Abstract
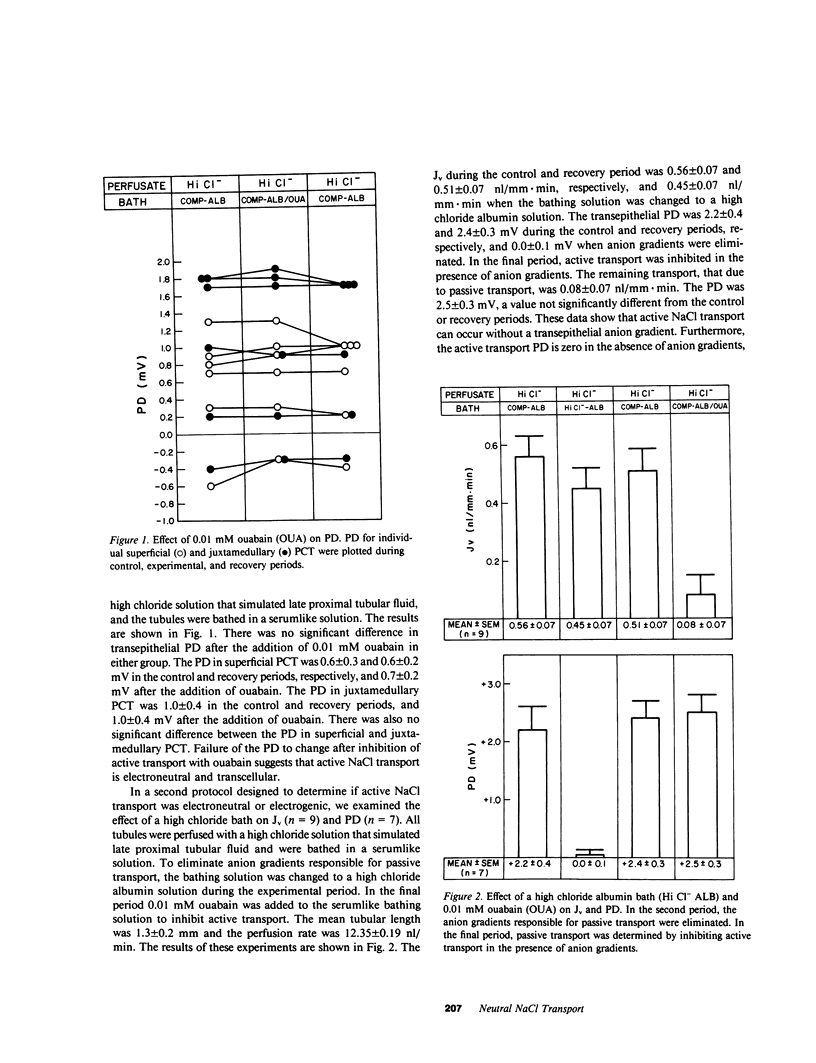
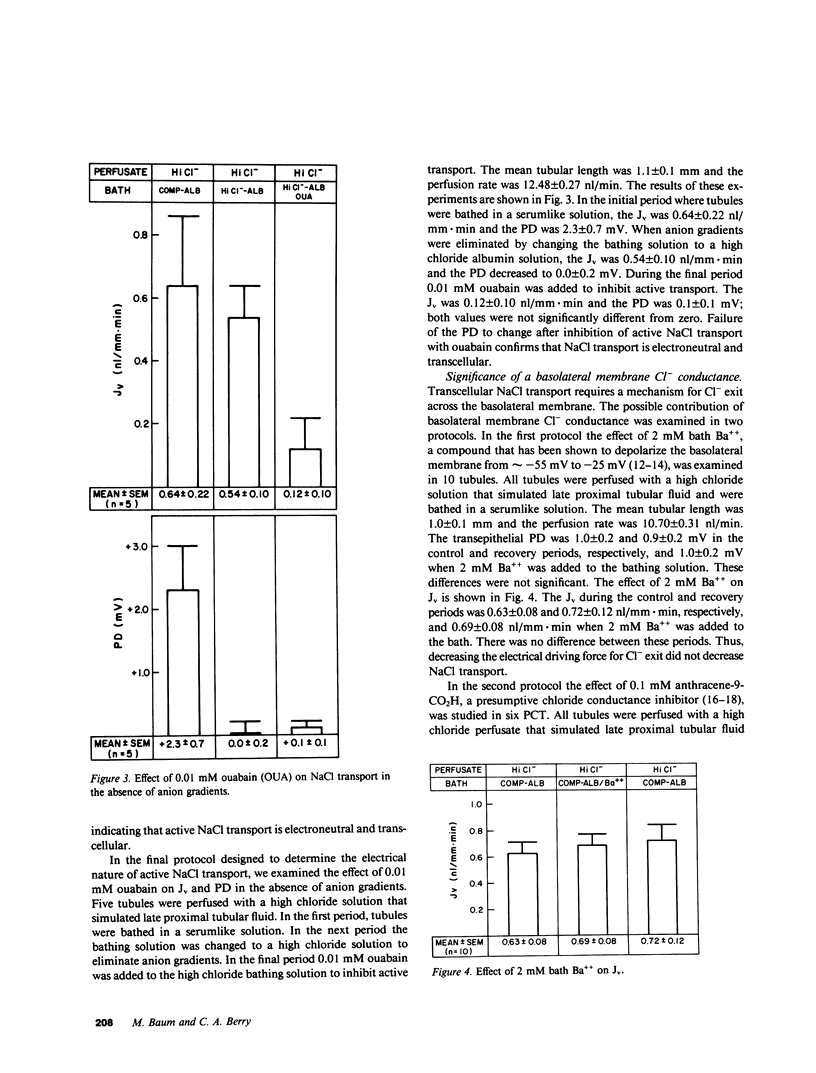
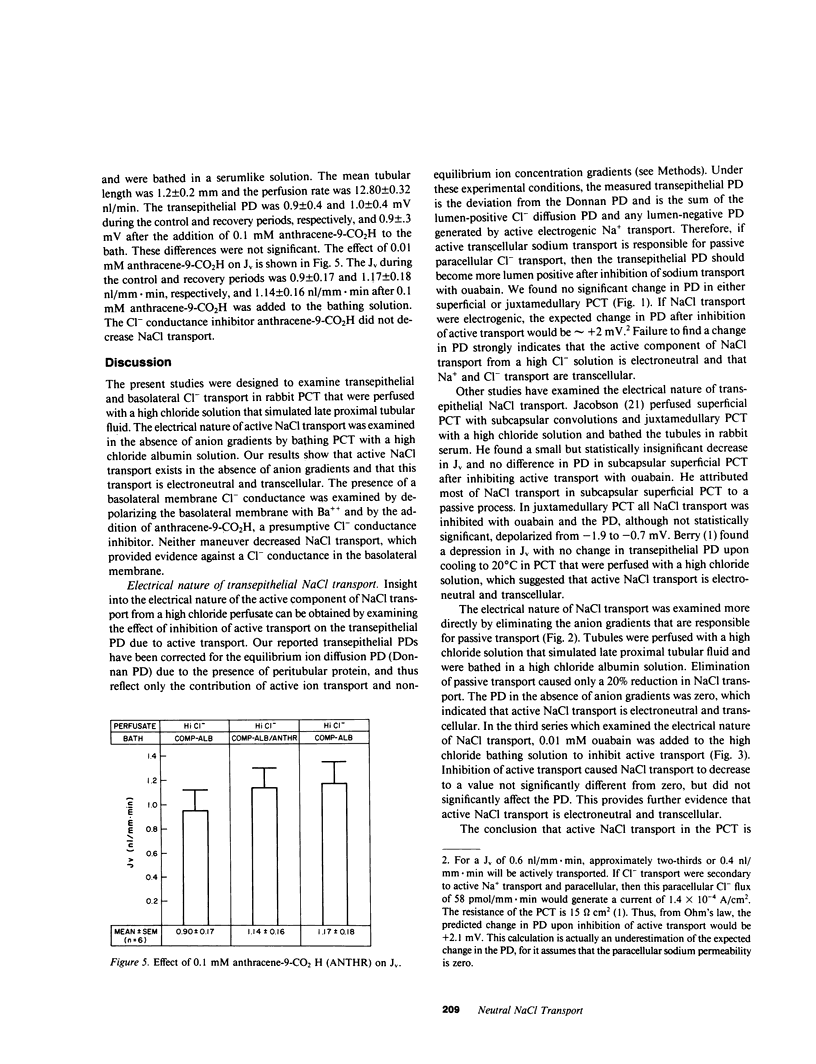
The electrical nature of active NaCl transport and the significance of a basolateral membrane chloride conductance were examined in isolated perfused rabbit proximal convoluted tubules (PCT). PCT were perfused with a high chloride solution that simulated late proximal tubular fluid and were bathed in an albumin solution that simulated rabbit serum in the control and recovery periods. The electrical nature of NaCl transport was examined by bathing the tubules in a high chloride albumin solution where there were no anion gradients. Volume reabsorption (Jv) during the control and recovery period was 0.56 and 0.51 nl/mm X min, respectively, and 0.45 nl/mm X min when the tubules were bathed in a high chloride bath. The transepithelial potential difference (PD) during the control and recovery periods averaged 2.3 mV, but decreased to 0.0 mV in the absence of anion gradients, which indicated that NaCl transport is electroneutral. Further evidence that NaCl transport is electroneutral was obtained by examining the effect of addition of 0.01 mM ouabain in PCT perfused and bathed with high chloride solutions. The Jv was 0.54 nl/mm X min in the control period and not statistically different from zero after inhibition of active transport. The PD was not different from zero in both periods. Two groups of studies examined the role of basolateral membrane Cl- conductance in NaCl transport. First, depolarizing the basolateral membrane with 2 mM bath Ba++ did not significantly affect Jv or PD. Second, the effect of the presumptive Cl- conductance inhibitor anthracene-9-CO2H was examined. Anthracene-9-CO2H did not significantly affect Jv or PD. In conclusion, these data show that NaCl transport in the PCT is electroneutral and transcellular and provide evidence against a significant role for basolateral membrane chloride conductance in the rabbit PCT.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bello-Reuss E. Electrical properties of the basolateral membrane of the straight portion of the rabbit proximal renal tubule. J Physiol. 1982 May;326:49–63. doi: 10.1113/jphysiol.1982.sp014176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. A., Cogan M. G. Influence of peritubular protein on solute absorption in the rabbit proximal tubule. A specific effect on NaCl transport. J Clin Invest. 1981 Aug;68(2):506–516. doi: 10.1172/JCI110282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry C. A. Lack of effect of peritubular protein on passive NaCl transport in the rabbit proximal tubule. J Clin Invest. 1983 Feb;71(2):268–281. doi: 10.1172/JCI110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi B., Kubota T., Sohtell M., Giebisch G. Intracellular potentials in rabbit proximal tubules perfused in vitro. Am J Physiol. 1981 Mar;240(3):F200–F210. doi: 10.1152/ajprenal.1981.240.3.F200. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Duffey M. E., Thompson S. M., Frizzell R. A., Schultz S. G. Intracellular chloride activities and active chloride absorption in the intestinal epithelium of the winter flounder. J Membr Biol. 1979 Nov 30;50(3-4):331–341. doi: 10.1007/BF01868896. [DOI] [PubMed] [Google Scholar]
- Duffey M. E., Turnheim K., Frizzell R. A., Schultz S. G. Intracellular chloride activities in rabbit gallbladder: direct evidence for the role of the sodium-gradient in energizing "uphill" chloride transport. J Membr Biol. 1978 Sep 19;42(3):229–245. doi: 10.1007/BF01870360. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Duffey M. E. Chloride activities in epithelia. Fed Proc. 1980 Sep;39(11):2860–2864. [PubMed] [Google Scholar]
- Goldin S. M. Active transport of sodium and potassium ions by the sodium and potassium ion-activated adenosine triphosphatase from renal medulla. Reconstitution of the purified enzyme into a well defined in vitro transport system. J Biol Chem. 1977 Aug 25;252(16):5630–5642. [PubMed] [Google Scholar]
- Green R., Bishop J. H., Giebisch G. Ionic requirements of proximal tubular sodium transport. III. Selective luminal anion substitution. Am J Physiol. 1979 Mar;236(3):F268–F277. doi: 10.1152/ajprenal.1979.236.3.F268. [DOI] [PubMed] [Google Scholar]
- Jacobson H. R. Characteristics of volume reabsorption in rabbit superficial and juxtamedullary proximal convoluted tubules. J Clin Invest. 1979 Mar;63(3):410–418. doi: 10.1172/JCI109317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucci M. S., Warnock D. G. Effects of anion-transport inhibitors on NaCl reabsorption in the rat superficial proximal convoluted tubule. J Clin Invest. 1979 Aug;64(2):570–579. doi: 10.1172/JCI109495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel W. Inhibition of potassium conductance by barium in frog skin epithelium. Biochim Biophys Acta. 1979 Apr 4;552(2):346–357. doi: 10.1016/0005-2736(79)90289-x. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Ritter M., Lang F., Guggino W. Anthracene-9-carboxylic acid inhibits renal chloride reabsorption. Pflugers Arch. 1983 Jul;398(2):172–174. doi: 10.1007/BF00581068. [DOI] [PubMed] [Google Scholar]
- Palade P. T., Barchi R. L. On the inhibition of muscle membrane chloride conductance by aromatic carboxylic acids. J Gen Physiol. 1977 Jun;69(6):879–896. doi: 10.1085/jgp.69.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector F. C., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol. 1983 May;244(5):F461–F471. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- Reuss L., Weinman S. A., Grady T. P. Intracellular K+ activity and its relation to basolateral membrane ion transport in Necturus gallbladder epithelium. J Gen Physiol. 1980 Jul;76(1):33–52. doi: 10.1085/jgp.76.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuss L., Weinman S. A. Intracellular ionic activities and transmembrane electrochemical potential differences in gallbladder epithelium. J Membr Biol. 1979 Sep 14;49(4):345–362. doi: 10.1007/BF01868991. [DOI] [PubMed] [Google Scholar]
- Shindo T., Spring K. R. Chloride movement across the basolateral membrane of proximal tubule cells. J Membr Biol. 1981 Jan 30;58(1):35–42. doi: 10.1007/BF01871032. [DOI] [PubMed] [Google Scholar]
- Sohtell M. Electrochemical forces for chloride transport in the proximal tubules of the rat kidney. Acta Physiol Scand. 1978 Aug;103(4):363–369. doi: 10.1111/j.1748-1716.1978.tb06229.x. [DOI] [PubMed] [Google Scholar]
- Spring K. R., Kimura G. Chloride reabsorption by renal proximal tubules of Necturus. J Membr Biol. 1978 Jan 18;38(3):233–254. doi: 10.1007/BF01871924. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Eveloff J. NaCl entry mechanisms in the luminal membrane of the renal tubule. Am J Physiol. 1982 Jun;242(6):F561–F574. doi: 10.1152/ajprenal.1982.242.6.F561. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Yee V. J. Chloride uptake by brush border membrane vesicles isolated from rabbit renal cortex. Coupling to proton gradients and K+ diffusion potentials. J Clin Invest. 1981 Jan;67(1):103–115. doi: 10.1172/JCI110002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J. Barium inhibition of basolateral membrane potassium conductance in tracheal epithelium. Am J Physiol. 1983 Jun;244(6):F639–F645. doi: 10.1152/ajprenal.1983.244.6.F639. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Hoshi T. Intracellular Cl- activity of the proximal tubule of Triturus kidney: dependence on extracellular ionic composition and transmembrane potential. Am J Physiol. 1983 Sep;245(3):F359–F366. doi: 10.1152/ajprenal.1983.245.3.F359. [DOI] [PubMed] [Google Scholar]



