpokpd mice 12 days post-immunization with Schistosoma eggs or in non-immunized controls (Ctrl). Each symbol represents one mouse. Significance determined by t-test (*: p <0.01). (c–f) Contour plots assess effector cells derived from naïve CD4+CD8− Thpok+/+ or Thpokpd T cells after 5-day culture in non-polarizing (THN) for expression of GATA3 versus Eomes (c), IL-4 versus IFN-γ (d), CD4 versus CD8α (e) and CD8α vs. CD8β (f). Wild-type CD8+ effectors derived in the same conditions are shown as a control in (c). (g) Granzyme B expression in effector T cells derived as in (c). (h) Immunoblot analysis of Runx protein expression in ex vivo naïve spleen CD4+ T (Thpok+/+, left lane) or Thpok+/+ and Thpokpd effector cells derived as in (c) (THN). β-actin expression control for loading. (i) Contour plots (right two columns) assess IL-4 versus IFN-γ expression in CD45.1 Thpok+/+ or CD45.2 Thpokpd effector cells, derived from sorted CD4+CD8− cells seeded in THN co-cultures at ratios indicated on the left. The leftmost column indicates the percentages of each genotype at the end of the culture. (j) Contour plots of IL-4 vs. IFN-γ expression in Thpokpd and Thpok+/+ effectors generated in THN (top) or TH2 (bottom) cultures, or in THN cultures supplemented by anti IFN-γ (20 µg/ml, middle row). (a–j) Profiles are representative of 3 (a, b), 5 (c–g), 2 (h–j) independent experiments.
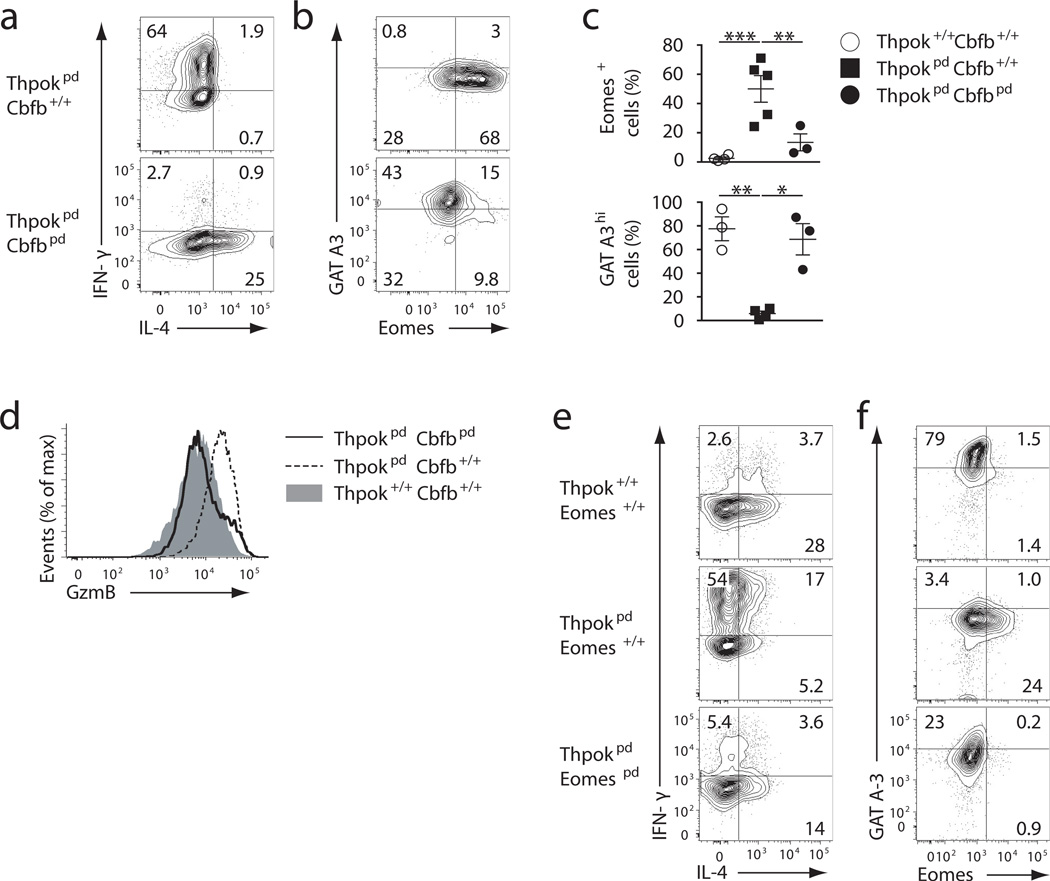
Accordingly, expression of IL-4, assessed by intra-cellular staining or ELISA of IL-4 secretion, and expression of GATA3 were markedly impaired when naive Thpokpd CD4+ T cells were activated in non polarizing conditions, which normally favor TH2 differentiation (Fig. 3c,d and Supplementary Fig. 3a,b). Instead, in these conditions, Thpokpd CD4+ T cells made IFN-γ and the cytotoxic enzyme Granzyme B (Fig. 3d, g), and expressed CD8 (both CD8α and CD8β) (Fig. 3e,f), and Eomesodermin (Eomes), a T-bet related transcription factor involved in IFN-γ production in cytotoxic cells28 (Fig. 3c). Analyses of Runx protein expression showed up-regulation of Runx3 in Thpokpd effector cells, which predominated over Runx1 (Fig. 3h), demonstrating that ThPOK is important to restrain Runx3 expression during T cell activation. Impaired production of IL-4 was not restored by co-culture with wild-type CD4+ T cells or by neutralizing IFN-γ activity during activation, indicating that it was intrinsic to ThPOK-deficient CD4+ T cells (Fig. 3i,j). A 10-fold excess of wild-type CD4+ T cells in the culture only modestly improved IL-4 expression by ThPOK-deficient CD4+ T cells activated under THN conditions (Fig. 3i). These experiments demonstrate a cell-intrinsic role for ThPOK in IL-4 production and TH2 differentiation. Disruption of Runx activity (by inactivating Cbfβ) restored IL-4 production in effector cells generated from naïve Thpokpd Cbfbpd CD4+ T cells activated under THN conditions (Fig. 4a), inhibited their expression of IFN-γ and Granzyme B and reversed the switch between GATA3 and Eomes expression (Fig. 4b,c,d and Supplementary Fig. 4a), These results indicate that ThPOK ‘protects’ TH2 differentiation by restraining Runx3 expression and the cytotoxic ‘diversion’ of activated CD4+ T effector cells.
Figure 4. ThPOK ‘protects’ TH2 differentiation by restraining Runx functions.
(a, b) Contour plots assess IL-4 versus IFN-γ (a) and GATA3 versus Eomes expression (b) in effector cells derived from naive Thpokpd Cbfb+/+and Thpokpd Cbfbpd CD4+ T cells after 5-day culture in THN conditions. (c) Percentage of Eomes- and GATA3-expressing cells among effectors of the indicated genotype cultured as in (b). Each symbol represents a distinct mouse. Significance was determined by t-test (*: P<0.05; **: P<0.005; ***: P<0.0005). (d) Granzyme B expression in cells prepared as in (a, b). (e, f) Contour plots show expression of IL-4 versus IFN-γ (e) and of GATA3 versus Eomes (f) on effector cells derived in the same conditions as in (a). (a–f) Representative of 3 experiments for each panel.
Because Eomes is downstream of Runx3 in the regulatory circuitry of cytotoxic cells, and also promotes IFN-γ production29,30, we examined its involvement in the cytotoxic diversion of Thpokpd T cells. We used the CD2-Cre transgene to generate Thpokpd Eomespd mice, in which ThPOK and Eomes were inactivated in mature T cells. As expected, because there is little or no Eomes expression in conventional thymocytes or naïve CD4+ T cells28, Eomes disruption did not affect steady-state thymic, spleen or LN CD4+ T cell populations (Supplementary Fig. 4b,c). T effector cells generated by activating naïve Thpokpd Eomespd in THN conditions made less IFN-γ but only marginally more IL-4 and GATA3 than their Thpokpd counterparts (Fig. 4e, f and Supplementary Fig. 4a). Thus, Eomes mediates IFN-γ production but not IL-4 repression caused by unrestrained Runx activity in ThPOK-deficient effectors. This agrees with our finding that the impaired TH2 differentiation of ThPOK-deficient cells was not the consequence of excessive IFN-γ production (Fig. 3j). We conclude from these experiments that ThPOK repression of Runx3 in CD4+ T cells is essential for Th2 responses, as it prevents Runx3-mediated activation of a cytotoxic gene expression program and, independently, the repression of IL-4.
ThPOK protects TH1 differentiation by constraining Runx functions
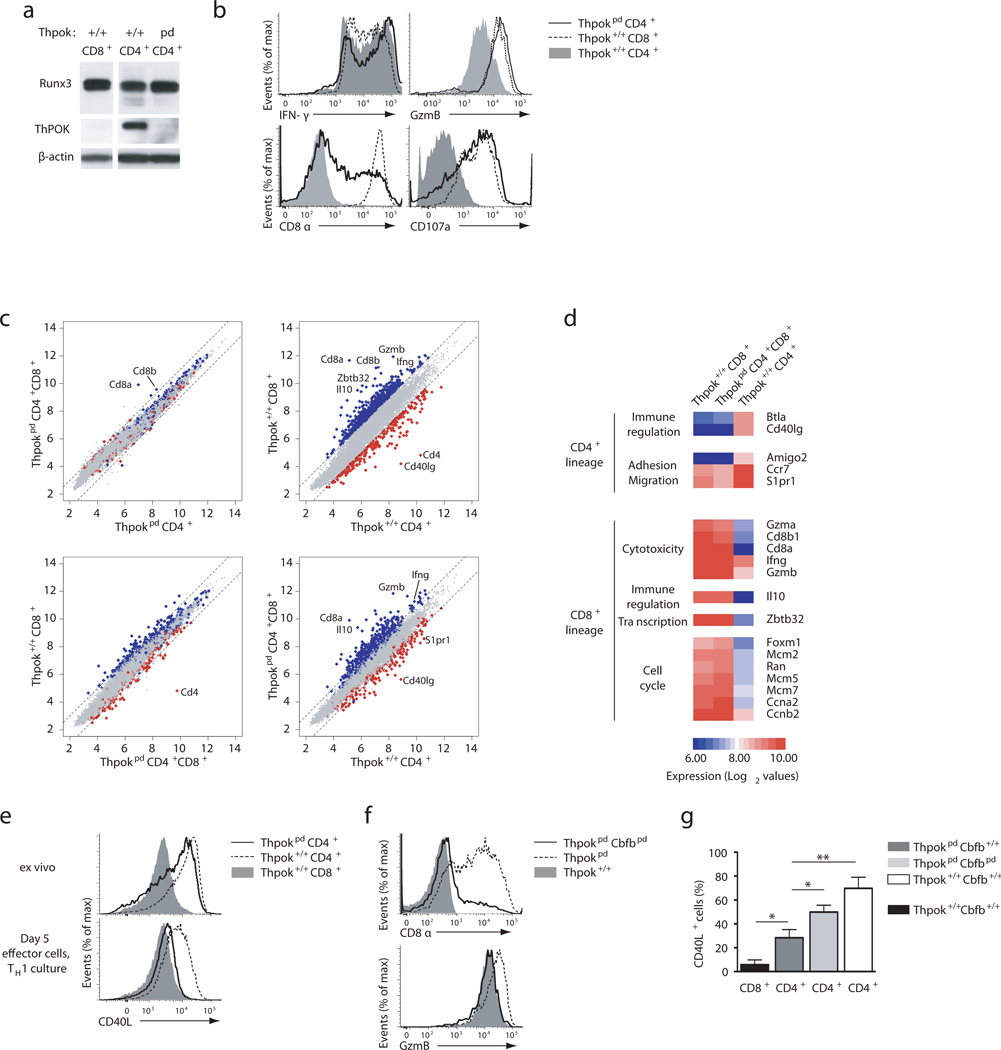
Because wild-type TH1 effector cells normally express Runx318,19, we investigated if ThPOK also affected TH1 differentiation. In T cells polarized in TH1 conditions, ThPOK disruption had little effect on Runx3 or IFN-γ expression (Fig. 5a,b). However, compared to their Thpok+/+ counterparts, Thpokpd effector cells generated in TH1 conditions had greater expression of CD8 and granzyme B and they externalized CD107a, a marker of cytotoxic cell degranulation31 (Fig. 5b). Of note, they retained CD4 expression (Supplementary Fig. 5a).
Figure 5. ThPOK antagonism of Runx activity is required for proper TH1 differentiation.
(a) Runx protein expression in effector T cells derived from spleen CD4+ or CD8+ Thpokpd or Thpok+/+ littermates cultured under TH1 conditions. The membrane was reprobed for ThPOK expression and β-actin control. (b) Overlaid histograms show expression of intra-cellular Granzyme B and IFN-γ, or surface CD8, and externalization of surface CD107a, in effectors derived under TH1 conditions from indicated sorted naïve T cells. (c) Scatter plots compare gene expression (log2 values, complete array gene set) in indicated effector cells derived in TH1 cultures. Dashed lines indicate 2-fold differential gene expression. Genes with 2-fold or greater expression in wild-type CD4+ vs. wild-type CD8+ cells (defined on top right plot) are shown in red and blue, respectively, in all plots. Relevant genes are indicated. (d) Heat map indicates relative expression (red-blue color scale, log2 values) on select genes defined by 3-fold or greater change in expression in Thpokpd CD4+CD8+ vs. wild-type CD4+CD8− effectors. The complete data set is shown in Fig. S5b. (e) Overlaid histograms show CD40L expression in sorted CD4+CD8− and CD4−CD8+ CD44lo T cells ex vivo (upper panel) and effector cells derived from these same subsets under TH1 conditions (lower panel). (f) Overlaid histograms show CD8 (top) or Granzyme B expression (bottom) in effector cells derived as in (e) from sorted CD4+CD8− cells of the indicated genotype. (g) Graph summarizes percentages of CD40L-expressing cells (after 3-hour in vitro stimulation) on effector T cells of the indicated genotype, derived as in (e); significance was determined by t-test (*: P<0.05, **: P<0.01). (e–g) Representative of 3 (a, b, e, f) and 6 (g) experiments.
These observations suggested that a comparison of the transcriptomes of wild-type and ThPOK-deficient TH1 effector cells would reveal how ThPOK affects gene expression independently of its effect on Runx3 expression. Thus, we used gene expression arrays to compare the transcriptional profiles of the following effector T cells, all generated in the same TH1 polarizing conditions:(i) wild-type TH1 CD4+ T cells, generated from naïve CD4+ T cells, (ii) Thpokpd CD4+CD8− and CD4+CD8+ cells, the two populations generated by activation of naive Thpokpd CD4+CD8− cells (Supplementary Fig. 5a), and (iii) wild-type cytotoxic CD8+ T cells, generated from naïve CD8+ T cells. Except for Cd8 genes, side by side comparisons showed no divergence between CD4+CD8− and CD4+CD8+ Thpokpd T effector cells generated in these conditions (Fig. 5c, top left, and Supplementary Fig. 5b). When we compared Thpokpd CD4+CD8+ to wild-type CD4+ and CD8+ T effector cells, Thpokpd CD4+CD8+ had a transcriptional profile that was very similar to that of wild-type CD8+ cytotoxic cells (Fig. 5c, bottom left), whereas they diverged from the wild-type CD4+ TH1 cells almost to the same extent as wild-type cytotoxic T cells did (Fig. 5c, right two plots). Thus, ThPOK is responsible for the bulk of differential gene expression between TH1 CD4+ and cytotoxic CD8+ T cells.
Hierarchical clustering distinguished two groups of genes differentially expressed between Thpokpd CD4+CD8+ (or CD4+CD8−) and wild-type CD4+ T effector cells (Fig. 5d and Supplementary Fig. 5b). The largest group represented genes up-regulated in each ThPOK-deficient subset in comparison to wild-type CD4+ T cells. In addition to prototypical cytotoxic genes such as perforin or Granzymes A and B, this group included most of the genes preferentially expressed in CD8+ T effector cells but which are not part of the conventional cytotoxic program, such as IL-10, Zbtb32 (encoding the transcription factor Rog) as well as cell cycle-related genes. The second group contained a smaller gene set that was down-regulated in each ThPOK-deficient subset compared to wild-type CD4+ T cells and comprised genes involved in effector T cell differentiation, such as Cd40lg or Vipr1 (encoding a receptor for the VIP peptide) and molecules involved in cell adhesion (Amigo2) or trafficking (Ccr7, S1pr1). Indeed, Thpokpd T effector cells (CD4+CD8+ and CD4+CD8−) had impaired induction of CD40L (Fig. 5e, bottom); of note, this contrasted with the conserved CD40L expression in naïve Thpokpd CD4+ T cells (Fig. 5e, top), which express little Runx3.
The repression of the cytotoxic program by ThPOK in TH1 effector cells supported the conclusion that this factor, in addition to repressing Runx3, restrained Runx-mediated activation of cytotoxic genes. Indeed, the cytotoxic gene up-regulation characteristic of ThPOK-deficient effector cells was reverted in Thpokpd Cbfbpd effector cells derived in TH1 cultures from naïve CD4+CD8− T cells (Fig. 5f).
In addition to activating effects, Runx proteins have repressive functions, notably on Cd4 and Thpok during T cell differentiation24. While ThPOK can antagonize Runx-mediated repression11,32, the physiological relevance of such antagonism has not been established, and was questioned by the continued expression of CD4 in Runx3-expressing ThPOK-deficient T effector cells (Supplementary Fig. 5a). Thus, we next examined if the ThPOK-dependence of helper effector gene expression, such as Cd40lg, was explained by ThPOK antagonism of Runx-mediated repression. Indeed, Cbfβ disruption enhanced CD40L expression in Thpokpd Cbfbpd TH1 effector cells (Fig. 5g). These observations demonstrate that ThPOK promotes effector gene expression at least in part by antagonizing Runx-mediated repression. We conclude from these experiments that ThPOK is essential to protect helper and restrain cytotoxic gene expression by antagonizing Runx3 functions.
Thpok is important for antigen-induced T cell responses in vivo
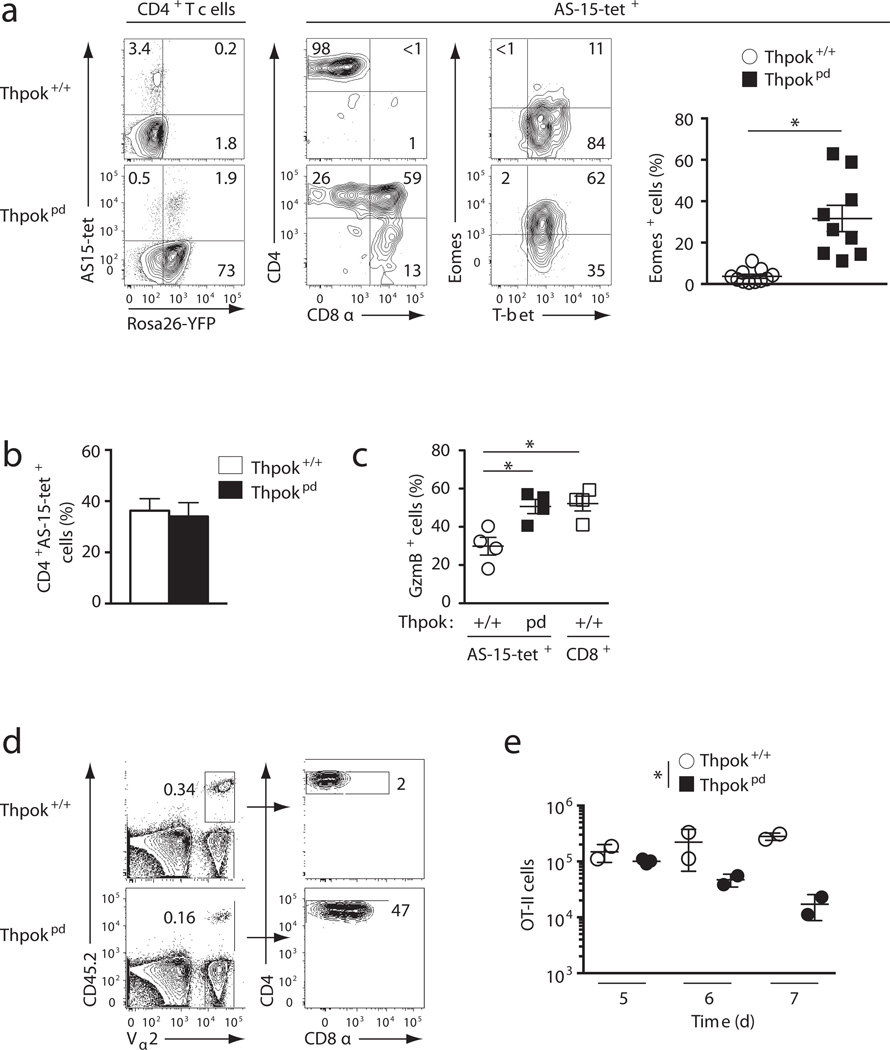
We took two approaches to examine how ThPOK expression affects CD4+ T cell responses in vivo. First, we evaluated responses to Toxoplasma gondii, an intra-cellular parasite generating a massive IFN-γ-mediated CD4+ TH1 response, during which we tracked T cells reacting against a specific MHC II-presented parasite epitope (AS-15)33. At the peak of the acute response, similar frequencies of IFN-γ-producing CD4+ T effector cells were found in both Thpokpd and wild-type controls (Supplementary Fig. 6a). ThPOK disruption did not affect the number of AS-15-reacting T cells (Fig. 6a,b) and did not increase parasite load (Supplementary Fig. 6b). However, consistent with the notion that ThPOK prevents cytotoxic gene expression in TH1 cells, Thpokpd effector cells were CD4+CD8+ and expressed granzyme B and Eomes (Fig. 6a,c and Supplementary Fig. 6c).
Figure 6. ThPOK is important for CD4+ T cell responses in vivo.
(a) Contour plots of AS-15-loaded I-Ab tetramer binding versus Rosa26-YFP expression on gated CD4+TCRβ+ spleen cells 10 days post-infection of Thpok+/+ and Thpokpd mice with T. gondii (left); expression of CD4 and CD8 (center) or Eomes and T-bet (right) on AS-15-specific cells. The graph on the right summarizes the percentage of Eomes-expressing cells among AS-15-reactive T cells; each symbol represents one mouse and significance was determined by t-test (*: P<10−4). Representative of 5 experiments. (b) Graph summarizes the number of CD4+ (CD8+ or CD8−) AS-15-specific spleen TCRβ+ cells 9–14 days after infection with T. gondii. Data is from 6 independent experiments. (c) Percentage (mean ± SEM) of granzyme B-expressing cells among AS-15-specific CD4+TCRβ+ cells as defined in (a) in day 14-infected mice.; open squares show expression in CD8+ T cells from infected Thpok+/+ mice. Data is from 2 independent experiments; significance was determined by t-test (*: P<10−4). (d) Contour plots (left) define Vα2+ CD45.2+ donor-derived cells in spleens from CD45.1+ ovalbumin-immunized wild-type hosts, 6 days after adoptive transfer of CD45.2+ OT-II TCR transgenic cells (either Thpok+/+ or Thpokpd). Donor cells are analyzed for CD4 and CD8 expression (right). Data is representative of 3 experiments. (e) Cumulative results of adoptive transfer experiments performed as in (d) and analyzed 5 to 7 days after transfer. Each symbol represents one recipient mouse. The difference between Thpok+/+ and Thpokpd responses was significant by 2-way ANOVA (*:P<0.005).
We considered the possibility that signals from other cells involved in T. gondii responses, including innate immune cells and CD8+ T cells, could help CD4+ T cells overcome ThPOK deficiency. Thus, in a second approach, we examined the cell intrinsic role of ThPOK in a defined, clonotypic CD4+ T cell response. We compared the antigen-driven expansion of ThPOK-deficient and -sufficient cells carrying the ovalbumin-specific OT-II TCR. We purified wild-type or Thpokpd CD4+CD8− OT-II T cells, and assessed their response after adoptive transfer into ovalbumin-immunized wild-type lympho-replete hosts. We found that the amplification of Thpokpd CD4+CD8− OT-II T cells was blunted compared to their wild-type counterparts, and that these Thpokpd cells re-expressed CD8, while maintaining CD4 (Fig. 6d,e). Thus, even if ThPOK is not required for the generation of antigen-responsive effector T cells, it is important for their proper expansion and differentiation.
Thpok and LRF redundantly maintain CD4+-lineage integrity
The puzzling persistence of CD4 expression in Thpokpd effector T cells, despite their high Runx3 expression, contrasted with their Runx-dependent repression of CD40L, or the Runx-dependent repression of CD4 in naïve CD4lo Thpokpd cells (Fig. 1h, i). This raised the possibility that the Cd4 locus was no longer sensitive to Runx-mediated repression in antigen-stimulated T cells. Consistent with this possibility, naïve CD4lo Thpokpd cells, in which CD4 repression is Runx-dependent (Fig. 1i) up-regulated CD4 upon activation (Supplementary Fig. 7a). An alternative possibility was that Cd4 expression in TH1 effector cells remained responsive to Runx-mediated repression, but was sustained by a redundant factor in the absence of ThPOK. The transcription factor LRF, which has a ThPOK-redundant role in promoting CD4+ T cell programming in the thymus16 represented an obvious candidate.
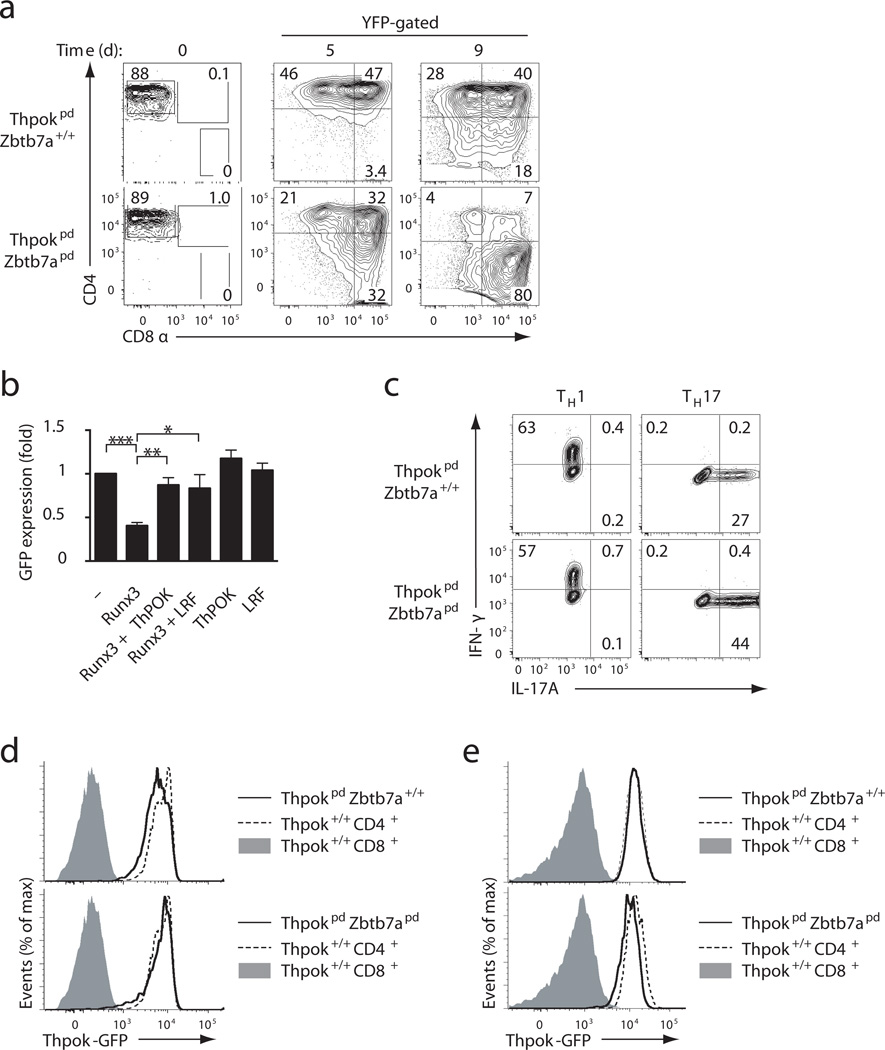
To distinguish between these possibilities, we generated Thpokfl/fl Zbtb7afl/fl mice carrying the CD2-Cre transgene (hereafter Thpokpd Zbtb7apd mice), in which both Thpok and Zbtb7a, which encodes LRF, are deleted in naïve peripheral T cells. The isolated disruption of LRF did not affect CD4+ T cell expression of CD4 or CD8, or functional differentiation (data not shown and Ref. 16). Compared to Thpokpd mice, Thpokpd Zbtb7apd mice showed little effect of Zbtb7a disruption on thymic development, but they had reduced peripheral CD4+CD8− and CD4lo T cell subsets (Supplementary Fig. 7b,c,d), suggesting that ThPOK and LRF redundantly contribute to maintain the CD4+ lineage. Following activation and culture in TH1 conditions, naive Thpokpd Zbtb7apd CD4+CD8− T cells gave rise to CD4−CD8+ effector cells, while most naïve Thpokpd CD4+CD8− T cells continued to express CD4 (Fig. 7a). Thus, post-thymic inactivation of ThPOK and LRF causes trans-differentiation of mature CD4+ into CD8+ T cells. In Thpokpd Zbtb7apd TH1 effector cells obtained from CD4+CD8− naïve cells, expression of Runx3 was not increased over that observed in their Thpokpd counterparts cells (Supplementary Fig. 7e). Rather, LRF antagonized Runx-mediated Cd4 repression. In transient transfection experiments that evaluate the activity of a GFP-based reporter construct including Cd4 cis-regulatory elements (promoter, proximal enhancer and silencer)24, Runx3 repressed Cd4 reporter activity, whereas ThPOK, while having no effect per se, prevented Runx-mediated repression32 (Fig. 7b). LRF was as efficient as ThPOK at antagonizing Runx-mediated repression of the Cd4 reporter (Fig. 7b). Of note, ThPOK and LRF disruption did not prevent TH17 differentiation in vitro (Fig. 7c), indicating that ThPOK and LRF were not required for TH17 differentiation in post-thymic cells, unlike their function in the thymus16. These results suggest that a transcriptional node defined by the overlapping activities of ThPOK and LRF supports CD4 expression in mature T cells.
Figure 7. ThPOK and LRF redundantly prevent CD4+ to CD8+ trans-differentiation.
(a) Contour plots show CD4 versus CD8 expression on Rosa26-YFP gated effector cells derived from CD4+CD8− ThpokpdZbtb7a+/+ (top) or ThpokpdZbtb7apd (bottom) naïve T cells (sorted populations, left), after 5 day (middle) or 9 day (right) culture under TH1 conditions. (b) Bar graph depicts Cd4 promoter activity in RLM-11 cells transfected with a GFP-based reporter plasmid for Cd4 gene expression, and indicated expression vectors. Data shows GFP mean fluorescence activity (mean ± SD from 3 independent experiments), gated on transfected cells and expressed relative to reporter-only control (top bar). Data significance was analyzed by t-test [P< 0.05(*), <0.01(**), <0.001(***)]. (c) Contours plots of IFN-γ versus IL-17A expression (gated on Rosa26-YFP+ cells) in effector cells derived from CD4+CD8− ThpokpdZbtb7a+/+ (top) or ThpokpdZbtb7apd (bottom) T cells after 5-day culture under TH1 (left) or TH17 conditions (right). (d, e) Solid line histograms show GFP expression from the ThpokGFP reporter in (d) Thpokpd Zbtb7a+/+ (top) or Thpokpd Zbtb7apd (bottom) CD44lo CD4+CD8− spleen T cells or (e) day 9 CD8-expressing effector cells derived as in (a) from naïve Thpokpd Zbtb7a+/+ (top) or Thpokpd Zbtb7apd (bottom) CD4+CD8−T cells. In both panels, plain line histograms are overlaid on CD4+CD8− (dashed line) or CD4−CD8+ (solid grey) counterparts from Thpok+/+Zbtb7a+/+ mice. Data is representative of 3 (a–c) or 2 (d, e) independent experiments.
Thpok expression is sustained independently of Thpok and LRF
Because ThPOK was proposed to protect expression of both Cd4 and Thpok genes11, the previous findings suggested that ThPOK and LRF would also contribute to sustain Thpok expression; that is, that the Thpok promoter would be inactive in Thpokpd Zbtb7apd trans-differentiated CD4−CD8+ cells. Cre-mediated inactivation of the Thpokfl allele excises most of its transcribed sequences, therefore preventing direct gene expression analyses. Thus, we used a ThpokGFP BAC reporter22, genetically independent from the Thpok locus, to test this possibility. Inactivation of ThPOK, or both ThPOK and LRF, had little or no effect on reporter expression in naïve CD4+ cells (Fig. 7d). In addition, the reporter was also expressed in Thpokpd Zbtb7apd TH1 effector cells, including those trans-differentiating into CD4−CD8+ cells (Fig. 7e). Thus, while ThPOK and LRF control lineage integrity and effector differentiation of CD4+ T cells, they are dispensable for Thpok gene expression.
Discussion
The present study demonstrates that the transcription factor ThPOK, redundantly with its homolog LRF, sustains the phenotypic and functional integrity of the CD4+ lineage. ThPOK’s impact in mature T cells is mediated both by its repression of Runx3 expression and its antagonism of Runx protein function. Thus, not only do ThPOK and LRF establish functional helper pre-programming in thymocyte16, they are continuously needed to maintain the integrity of helper T cell responses.
Using a novel mouse strain which targets Cre expression to naïve T cells with greater selectivity than existing lines34, we demonstrate that the ThPOK-LRF transcriptional ‘node’ is required to maintain CD4+ lineage integrity beyond the thymic checkpoint that defines CD4+-lineage commitment. The contribution of ThPOK and LRF to CD4 expression is important because CD4 is needed for the survival and fitness of mature CD4+ T cells35. Conversely, repression by ThPOK of genes encoding CD8α and CD8β fits with previous reports that ThPOK functionally and physically targets Cd8 enhancers36–38. However, our findings underscore a fundamental difference between CD4+ and CD8+ T cells for coreceptor gene repression. Once established in CD8SP thymocytes by recruitment of the Cd4 repressor Runx3 to the Cd4 silencer, Cd4 silencing is epigenetically maintained independently of Runx3 or the silencer24,39. This contrasts with the active repression of Cd8 in CD4+ T cells.
Our study demonstrates that ThPOK broadly represses cytotoxic gene expression and thereby avoids the ‘cytotoxic diversion’ of TH2 effectors. The balance between Runx3 and GATA3 controls TH1-TH2 differentiation in CD4+ T cells18,19,30. Runx molecules inhibit IL-4 and promote IFN-γ expression presumably both directly and indirectly, by binding to Il4 and Ifng genes and controlling expression of GATA3 and T-bet. GATA3 antagonizes both direct and indirect effects, at least in part through direct binding to Runx3 molecules30. By limiting the amount of Runx3 produced during CD4+ T cell activation, ThPOK is critical for TH2 differentiation. ThPOK disruption results in Runx-dependent Eomes expression, which is needed for IFN-γ production but not to prevent IL-4 expression. This finding supports the conclusions that Il4 repression and Ifng activation are mechanistically independent: the former being principally mediated by Runx3 whereas Ifng activation by Runx3 requires Eomes or T-bet, even though Runx3 binds the Ifng gene18,30
In addition to repressing Runx3, ThPOK antagonizes Runx protein activity, the latter function underpinning ThPOK’s support for Cd40lg expression and its impact on TH1 differentiation. While such antagonism of Runx function had been identified in cell lines and thymocytes11,32, our study establishes its physiological relevance by comparing the transcriptome of ThPOK-deficient and -sufficient cells with equivalent expression of Runx proteins. Future experiments will explore whether such mechanisms are important for long term control of intra-cellular pathogens which relies on TH1 responses, including of T. gondii. The central role of CD4+ effector cells for long term T. gondii control41, including providing help to CD8+ T, B, and myeloid cells, notably through CD40L40, suggests that the function of ThPOK in maintaining the TH1 circuitry may be essential to coordinate such responses.
ThPOK was reported to ‘protect’ the colitogenic potential of CD4+ T cells in adoptive cell transfer colitis in alymphoid mice17, a function assigned to ThPOK promoting the differentiation of colitogenic TH17 cells. However, both that report17 and our study found that ThPOK is not needed for in vivo TH17 responses. Furthermore, ThPOK inhibits, rather than protects, TH17 differentiation in invariant natural killer (iNK T) cells42. We note that ThPOK’s control of CD40L in TH1 effectors provides an alternative rationale for its impact on adoptive transfer colitis. Indeed, TH1 cells are instrumental in this disease, in which CD40L is critical to induce gut inflammation43,44.
It was recently proposed that the expression of Thpok in CD4+ T cells is subject to regulation, namely that Runx3 up-regulation by environmental signals represses Thpok in CD4+ effector cells by activating a Thpok silencer17,38. However, even though the Thpok silencer includes binding sites for Runx molecules13, such repression did not in fact require Runx317. Nonetheless, given that ThPOK has been reported to protect the Thpok gene from repression by Runx molecules11, we expected that inactivation of ThPOK, or ThPOK and LRF, would facilitate Thpok repression by Runx3. Contrary to this prediction, the Thpok locus remained transcriptionally active in Runx3-expressing ThPOK-LRF double-deficient cells, challenging the idea that Runx3 expression initiates Thpok silencing in effector T cells. Rather, such silencing, if physiologically relevant, could be caused by signals targeting other repressors of Thpok expression45,46.
In primate T cells, CD4 serves as receptor for the human immunodeficiency virus (HIV) and its simian homologs (SIV)47. Notably, it was found that antigen-experienced MHC II-restricted cells convert from a CD4+CD8− to a CD4−CD8αloβ− phenotype in African green and Patas monkeys48,49. Although such conversion is associated with reduced expression of helper genes, it is believed to be beneficial by protecting the helper T cell population from SIV infection and the ensuing immunodeficiency. The present study provides a conceptual framework to investigate the mechanisms of this conversion, in which the association of functional changes with a concerted switch of CD4 and CD8 expression suggests the possible involvement of Runx molecules and their control by ThPOK or related factors.
In summary, our findings emphasize the inherent instability of the CD4+ lineage, that ThPOK, together with LRF, continuously protects from CD8+ trans-differentiation. They indicate that changes in expression or activity of these factors have broad consequences on T helper functions, making them potential targets for physiological control and therapeutic intervention.
Online Methods
Mice
CD2-cre mice were generated as previously described by inserting a Cre cDNA into a CD2-based transgenic cassette8. Mice carrying floxed alleles for Zbtb7b10, Zbtb7a50 (gift from P.P. Pandolfi), Eomes51 (gift from S. Reiner), Cbfb19 (purchased from the Jackson laboratory) or Runx3dYFP (gift from D. Littman) and ThpokGFP reporters12,22 were all previously described. Rosa26YFP (Ref. 52) and OT-II (Ref. 53) mice were purchased from Jackson Laboratories. CD45.1 and CD45.2 C57BL/6 mice were obtained from the National Cancer Institute Animal Production Facility. All transgenic mice were maintained were heterozygous for the transgene. Mice were housed in specific pathogen-free facilities and analyzed between 6 and 16 weeks of age. Animal procedures were approved by the NCI Animal Care and Use Committee.
Antibodies
Antibodies for the following specificities were purchased either from Affymetrix eBiosciences or BD Pharmingen: CD4 (RM4.4 or GK1.5), CD8α (53-6-7), CD8β (53-5.8), CD24 (M1/89), TCRβ (H57-597), CD40L (MR1), CD107a (1D4B), GATA3 (TWAJ), Tbet (4B10), eomes (Dan11mag), IL4 (11B11), CD45.2 (104), Va2 (B20.1), IFN-g (XMG1.2), IL17a,(eBio17B7) CD44 (IM7). Antibodies specific for Granzyme B (GB12) were purchased from Invitrogen. TGME49 Class II I-Ab tetramers loaded with the AS-15 peptide, and control Clip peptide-loaded I-Ab tetramers were obtained from the NIH tetramer facility.
Cell preparation and staining
Lymph node, spleen, thymus and intestinal lymphocytes were prepared and stained as previously described10,22,54. Flow cytometry data were acquired on LSR II or LSR Fortessa cytometers (BD Biosciences) and analyzed with FlowJo (TreeStar) software. Dead cells and doublets were excluded by DAPI and forward scatter height by width gating. Purification of lymphocytes by cell sorting was performed on a FACSAria (BD Biosciences). Analyses of intracellular cytokines and transcription factors was performed as previously described10,16.
In vitro analysis of gene and protein expression
Analysis of CD40L expression was performed as previously described16. Effector T cells were generated from sorted CD44lo (naïve) T cells from spleen or LN as previously described10, except that activating and blocking antibodies were purchased from BioXCell. Transfection of RLM-11 cells was performed as previously described32. Analysis of ThPOK and Runx protein expression was performed by Western blot as previously described using an antiserum against ThPOK22 and anti-Runx antibody (EPR3099, Epitomics). Membranes were subsequently stripped and blotted with anti β-actin (AC-15, Sigma) to control for lane loading. Quantification of cytokine production by ELISA was performed on culture supernatants 5 days after activation without additional restimulation, using mouse DuoSet ELISA kits (R&D Systems) and following the manufacturer’s instruction.
Adoptive transfer and immunization
For analyses of coreceptor expression in naïve cells, sorted CD45.2+ CD44lo CD4+CD8− T cells (2×106) from spleen and or lymph nodes of Thpok+/+ or Thpokpd mice were injected intravenously into CD45.1 recipients, which were analyzed 14 days later. For OVA-specific responses, 30,000 sorted CD45.2+ CD44lo CD4+CD8− T cells from spleen and or lymph nodes of OT-II TCR transgenic Thpok+/+ or Thpokpd mice were injected into CD45.1 recipients, followed by immunization with 100ug OVA emulsified in complete Freund’s adjuvant administered by intraperitoneal (i.p.) injection 18 hours after adoptive transfer. Mice were analyzed 4–6 days post-immunization.
Schistosoma mansoni infections were performed essentially as previously described26. Briefly, mice were injected i.p. with 2500 inactivated (freeze-thaw) eggs, then boosted 8 days later with a second dose of 2500 inactivated egg dose. Eggs were stored at −70°C before use. Mice were analyzed 12–14 days after the initial injection.
Granule exocytosis assay
In vitro activated T cells from 5 day cultures were re-stimulated on anti-CD3 coated plates, for 4 hours at 37°C, 5% CO2 in the presence of PE-labeled anti-CD107a. Cells were harvested, washed and surface stained before flow cytometric analysis.
Infections
Infection of mice with C. rodentium was performed as previously described.55 Mice were analyzed between 8 to 12 days post-infection. ME-49 clone C1 of T. gondii (kindly provided by Dr M.E. Grigg, NIAID) was obtained by electroporation of the parental ME-49 type II strain (ATCC 50840) with the red fluorescent protein (RFP) and was used for production of tissue cysts in C57BL/6 mice. Mice were infected with 10 cysts by oral gavage. Tissues were harvested between 9 and 14 days post-infection and analyzed as described56. Parasite load was evaluated by the frequency of RFP-expressing cells in the spleen of infected animals.
Microarray analyses
Sorted naïve (CD44lo) CD4+CD8− and CD4−CD8+ T cells, from wild-type mice, and CD4+CD8− cells, from Thpokpd mice were activated in vitro in TH1 conditions and sorted again 4 days later into purified CD4+CD8− and CD4−CD8+ (wild-type) and CD4+CD8− and CD4+CD8+ (Thpokpd) subsets. Total RNA was extracted from sorted subsets and processed for microarray analyses (Affymetrix Mouse Exon 1.0 ST array) at the NCI microarray facility, following the manufacturer’s recommendation. Data is from 3 replicates (except wild-type CD4−CD8+ cells, for which two samples only were processed) generated from two distinct cell preparations. Data was analyzed with Partek Genomic Suite and deposited in the GEO database under accession number GSE57846.
Statistical analyses
All statistical analyses were performed using Prism software. Bars in graphs indicate average ± SEM. Except where otherwise indicated, comparisons were performed by two-tailed unpaired t-test; F-test was performed to check the assumption of equal variance. Two-tailed paired t-test was used where biologically appropriate (e.g. cytokine assays processed in parallel). Significance levels (P-values) are indicated on figures. For statistical comparisons, sample size was always greater than 3 and determined empirically based on pilot analyses. We used neither randomization nor blinding since comparisons involve mice of distinct genotypes; animals were excluded from analyses only on the basis of age or poor health status unrelated to the experiment.
Supplementary Material
Acknowledgements
We thank T. Ciucci for experimental assistance and discussions, T.-A. Lewis for expert animal care and genotyping, M. McGinty and Q. Xiao for technical assistance, X. Wu for microarray analyses, D. Littman, P.P. Pandolfi and S. Reiner for mice, M. Grigg for the T. gondii strain, J. Grainger, T. Hand and S. Spencer for useful discussions, and J. Ashwell and J. Brenchley for reading the manuscript. Supported by the Intramural Research Programs of the National Cancer Institute, Center for Cancer Research, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Database accession numbers
Microarray data was deposited in the GEO database under accession number GSE57846.
The authors declare no competing financial interests.
Author Contributions
M.V., L.W., Y.B. and R.B designed the research; M.V., L.W., N.B, A.C.C, Y.X, L.C.W, and E.W. performed and analyzed experiments; K.-D.S. and P.E.L constructed and provided CD2-Cre mice; R.B. supervised the research; M.V. and R.B wrote the manuscript.
References
- 1.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masopust D, Vezys V, Wherry EJ, Ahmed R. A brief history of CD8 T cells. Eur J Immunol. 2007;37(Suppl 1):S103–S110. doi: 10.1002/eji.200737584. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter AC, Bosselut R. Decision checkpoints in the thymus. Nat Immunol. 2010;11:666–673. doi: 10.1038/ni.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taniuchi I, et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- 6.Woolf E, et al. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci U S A. 2003;100:7731–7736. doi: 10.1073/pnas.1232420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He X, et al. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 8.Sun G, et al. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol. 2005;6:373–381. doi: 10.1038/ni1183. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Bosselut R. CD4–CD8 lineage differentiation: Thpok-ing into the nucleus. J Immunol. 2009;183:2903–2910. doi: 10.4049/jimmunol.0901041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, et al. The zinc finger transcription factor Zbtb7b represses CD8-lineage gene expression in peripheral CD4+ T cells. Immunity. 2008;29:876–887. doi: 10.1016/j.immuni.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muroi S, et al. Cascading suppression of transcriptional silencers by ThPOK seals helper T cell fate. Nat Immunol. 2008;9:1113–1121. doi: 10.1038/ni.1650. [DOI] [PubMed] [Google Scholar]
- 12.Egawa T, Littman DR. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat Immunol. 2008;9:1131–1139. doi: 10.1038/ni.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Setoguchi R, et al. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science. 2008;319:822–825. doi: 10.1126/science.1151844. [DOI] [PubMed] [Google Scholar]
- 14.Davies JM, et al. Novel BTB/POZ domain zinc-finger protein, LRF, is a potential target of the LAZ-3/BCL-6 oncogene. Oncogene. 1999;18:365–375. doi: 10.1038/sj.onc.1202332. [DOI] [PubMed] [Google Scholar]
- 15.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter AC, et al. The Transcription Factors Thpok and LRF Are Necessary and Partly Redundant for T Helper Cell Differentiation. Immunity. 2012;37:622–633. doi: 10.1016/j.immuni.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4(+) T cell immunity. Nat Immunol. 2013;14:271–280. doi: 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djuretic IM, et al. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat Immunol. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 19.Naoe Y, et al. Repression of interleukin-4 in T helper type 1 cells by Runx/Cbf beta binding to the Il4 silencer. J Exp Med. 2007;204:1749–1755. doi: 10.1084/jem.20062456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lucas B, Vasseur F, Penit C. Normal sequence of phenotypic transitions in one cohort of 5-bromo-2'-deoxyuridine-pulse-labeled thymocytes. Correlation with T cell receptor expression. J Immunol. 1993;151:4574–4582. [PubMed] [Google Scholar]
- 21.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, et al. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells. Nat Immunol. 2008;9:1122–1130. doi: 10.1038/ni.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guy-Grand D, Vassalli P. Gut intraepithelial T lymphocytes. Curr Opin Immunol. 1993;5:247–252. doi: 10.1016/0952-7915(93)90012-h. [DOI] [PubMed] [Google Scholar]
- 24.Taniuchi I, Ellmeier W. Transcriptional and epigenetic regulation of CD4/CD8 lineage choice. Adv Immunol. 2011;110:71–110. doi: 10.1016/B978-0-12-387663-8.00003-X. [DOI] [PubMed] [Google Scholar]
- 25.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 26.Oswald IP, et al. IL-12 inhibits Th2 cytokine responses induced by eggs of Schistosoma mansoni. J Immunol. 1994;153:1707–1713. [PubMed] [Google Scholar]
- 27.Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev Immunol. 2009;9:125–135. doi: 10.1038/nri2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearce EL, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 29.Cruz-Guilloty F, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yagi R, et al. The transcription factor GATA3 actively represses RUNX3 protein-regulated production of interferon-gamma. Immunity. 2010;32:507–517. doi: 10.1016/j.immuni.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Betts MR, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 32.Wildt KF, et al. The transcription factor zbtb7b promotes CD4 expression by antagonizing runx-mediated activation of the CD4 silencer. J Immunol. 2007;179:4405–4414. doi: 10.4049/jimmunol.179.7.4405. [DOI] [PubMed] [Google Scholar]
- 33.Grover HS, et al. The Toxoplasma gondii peptide AS15 elicits CD4 T cells that can control parasite burden. Infect Immun. 2012;80:3279–3288. doi: 10.1128/IAI.00425-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang DJ, et al. Selective expression of the Cre recombinase in late-stage thymocytes using the distal promoter of the Lck gene. J Immunol. 2005;174:6725–6731. doi: 10.4049/jimmunol.174.11.6725. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q, Strong J, Killeen N. Homeostatic competition among T cells revealed by conditional inactivation of the mouse Cd4 gene. J Exp Med. 2001;194:1721–1730. doi: 10.1084/jem.194.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkinson SR, et al. Expression of the transcription factor cKrox in peripheral CD8 T cells reveals substantial postthymic plasticity in CD4–CD8 lineage differentiation. J Exp Med. 2007;204:267–272. doi: 10.1084/jem.20061982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rui J, Liu H, Zhu X, Cui Y, Liu X. Epigenetic Silencing of Cd8 Genes by ThPOK-Mediated Deacetylation during CD4 T Cell Differentiation. J Immunol. 2012 doi: 10.4049/jimmunol.1201077. [DOI] [PubMed] [Google Scholar]
- 38.Mucida D, et al. Transcriptional reprogramming of mature CD4(+) helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat Immunol. 2013;14:281–289. doi: 10.1038/ni.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou YR, et al. Epigenetic silencing of CD4 in T cells committed to the cytotoxic lineage. Nat Genet. 2001;29:332–336. doi: 10.1038/ng750. [DOI] [PubMed] [Google Scholar]
- 40.Reichmann G, et al. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect Immun. 2000;68:1312–1318. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dupont CD, Christian DA, Hunter CA. Immune response and immunopathology during toxoplasmosis. Semin Immunopathol. 2012;34:793–813. doi: 10.1007/s00281-012-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engel I, Zhao M, Kappes D, Taniuchi I, Kronenberg M. The transcription factor Th-POK negatively regulates Th17 differentiation in Valpha14i NKT cells. Blood. 2012;120:4524–4532. doi: 10.1182/blood-2012-01-406280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z, et al. Prevention of experimental colitis in SCID mice reconstituted with CD45RBhigh CD4+ T cells by blocking the CD40-CD154 interactions. J Immunol. 2000;164:6005–6014. doi: 10.4049/jimmunol.164.11.6005. [DOI] [PubMed] [Google Scholar]
- 44.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakaguchi S, et al. The zinc-finger protein MAZR is part of the transcription factor network that controls the CD4 versus CD8 lineage fate of double-positive thymocytes. Nat Immunol. 2010;11:442–448. doi: 10.1038/ni.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong Y, Bosselut R. CD4–CD8 differentiation in the thymus: connecting circuits and building memories. Curr Opin Immunol. 2012;24:139–145. doi: 10.1016/j.coi.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sattentau QJ, Weiss RA. The CD4 antigen: physiological ligand and HIV receptor. Cell. 1988;52:631–633. doi: 10.1016/0092-8674(88)90397-2. [DOI] [PubMed] [Google Scholar]
- 48.Beaumier CM, et al. CD4 downregulation by memory CD4+ T cells in vivo renders African green monkeys resistant to progressive SIVagm infection. Nat Med. 2009;15:879–885. doi: 10.1038/nm.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vinton C, et al. CD4-like immunological function by CD4- T cells in multiple natural hosts of simian immunodeficiency virus. J Virol. 2011;85:8702–8708. doi: 10.1128/JVI.00332-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maeda T, et al. Role of the proto-oncogene Pokemon in cellular transformation and ARF repression. Nature. 2005;433:278–285. doi: 10.1038/nature03203. [DOI] [PubMed] [Google Scholar]
- 51.Intlekofer AM, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 54.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spencer SP, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science. 2014;343:432–437. doi: 10.1126/science.1247606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.