Abstract
Background
Arsenic trioxide (As2O3) is highly effective in treating acute promyelocytic leukemia (APL), but shows more variable therapeutic efficacy for other types of hematological malignancies. Previously, we reported that As2O3 selectively eliminates pathogenic B220-expressing T cells in autoimmune MRL/lpr mice. We investigated herein the relationship between As2O3 sensitivity of leukemic T-cell lines and the expression levels of the B220 isoform of transmembrane tyrosine phosphatase CD45.
Methods
GSH content, O2- production, and B220, HSP70, Fas and FasL membrane expression was measured by flow cytometry. Subcellular localization of B220 was determined by imaging flow cytometry. Cell death was analyzed by morphological changes, annexin V and propidium iodide staining, and caspase 8 and 9 activation. B220 mRNA expression was analyzed by RT-PCR. Activated NF-κB p50 was quantified by a DNA binding ELISA.
Results
We selected human (Jurkat, Jurkat variant J45.01, HPB-ALL) and mouse (EL-4, BW5147, L1210) T-cell lines for their marked differences in As2O3 sensitivity over a large range of doses (1 to 20 μM). Differences in redox status cannot explain the dramatic differences in As2O3 sensitivity observed among the T-cell lines. Unexpectedly, we found that B220 is differentially induced on As2O3-treated T-cell lines. As2O3 treatment for 24 h induced low (HPB-ALL), intermediate (Jurkat) and high (EL-4, BW5147) levels of B220 membrane expression, membrane-bound HSP70 and cell death, but inhibited NF-κB p50 nuclear translocation. When high levels of B220 expression were achieved with low doses of As2O3, the T-cell lines died by apoptosis only. When high doses of As2O3 were required to induce B220 expression, leukemic T cells died by both apoptosis and necrosis.
Conclusions
Cellular redox status is not essential for As2O3 sensitivity of leukemic T cells, suggesting the existence of additional factors determining their sensitivity to As2O3 cytotoxicity. Phosphatase B220 could be such a factor of sensitivity. As2O3 treatment inhibits NF-κB p50 nuclear translocation, and induces B220 expression and cell death in a dose and time dependent manner. The levels of B220 induction on leukemic T cells strictly correlate with both the extent and form of cell death, B220 might therefore play a checkpoint role in death pathways.
Electronic supplementary material
The online version of this article (doi:10.1186/1476-4598-13-251) contains supplementary material, which is available to authorized users.
Keywords: As2O3-based therapy, Leukemia, T lymphocyte, Membrane tyrosine-phosphatase B220/CD45R, Membrane-bound HSP70, Fas/Fas ligand pathway, Cell death, Caspase activation, NF-κB p50
Background
Arsenic trioxide (As2O3) shows impressive efficacy in the treatment of patients with acute promyelocytic leukemia (APL) [1–4]. As2O3 induces clinical remission in APL patients by multiple mechanisms [5]. As2O3 promotes cell differentiation at low concentrations [6, 7], whereas it induces apoptosis at higher concentrations [8]. The high sensitivity of the APL cell line NB4 to As2O3-induced cytotoxicity is associated to its low content of reduced glutathione (GSH) and increased production of reactive oxygen species (ROS) [9–11]. Although most studies have been focused on the APL, As2O3 could be beneficial against various hematopoietic malignancies and solid tumors [12]. Moreover, we have shown that As2O3 also possesses immunomodulatory properties, and might be a therapeutic agent for autoimmune diseases. Indeed, As2O3 selectively eliminates the pathogenic B220-expressing double negative (DN) CD4-CD8- T cells that accumulate in autoimmune MRL/lpr mice due to the lpr mutation of the death receptor Fas [13, 14].
In normal murine and human T cells, CD4+ and CD8+ effector T cells massively induce the expression of transmembrane tyrosine phosphatase B220 before undergoing apoptosis by the Fas/Fas ligand (FasL) pathway [15, 16]. In Fas-deficient mice and patients, CD4+ and CD8+ effector T cells also express the B220 molecules at their surface, but then they downregulate their CD4 or CD8 molecules while maintaining B220 plasma membrane expression. B220 (or CD45RABC) is one of the five isoforms of the transmembrane tyrosine phosphatase CD45 found on lymphocytes. CD45 isoforms are generated by cell-type and activation-state specific alternative splicing of exons 4/A, 5/B, and 6/C encoding domains at the NH2-terminus. Naive T cells express high molecular weight CD45 isoforms (CD45RA or CD45RB) containing the A domain in humans or the B domain in mice whereas effector/memory T cells expressed the low molecular weight isoform CD45RO lacking extracellular domains A, B and C. All CD45 isoforms share the same intracellular region, which contains two phosphatase domains. Although the function of each isoform remains unknown, it is well established that CD45 phosphatase activity is crucial for lymphocyte development, and antigen and cytokine receptor signaling [17–19]. CD45 might also regulate apoptosis of T and B lymphocytes [20–22].
In this study, we found that murine (EL-4, BW5147, L1210) and human (Jurkat, CD45-deficient Jurkat variant, HPB-ALL) leukemic T-cell lines dramatically differed in their sensitivity to As2O3-induced cell death. In contrast with previous findings in APL cell line NB4 [9, 10], these differences in As2O3 sensitivity are independent of intracellular GSH content and O2- production. Unexpectedly, we found that As2O3 differently induced B220 cell surface expression in the leukemic T-cell lines in a dose- and time-dependent manner. Moreover, the levels of B220 expression correlated with the sensitivity of these T-cell lines to As2O3. Induction of B220 membrane expression by As2O3 treatment is reminiscent of that observed on antigen-activated normal T-cell blasts before undergoing apoptosis [15, 16]. Therefore, the leukemic T-cell lines were activated with calcium ionophore A23187, which triggers both cell activation and cell death. Calcium ionophore A23187 also induced B220 expression and cell death, but with reverse efficiencies in the leukemic T-cell lines compared to As2O3. In addition, T-cell lines treated with A23187 most probably died by an activation-induced cell death mechanism since the T-cell activation marker CD69 is expressed before B220 expression and cell death. In contrast, CD69 was not detected on As2O3-treated cells, indicating that B220 expression occurs independently of leukemic T-cell activation. Surprisingly, we found that B220 is expressed constitutively on L1210 T cells. L1210 cells were highly sensitive to A23187 treatment whereas they were highly resistant to As2O3 cytotoxicity, indicating that the constitutive high-level expression of B220 did not favor cell death triggered by As2O3. B220 induction on the T-cell lines after treatment with As2O3 or calcium ionophore A23187 strictly correlates with sensitivity to cell death, emphasizing the role of B220 as a proapoptotic factor. However, our data indicate that As2O3 and A23187 trigger B220 induction and cell death through different upstream signaling pathways. Different signaling pathways, such as the c-Jun NH2-terminal kinase, have been implicated as mediators of the cytotoxic effects of As2O3 in the APL cell line NB4 [23]. Here, we show that high induction of B220 expression on leukemic T cells is a determining factor leading to As2O3-triggered cell death. Thus we hypothesize that B220 might play a checkpoint role in death pathways.
Results
Human and mouse leukemic T-cell lines exhibit different sensitivities to As2O3-induced growth inhibition and cytotoxicity
In addition to APL-derived NB4 cells, As2O3 kills various cancer cell types [12]. However, As2O3 sensitivity varies considerably among tumor cell lines. In the present study, we determined the sensitivity to As2O3-induced cytotoxicity and growth inhibition of human (Jurkat and HPB-ALL) and murine (EL-4, BW5147 and L1210) leukemic T-cell lines. The five T-cell lines along with APL-derived NB-4, known for its high sensitivity to As2O3 cytotoxicity, were treated with As2O3 in doses ranging from 1 to 20 μM for 12, 24 and 48 h. Then, the percentage of living cells was determined by Annexin V and propidium iodide (PI) staining, and flow cytometry. We rapidly abandoned treatments for 12 h and 48 h. Indeed, 100% EL-4 and BW5147 cells were killed after 24 h of treatment with low doses of As2O3, whereas HPB-ALL and L1210 cells showed no sensitivity to As2O3 cytotoxicity before 24 h of treatment, even with high doses (data not shown). Because 24 h of treatment was the optimal time to observe significant differences in As2O3 cytotoxicity among T-cell lines, this duration was used in subsequent experiments. Sensitivity to As2O3 varied considerably among the T-cell lines (Figure 1A). While around 3% NB4, 6% EL-4 and 13% BW5147 cells remained alive (Annexin V- and PI-) at a concentration of 4 μM As2O3, remarkably, the percentages of living cells were around 65% for Jurkat cells, and between 80% and 90% for HPB-ALL and L1210 cells (Figure 1A). In addition, even at a dose of 20 μM, 20% L1210 and 40% HPB-ALL cells remained Annexin V- and PI- (Figure 1A). The marked differences in As2O3 sensitivity among the T-cell lines can also be shown by calculating the concentration of As2O3 that killed 50% of the cells (IC50 value). The IC50 value of As2O3 was 1.5 μM for EL-4, 2.5 μM for BW5147, 6 μM for Jurkat, 13 μM for L1210 and 15 μM for HPB-ALL cells compared with 1.5 μM for the highly sensitive APL-derived NB4 cells (Figure 1A). As2O3 cytotoxicity was also evaluated through flow cytometric analysis of physical characteristics of As2O3-treated T-cell lines using forward (FSC) and side (SSC) scatters since dying or dead cells have lower FSC and higher SSC than living cells. Depending upon the dose of As2O3, two populations were observed on the dot plots for FSC vs. SSC. The one containing large cells (region R2) encompassed live cells, and the other containing smaller cells (region R1) encompassed dying/dead cells (Additional file 1: Figure S1A). We observed that 50% of the cells of region R2 shifted to region R1 at 2.5 to 3 μM As2O3 for BW5147 and EL-4 cells, 6 μM for Jurkat cells, and 15 μM for L1210 and HPB-ALL cells compared with 1.5 μM for the APL cell line NB4 (Figure 1B). In parallel, we determined As2O3-induced cell growth inhibition by counting the total cell number in each condition using flow cytometry. IC50 value for growth inhibition was around 1 μM for NB4 and EL-4 cells, 3.5 μM for BW5147 cells, 8 μM for Jurkat, 15 μM for L1210 and HPB-ALL cells. In summary, EL-4 and BW5147 cells, and to a lesser extent Jurkat cells, are among the most sensitive T-cell lines to As2O3-induced cell growth inhibition and cytotoxicity, whereas L1210 and HPB-ALL cells are the most resistant.
Figure 1.
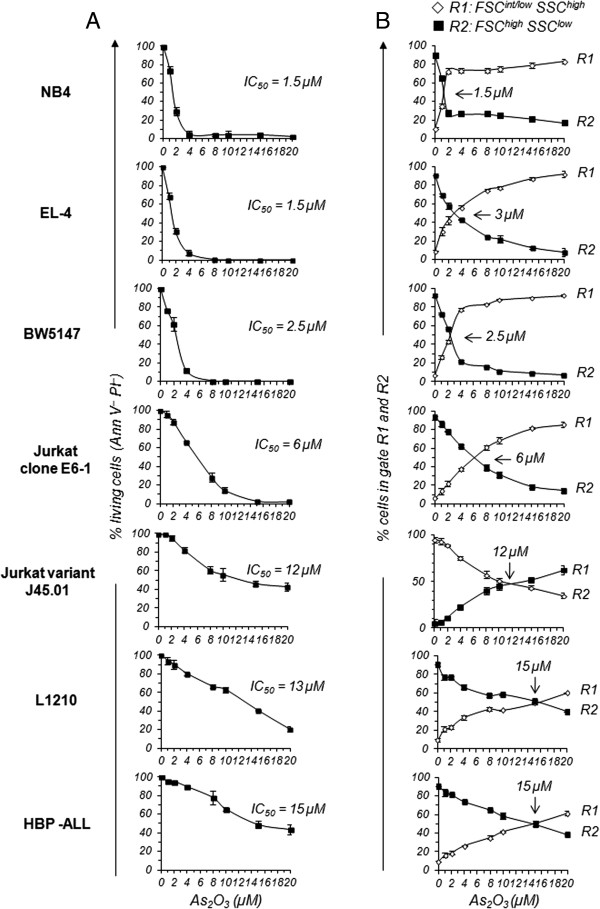
Viability and cell morphology of various T-cell lines cells upon As 2 O 3 -treatment. (A) APL-derived NB4 cells as well EL-4, BW5147, L1210, Jurkat (clone E6-1), CD45-deficient Jurkat variant (J45.01) and HPB-ALL T-cell lines were treated with As2O3 for 24 h with doses ranging from 1 to 20 μM. T-cell lines were then stained with Annexin V and PI, and the percentages of living cells (Ann V- PI-) determined by flow cytometry. At least 20,000 events were analyzed for each sample. Graphs show mean percentages ± SE of Ann V- PI- cells from more than 4 independent experiments. IC50 in the graphs indicates the concentration of As2O3 that kills 50% of the cells. (B) Cells were analyzed by flow cytometry with respect to size (FSC) and granulosity (SSC). Regions R1 and R2 identified on a FSC vs. SSC dot plot encompassed cells with FSCintermediate(int)/lowSSChigh and FSChighSSClow, respectively. At least 20,000 events were analyzed for each sample. Graphs show mean percentages ± SE of cells located in region R1 (◊) and R2 (■) at the indicated concentration of As2O3. Numbers in the graphs indicate the concentration of As2O3 at which 50% of the cells shifted from region R2 to region R1. Four independent experiments were performed for NB4 and J45.01 cells, and more than 10 for EL-4, BW5147, L1210, Jurkat and HPB-ALL cells.
Lack of evidence for the implication of GSH and O2- in As2O3-induced cytotoxicity in T-cell lines
As2O3 is able to impair the function of the mitochondrial respiratory chain, leading to increased O2- production [24]. O2- is neutralized by intracellular GSH, which is the major antioxidant produced by the cell. The detoxification function of intracellular GSH also includes the cellular efflux of As2O3 [25]. Therefore, we performed flow cytometry analysis of intracellular GSH content and O2- production in the T-cell lines before and after treatment with As2O3 (Figure 2). APL-derived NB4 cells were used as a reference because intracellular redox status has been shown to be important in APL sensitivity to As2O3 [9, 10]. As observed in many cancer cells [25], the untreated cell lines showed considerable heterogeneity in the intrinsic levels of O2- production, with untreated NB4 cells displaying the lower level of O2- (Figure 2A). In As2O3-treated APL-derived NB4 cells, O2- production increased with the dose of arsenic (Figure 2B). In As2O3-sensitive EL-4 and BW5147 T-cell lines, O2- production was not increased by As2O3 treatment even at the high dose of 15 μM (Figure 2B). In contrast, in As2O3-resistant Jurkat and L1210 T-cell lines, O2- production was markedly increased (Figure 2B). Therefore, these data indicate that the differences in As2O3 sensitivity between EL-4, BW5147, Jurkat and L1210 cells are not directly linked to increased production of O2-.
Figure 2.

Intracellular levels of O 2 - and GSH in cell lines before and after As 2 O 3 treatment. Intracellular levels of O2 - and GSH were measured using CMFDA or DHE fluorescent probes and flow cytometry. (A) Untreated APL-derived NB4 cells and T-cell lines were examined for intrinsic levels of O2 - production and GSH content. Histograms obtained with specific fluorescent probes (colored histograms) are overlaid on fluorescence histograms of unstained cells (black dotted histogram). A total of 20,000 events were analyzed for each histogram. Graphs report mean ± SE (n = 3 independent experiments) of integrated MFI values for O2 - production and GSH content. The integrated MFI is calculated by multiplying the frequency of positive cells by the MFI of O2 - and GSH. Asterisks denote statistically significant differences for levels of GSH or O2 - between leukemic T-cell lines and APL-derived NB4 cells: *p ≤0.05; ***p ≤0.001. (B) O2 - production and GSH content were examined in APL-derived NB4 cells and T-cell lines treated with 1 μM to 15 μM As2O3 for 24 h. Graphs report mean ± SE (n = 3 independent experiments) of integrated MFI values for O2 - production and GSH content.
As expected, As2O3-sensitive APL-derived NB4 cells displayed extremely low level of intrinsic GSH content (Figure 2A). In contrast, the intrinsic GSH content in untreated EL-4 and BW5147 T-cell lines were significantly higher than in untreated HPB-ALL and L1210 T-cell lines (Figure 2A), although L1210 and HPB-ALL cells were considerably more resistant to the cytotoxic effect of As2O3 than EL-4 and BW5147 cells. At the dose of 1 μM As2O3, levels of GSH were unaffected by the treatment in the extremely sensitive NB4 cells, but were markedly decreased in As2O3-sensitive BW5147 T cells and also, although to a lesser extent, in the As2O3-resistant Jurkat cells (Figure 2B). Furthermore, in the extremely resistant L1210 T cells, GSH production was unchanged even at the high dose of 15 μM As2O3 (Figure 2B). Therefore, our data indicate that intracellular GSH content alone cannot account for the differences in As2O3 sensitivity observed among the five T-cell lines.
Induction of B220/CD45R expression on As2O3-treated human and murine T-cell lines
Normal effector T cells undergoing apoptosis at the end of an immune response express B220 on their cell surface. These apoptotic B220+ T cells are solely found within the FSCint/low SSChigh subset by flow cytometry analysis [15, 16]. Interestingly, we observed that As2O3 is also able to induce cell-surface expression of B220 on EL-4, BW5147, Jurkat and HPB-ALL T-cell lines in a dose-dependent manner (Figure 3). However, B220 expression levels differed significantly among these four T-cell lines. Thus, a significantly higher percentage of EL-4 cells, and to a lesser extent of BW5147 and Jurkat cells, expressed B220 compared to HPB-ALL cells (for instance, see 2 μM dose in Table 1 or 4 μM dose in Figure 3). B220+ leukemic T cells were found in both gate R1 and R2 that contained FSCint/low SSChigh dying cells (Figure 3) and FSChigh SSClow living cells (data not shown), respectively, although the numbers were significantly higher in gate R1. As2O3 also induces B220 expression at the mRNA level in a dose- and time-dependent manner. High levels of B220 mRNA were found in EL-4 and BW5147 within 3 h of treatment with 1 μM As2O3. Higher doses of As2O3 (e.g., 4 μM for 3 h) or longer treatment (e.g., 1 μM for 9 h) resulted in increased levels of B220 mRNA (Additional file 1: Figure S1C). In contrast, As2O3 did not induce B220 expression on APL-derived NB4 cells (data not shown). To confirm that A2O3-induced cytotoxicity in leukemic T cells and B220 expression are mechanistically related, we analyzed CD45-deficient Jurkat cells (clone J45.01) for their As2O3 sensitivity over a large range of doses (1 to 20 μM). As shown in Additional file 1: Figure S1B, J45.01 T-cell line displays a strong reduction in the expression level of CD45 per cell (MFI) compared to wild-type Jurkat cells (MFI J45.01: 40; MFI Jurkat: 1528; MFI isotype control: 5). The IC50 value for cytotoxicity was around 12 μM for J45.01 compared to 6 μM for wild-type Jurkat cells, corroborating the critical role of the B220 isoform of CD45 in the resistance/sensitivity of leukemic T cells to As2O3.
Figure 3.

Induction of phosphatase B220/CD45R and HSP70 on As 2 O 3 -treated T-cell lines. EL-4, BW5147, Jurkat, CD45-deficient Jurkat variant (J45.01) and HPB-ALL T-cell lines were treated with As2O3 for 24 h in doses ranging from 1 to 20 μM. (A) T-cell lines were then stained with PE-conjugated anti-B220/CD45R mAb or PE-conjugated rat IgG2a isotype control, and then analyzed by flow cytometry with respect to size (FSC) and B220 expression. FSC vs. B220 contour plots were used to determine the percentage of B220+ cells in region R1 (FSCint/lowSSChigh). At least 20,000 events were analyzed per sample. Squares in contour dots contain B220+ cells, and are drawn relative to the location of the cells in FSC vs. IgG2a isotype control contour plots. Numbers in the squares indicate the percentages of B220+ cells. Graphs report mean percentages ± SE (n =10 independent experiments) of B220+ cells versus the dose of As2O3. (B) T-cell lines were stained with APC-conjugated anti-B220 and FITC-conjugated anti-HSP70 antibodies or FITC-conjugated IgG and APC-conjugated IgG2a isotype control. FSC vs. SSC dot plots were used to define gates R1 and R2 with FSCint/lowSSChigh and FSChighSSClow, respectively. HSP70 vs. B220 dot plots were then gated in R1 and R2 to determine the percentages of cells expressing HSP70, B220 or both. At least 20,000 events were analyzed for each sample. Graphs report the percentages of cells co-expressing B220 and HSP70 in gates R1 (◊) or R2 (■) versus the dose of As2O3.
Table 1.
Opposite effects of As 2 O 3 or Calcium ionophore A23187 on a panel of leukemic T-cell lines
| As 2O 3 (2 μM*) | Calcium ionophore A23187 (100 nM) | |||||||
|---|---|---|---|---|---|---|---|---|
| T-cell lines | B220 upregulation** | CD69 upregulation** | HSP70 upregulation** | Cell death*** | B220 upregulation** | CD69 upregulation** | HSP70 upregulation** | Cell death*** |
| EL-4 | 63 | No | 35 | 70 | 7 | Yes | no | 1 |
| BW5147 | 30 | No | 20 | 39 | 19 | Yes | no | 25 |
| Jurkat clone E6-1 | 22 | No | 10 | 12 | 30 | Yes | no | 28 |
| HPB-ALL | 10 | No | 3 | 5 | 80 | Yes | no | 75 |
| L1210 | Constitutive expression | No | 5 | 10 | Constitutive expression | Yes | no | 55 |
*a clinically relevant concentration of As2O3.
**percentage of positive cells determined by flow cytometry.
***percentage of PI+ cells (either Annexin V- or Annexin V+) determined by flow cytometry.
Unexpectedly, we also found that L1210 T cells constitutively express B220 at the plasma membrane. L1210 cells undoubtedly belong to the T-cell lineage because they coexpress the mouse pan T-cell marker CD90 (Additional file 2: Figure S2A). To the best of our knowledge, we have shown for the first time that B220 can be constitutively expressed at high levels on T cells, in the absence of external stimulation. As2O3 treatment did not change either the percentages of B220+ L1210 cells, or the expression level of B220 per cell (MFI) (Additional file 2: Figure S2B).
Furthermore, we have compared the subcellular localization and distribution of B220 molecules that were either constitutively expressed on L1210 cells or As2O3-induced on EL-4 cells, using ImageStream device, which allows simultaneous flow cytometry and fluorescence microscopy analysis on a large numbers of cells. Whole cell and nuclear boundaries were identified using brightfield image and DAPI staining, respectively. In the absence of As2O3 treatment, no labeling with anti-B220/CD45R mAb was observed in EL-4 cells (Figure 4A). In contrast, in untreated L1210 cells and 2 μM As2O3-treated EL-4 cells, B220 molecules were expressed at high levels with a restricted localization to the cell surface (Figure 4A and B), confirming the results obtained by flow cytometry. However, B220 molecules appeared less uniformly distributed over the plasma membrane of EL-4 cells (Figure 4A) than L1210 cells (Figure 4B). Thus, L1210 cells showed a uniform fluorescence at the periphery of the cell, whereas a variable number of bright spots of fluorescence were distributed at the periphery of EL-4 cells.
Figure 4.
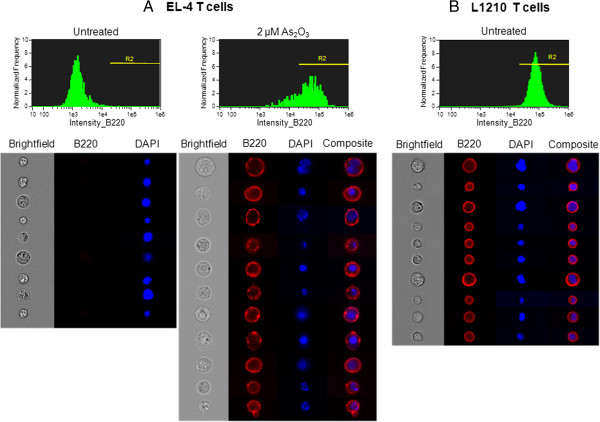
Subcellular localization of constitutively expressed and As 2 O 3 -induced B220/CD45R molecules. Untreated and As2O3-treated (2 μM for 24 h) EL-4 cells (panel A), and untreated L1210 cells (panel B) were stained with PE-conjugated anti-B220/CD45R mAb and DAPI nuclear dye. Samples were then analyzed on the ImageStream system. At least 10,000 images were collected per sample at 40× and 60× magnification. B220-PE positive cells were gated (gate R2) according to the principle for flow cytometry as shown on histogram. Background staining was determined using PE-conjugated rat IgG2a isotype control. Representative cell images of brightfield illumination, B220-PE (red) and DAPI (blue) fluorescence and composite images of B220/DAPI are shown on panel A and B.
Duration of B220/CD45R membrane expression after As2O3 treatment
To determine the duration of B220 expression following As2O3 treatment, EL-4 and Jurkat cells were cultured in the presence of 1 to 8 μM As2O3 for 24 h. Cells were then extensively washed to eliminate As2O3, and cultured in complete medium for 9 additional days. As2O3 treatment for 24 h induced B220 expression in a dose-dependent manner (Additional file 3: Figure S3, graphs), particularly on EL-4 cells, corroborating the findings reported in Figure 3. On day 1 post-treatment, and at concentrations of 1 μM and 8 μM As2O3, the percentages of B220+ EL-4 cells were around 50% and 95%, respectively. One week later, the percentage of B220+ EL-4 cells fell below 15% at 1 μM As2O3, whereas it remained at 90% at the concentration of 8 μM (Additional file 3: Figure S3, graphs). Likewise in Jurkat cells, on day 7 post-treatment, around 50% of cells treated with 8 μM As2O3 expressed B220 (Additional file 3: Figure S3, dot plots and graph), while B220 could no longer be detected on Jurkat cells treated with 1 to 4 μM As2O3 (Additional file 3: Figure S3, graph). The loss of B220 expression on EL-4 and Jurkat cells was accompanied by a decreased cell number in gate R1 (FSCint/low SSChigh) and an expansion of viable cells in gate R2 (Additional file 3: Figure S3, dot plots, see days 7 and 9). The duration of B220 expression in gate R1 was dependent on the dose of As2O3 treatment. Thus, a longer duration of B220 expression was associated with a higher concentration of As2O3, and a higher number of B220+ cells at the time of As2O3 removal. The decrease in the percentages of B220+ cell population could be due to the death of B220+ cells, and/or the proliferation of the remaining B220- cells. However, even on day 9 post-treatment, EL-4 and Jurkat cells had not fully recovered their initial proliferative capacity (data not shown). Our data suggest that dead B220+ T cells release their As2O3 content into the medium, which is subsequently recaptured by living B220-negative T cells. The amount of released As2O3 is dependent on the dose used at the time of treatment.
Expression of membrane-bound HSP70 on As2O3-treated T-cell lines parallels the induction of B220/CD45R
Heat shock protein 70 (HSP70) is upregulated intracellularly in respond to a variety of stresses including chemical or chemotherapeutic agents. HSP70 proteins can also be found at the cell surface of some tumor cells. Notably, membrane-bound HSP70 has been shown to elicit antitumor immunity [26, 27]. Therefore, HSP70 and B220 expression was determined by flow cytometry on the plasma membrane of the T-cell lines treated with As2O3 for 24 h in doses ranging from 1 to 20 μM. HSP70 was not detected on untreated T-cell lines (Figure 3B). As2O3 treatment upregulated membrane-bound HSP70 in EL-4, BW5147, Jurkat and HPB-ALL cells in a dose-dependent manner. The expression pattern of HSP70 was parallel to that of B220, and HSP70 was expressed in B220+ cells only (Figure 3B). As observed for B220 expression, a significantly higher percentage of EL-4 cells, and to a lesser extent of Jurkat cells, than HPB-ALL cells expressed HSP70 (Figure 3B) (Table 1). L1210 cells, constitutively expressing B220, had a low level of expression of membrane-bound HSP70 (Additional file 2: Figure S2C) similar to HSP70 levels observed in HPB-ALL cells. Therefore, As2O3 treatment induced both HSP70 and B220 membrane expression and the levels of expression correlated with leukemic T-cell lines sensitivity to As2O3.
B220/CD45R expression on As2O3-treated leukemic T cells is not a direct consequence of T-cell activation unlike the expression induced by the calcium ionophore A23187
The induction of B220 on EL-4, BW5147, Jurkat and HPB-ALL leukemic T cells treated with As2O3 is suggestive of the induction observed on normal T cells entering apoptosis after repeated activation by their antigen. T-cell activation can be evidenced by measuring the expression of surface marker CD69 [28]. Therefore, we have analyzed the induction of CD69 on the T-cell lines treated with As2O3 as well as with PMA and calcium ionophore A23187 as positive controls. As expected, the activation of leukemic T cells with 10 ng/ml of PMA for 5 h induced a strong expression of the activation marker CD69 in cells within region R2 only, which encompasses living cells. In contrast, we did not detect the expression of CD69 by flow cytometry on the cell surface of As2O3-treated leukemic T cells, whatever the doses tested (1 to 20 μM, data not shown), suggesting that the induction of B220 expression by As2O3 is not a direct consequence of T-cell activation.
As observed with PMA, activation of the leukemic T-cell lines with 100 nM to 800 nM of the calcium ionophore A23187 for 24 h induced the expression of CD69 on EL-4, BW5147, L1210, and Jurkat cells and also, to a lesser extent, on HPB-ALL cells (Table 1). However, the number of CD69+ cells decreased with the dose of A23187, and hardly any CD69+ cells were detected at the higher dose of A23187 (data not shown). In contrast, a dose-dependent expression of B220 was detected on EL-4, BW5147, Jurkat and HPB-ALL cells, although significant differences were observed in the percentages of B220+ cells among T-cell lines (Figure 5). Thus, 50% of B220+ cells were detected at a dose of A23187 around 50 nM for HPB-ALL cells, 100–130 nM for Jurkat cells, 275 nM for BW5147 and 600 nM for EL-4 cells (Figure 5, see B220 induction). Interestingly, at around the same dose of A23187 that induced B220 expression, 50% of HPB-ALL, Jurkat, BW5147 and EL-4 cells died (Annexin V and PI staining, Figure 5, see Cell viability) or shifted from region R2 to R1 on the FSC vs. SSC dot plots (Figure 5, see Cell morphology). In contrast to As2O3, the constitutive expression of B220 on L1210 T cells renders these cells remarkably sensitive to A23187 (Figure 5, see Cell viability and Cell morphology). Therefore, B220 expression level strictly correlates with the susceptibility of T cells to die following A23187 activation, suggesting an interesting link between transmembrane phosphatase B220 expression and cell death. Moreover, in contrast with As2O3 treatment, membrane-bound HSP70 was not upregulated following treatment with calcium ionophore A23187, even on the B220+ T-cell subpopulations (data not shown).
Figure 5.
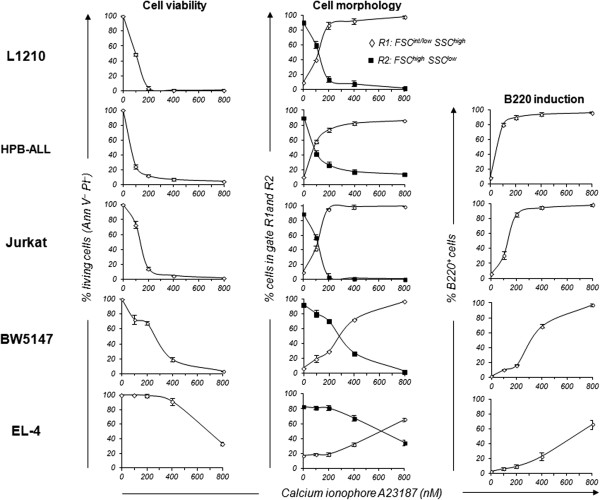
Cell viability, cell morphology and B220/CD45R induction in T-cell lines stimulated with calcium ionophore A23187. Jurkat, HPB-ALL, L1210, EL-4 and BW5147 cells were treated with Calcium ionophore A23187 for 48 h in doses ranging from 100 to 800 nM. Cell viability measurement. Untreated and A23187-treated cells were stained with Annexin V and PI, and the percentages of living cells (Ann V- PI-) determined by flow cytometry. Graphs show mean percentages ± SE of Ann V- PI- from 4 independent experiments. Cell morphology analysis. Untreated and A23187-treated cells were analyzed by flow cytometry with respect to size (FSC) and granulosity (SSC). Regions R1 (◊) and R2 (■) identified on FSC vs. SSC dot plots encompassed cells with FSCint/lowSSChigh and FSChighSSClow, respectively. B220 induction. Untreated and A23187-treated T-cell lines were stained with anti-B220/CD45R mAb and analyzed by flow cytometry. Graphs report mean percentages ± SE (n = 4 independent experiments) of B220+ EL-4, BW5147, Jurkat and HPB-ALL cells at the indicated concentration of A23187. At least 20,000 events were analyzed per sample in all experiments reported in the graphs.
Relative contributions of apoptosis and necrosis in T leukemia cell death induction by As2O3 or calcium ionophore A23187
It is widely considered that As2O3 exerts its cytotoxic activity by triggering cell apoptosis [12]. However, it has been reported that As2O3 can induce cell death independently of caspase activity [29, 30]. We have determined whether the apoptotic and/or necrotic pathways were activated upon As2O3 or calcium ionophore A23187 treatment in EL-4, Jurkat, HPB-ALL and L1210 cells. Because BW5147 cells displayed similar sensitivity to As2O3 and A23187 as EL-4 and Jurkat cells, respectively, the latter two T-cell lines were preferentially chosen for subsequent experiments. In the presence of As2O3 or A23187, no PI+ cells (either Annexin V- or Annexin V+) were detected in the B220-negative cell subpopulation (data not shown). In contrast, in the B220+ cell subpopulation, the IC50 value of As2O3 that causes either apoptotic or necrotic cell death was 1.5 μM for EL-4 cells, 8 μM for Jurkat cells and 9 μM for L1210 cells. For B220+ HPB-ALL cells, even at the higher dose of 20 μM As2O3, only 39 ± 2.5% of the cells were killed (Figure 6A). Furthermore, to discriminate between late apoptosis (PI+/Annexin V+) and necrosis (PI+/Annexin V-), we analyzed the levels of active initiator caspases 8 and 9 in the B220+ PI+-cell subpopulation. Significative differences were observed between the T-cell lines, even though the levels of active caspase 8 and/or 9 increased with the dose of As2O3 (Figure 6B and C). Thus, EC50 for caspase 8 activation was 2 μM for EL-4 cells and 6.5 μM for Jurkat cells (Figure 6B). To activate caspase 9 in these T-cell lines, almost twice as much As2O3 was required compared with caspase 8 (Figure 6C). Even at the higher dose of 20 μM As2O3, only 16 to 19% of HPB-ALL cells and ~40% of L1210 cells contained active caspase 8 or caspase 9 (Figure 6B and C). Since As2O3 can also induce cell death independently of caspase activity [30], we have determined the percentages of necrotic cells (PI+/Annexin V- or PI+/active Caspase-). No necrotic cells were detected in As2O3-treated EL-4 cells. In contrast, 4 to 29% necrotic cells were detected in As2O3-treated Jurkat, HPB-ALL and L1210 cells (Figure 6D). In agreement with Figure 4, the IC50 value of calcium ionophore A237187 that cause either apoptotic or necrotic cell death was between 80 nM and 150 nM for L1210, HPB-ALL and Jurkat cells compared with around 600 nM for EL-4 cells (Figure 6A). The relative contributions of apoptotic and necrotic cell death in calcium ionophore A23187 cytotoxicity showed an opposite pattern compared with As2O3 cytotoxicity. In the presence of calcium ionophore A23187, up to 32% necrotic cells were detected in EL-4 cells, whereas no necrotic cells were detected in HPB-ALL cells and less than 8% were detected in L1210 and Jurkat cell lines (Figure 6D). Moreover, EL-4 cells did not contain active caspase 8 and 9, whereas high levels of active caspase 8 and 9 were detected in L1210, HPB-ALL, and Jurkat T-cell lines (Figure 6B and C).
Figure 6.
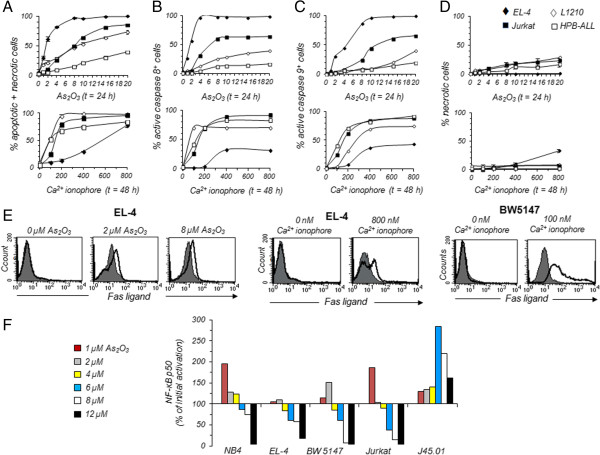
Contribution of apoptosis, necrosis and NF-κB p50 pathways in the cytotoxicity of As 2 O 3 or calcium ionophore A23187. (A to E) EL-4 (♦), L1210 (◊), Jurkat (■) and HPB-ALL (□) T-cell lines were treated with 1 to 20 μM As2O3 for 24 h or 100 to 800 nM A23187 for 48 h. Cells treated with As2O3 or A23187, or left untreated, were subsequently stained with PI and CaspaTag Caspase 8 or Caspase 9 fluorescein in situ fluorescence assay kit. The stained cells were analyzed by flow cytometry in order to determine the percentages of dead cells (either apoptotic or necrotic cells, graphs A) (n = 5 independent experiments), the percentages of PI+ apoptotic cells containing active caspase 8 (graphs B) or active caspase 9 (graphs C), the percentage of necrotic cells (graphs D). Human or murine FasL expression was detected using anti-FasL mAb and flow cytometry (histograms E). Histograms obtained with PE-conjugated Armenian hamster (clone MFL3) or mouse (clone NOK-1) anti-FasL mAb (open histogram) are overlaid on histograms obtained with PE-conjugated Armenian hamster or mouse IgG1 isotype control (shaded histogram) (n = 3 independent experiments). At least 20,000 events were analyzed for each sample. (F). Nuclear extracts were prepared from NB4, EL-4, BW5147, Jurkat and J45.01 cells treated with 1 to 12 μM As2O3 for 24 h. Levels of NF-κB p50 in 5 μg of nuclear extracts were quantified by a DNA binding ELISA assay. Results are representative of two other experiments.
To summarize, when high levels of B220 membrane expression were achieved with low doses of As2O3 or calcium ionophore A23187, the leukemic T-cell lines died by apoptosis only. When high doses of As2O3 or calcium ionophore A23187 were required to induce B220 expression, the T-cell lines died by both apoptosis and necrosis. The constitutive expression of B220 on L1210 cells did not favor apoptosis or necrosis, and cells died by a combination of the two. Taken together, our data suggest that transmembrane tyrosine phosphatase B220 plays a checkpoint role in apoptotic pathways since its expression is markedly and rapidly induced on the surface of T cells undergoing apoptosis.
Expression of Fas and Fas ligand upon treatment with As2O3 or A23187
The presence of active initiator caspase 8 in the T-cell lines treated with A23187 or As2O3 suggested that cells entered apoptosis upon engagement of death receptors such as Fas. In normal T cells, Fas receptor is constitutively expressed whereas its ligand, FasL, is expressed after T cell activation. The five T-cell lines were treated or not with As2O3 or calcium ionophore A23187 for 24 h and 48 h, and the levels of Fas and FasL expression were determined by flow cytometry. Fas receptor is strongly expressed on untreated Jurkat cells, weakly expressed on untreated HPB-ALL cells, and not detected on untreated EL-4, BW5147 and L1210 cells. No significant modulation in Fas receptor expression was observed after treatment with As2O3 or A23187 (data not shown). Its ligand, FasL, is not expressed on untreated T-cell lines. Upon treatment with 2 μM As2O3 for 24 h, only the highly As2O3-sensitive EL-4 T-cell line expressed FasL, with 100% of EL-4 cells being FasL positive although weakly (Figure 6E). FasL+ EL-4 cells were equally detected in regions R1 and R2 (data not shown), suggesting that FasL is expressed before apoptosis. FasL-expressing BW5147, L1210, Jurkat and HPB-ALL cells were not detected even after 48 h of treatment with As2O3 (data not shown).
Treatment with the calcium ionophore A23187 upregulated FasL expression on EL-4, BW5147 and L1210 cells (Figure 6E and Additional file 2: Figure S2D), but not on Jurkat and HPB-ALL cells (data not shown). While 24 h of treatment were sufficient to induce FasL expression on BW5147 and EL-4 cells, 48 h were required to induce such expression on L1210 cells (Additional file 2: Figure S2D). However, the expression levels of FasL were significantly higher on BW5147 cells than on EL-4 and L1210 cells. In addition, FasL upregulation was achieved using a concentration of calcium ionophore A23187 around 4 and 8 times higher in EL-4 cells and L1210 cells, respectively, than in BW5147 cells. FasL-expressing EL-4, BW5147 and L1210 cells were equally detected in regions R1 and R2 (data not shown). The expression of FasL on EL-4 cells treated with As2O3 or A23187, and on BW5147 and L1210 cells only when treated with A23187, strongly suggest that As2O3 and A23187 trigger FasL expression through different signaling pathways. Furthermore, the absence of either Fas or FasL, or both, on A2O3- or A23187-treated cells indicates that their activation of caspase 8 is independent of the Fas/FasL pathway and must depend on other death pathway.
As2O3 treatment represses nuclear translocation of NF-κB p50
NF-κB activity regulates the apoptosis of various cancer cell lines. Activation of NF-κB can promote or prevent apoptosis, depending on the stimuli utilized and the cell type [31]. Conflicting results have been published on the activation status of NF-κB in As2O3-treated leukemia T cells. One report has shown that As2O3 treatment markedly decreases constitutive NF-κB activation [32]. In another study, the authors could not detect any decrease in the translocation of the p65 subunit of NF-κB [33]. This discrepancy may be due to differences in the doses of As2O3 used to treat leukemic T cells. Therefore, we have quantified NF-κB p50 in nuclear extracts from NB4, EL-4, BW5147, Jurkat (clone E6-1) and CD45-deficient variant (clone J45.01) treated with a large range of doses (i.e. 1 μM to 12 μM). We found that As2O3 treatment represses nuclear translocation of NF-κB p50 in As2O3-treated T cells in a dose-dependent manner (Figure 6F). Importantly, nuclear translocation of NF-κB p50 was strongly increased in the CD45-deficient Jurkat T-cell line (clone J45.01) treated with As2O3 compared to wild-type Jurkat cells (Figure 6F). Thus, after a treatment with 6 μM As2O3 (IC50 value for the cytotoxic effect of As2O3 on Jurkat cells) J45.01 cells contained markedly more nuclear NF-κB p50 (about 3 times more) than wild-type Jurkat cells, suggesting a link between B220 and NF-κB signaling pathways.
Discussion
In APL-derived NB4 cells, As2O3 triggers apoptosis at concentrations of 0.5 to 2 μM [8]. The cytotoxic properties of As2O3 are not restricted to APL, and As2O3 induces apoptosis in various types of hematopoietic and solid tumors [12]. However, hematologic tumor cells vary considerably in their sensitivity to As2O3. To gain insight into the mechanisms underlying the As2O3 sensitivity of malignant T cells, we firstly selected two human (Jurkat and HPB-ALL) and three murine (EL-4, BW5147 and L1210) T-cell lines for their marked differences in sensitivity to As2O3 cytotoxicity over a large range of doses (i.e. 1 μM to 20 μM). Thus, 50% of EL-4 cells are killed at a clinically relevant concentration of about 1–2 μM As2O3, whereas concentrations of ~2.5 μM As2O3 for BW5147, ~6 μM As2O3 for Jurkat and ~12–15 μM As2O3 for L1210 and HPB-ALL T-cell lines are required to kill approximately 50% of the cells. Using this tumor panel, we have shown that: 1) differences in the intracellular levels of GSH and O2- are not sufficient to account for their differences in As2O3 sensitivity; 2) transmembrane tyrosine phosphatase B220 is induced on EL-4, BW5147, Jurkat and HPB-ALL T-cell lines, but not APL-derived NB4 cells, upon treatment with As2O3 in a dose and time dependent manner; 3) the degree of B220 induction on the T-cell lines is strongly correlated with the sensitivity to As2O3 cytotoxicity; 4) surprisingly, B220 is constitutively expressed on the L1210 T-cell line; 5) membrane-bound HSP70, known to induce antitumor immunity, is upregulated by As2O3 in parallel with B220 induction; 6) initiator caspases 8 and 9 are activated by As2O3 in the T-cell lines where this activation parallels B220 induction, but not in L1210 cells; 7) As2O3 represses nuclear translocation of NF-κB p50 in a dose dependent manner; 8) FasL upregulation by As2O3 is found on Fas-negative EL-4 cells only, indicating that caspase 8 activation is most probably independent of the Fas/FasL pathway [34]. However, the absence of FasL on some T-cell lines (either human or murine) might be due to the rapid shedding of FasL by protease activities induced by As2O3 or calcium ionophore A23187, as we have reported for TNF-α and CD62L upon treatment with Ionomycin or ATP [35].
Contradictory data have been published on the efficacy of As2O3 treatment against tumors belonging to the lymphoid lineages. Evidence for a pro-apoptotic effect of As2O3 against human malignant T- and B-cell lines [36], cutaneous T-cell lymphoma [37] or HTLV-I-associated adult T-cell leukemia [38] was provided by in vitro studies. However, it has been shown, in a multi-institution phase II study, that As2O3 exhibits limited efficacy against lymphoid malignancies, even though the patients received ascorbic acid in addition to As2O3 [39]. The authors of this clinical study expected that agents such as ascorbic acid, which depletes intracellular GSH, could potentiate arsenic-induced apoptosis, since it has been described previously [10] that the sensitivity of cells to As2O3 are inversely correlated with their intracellular GSH content. As2O3 interacts with sulfhydryl (SH) groups of biologically active molecules. The binding of As2O3 to the SH group of GSH could cause a drastic decrease in the capacity to scavenge ROS in cells with a low basal GSH content, resulting in overproduction of intracellular ROS that could trigger cell apoptosis. However, our present study shows that EL-4 and BW5147 T-cell lines, which were the most sensitive to As2O3-induced cytotoxicity among the cells lines studied (IC50 of approximately 1 to 2.5 μM) had the highest baseline GSH content (Figure 2). Likewise, L1210 and HPB-ALL T cells that were significantly more resistant to As2O3 cytotoxicity (IC50 of approximately 12 to 15 μM) had significantly lower baseline GSH content than EL-4 and BW5147 cells (Figure 2). In the presence of As2O3, intracellular GSH content was differently modulated in the T-cell lines, but without any correlation with their As2O3 sensitivity. Thus, GSH level in the highly sensitive NB4 cell line was unaffected by the dose of 1 μM As2O3, whereas it was markedly decreased in the BW5147 T-cell line and also, although to a lesser extent, in the resistant Jurkat cells (Figure 2). Because intracellular GSH content did not appear to be a key factor in determining As2O3 sensitivity of these five leukemia T-cell lines, we further explored the evolution of O2- production upon treatment with As2O3. The five T-cell lines showed a great heterogeneity in levels of intrinsic O2- production (Figure 2), which, interestingly, were 2 to 8 times higher than in APL-derived NB4 cells. As expected, the levels of O2- were significantly increased in APL-derived NB4 cells in the presence of As2O3. In T-cell lines, the situation was more complex. While the levels of O2- increased significantly, in Jurkat, L1210, and to a lesser extent HPB-ALL T cells treated with As2O3, they remained unchanged in EL-4 and BW5147 T cells. Therefore, the changes of O2- production upon treatment with As2O3 are not correlated with the differences in As2O3 sensitivity that we observed among the five T-cell lines. In contrast to other tumor cells [5], neither the intracellular GSH content nor the production of O2- had any decisive effect on As2O3-induced apoptosis of the five T-cell lines studied. Therefore, our findings suggest that the depletion of leukemic T cells in their intracellular GSH content by ascorbic or butyric acid are not necessarily relevant to potentiate the cytotoxic effect of As2O3. Furthermore, it has been reported that the expression level of aquaglyceroporin (AQP)9, a transmembrane protein that controls arsenic transport, correlated positively with As2O3-induced cytotoxicity in myeloid and lymphoid leukemia cell lines [40]. In contrast, the overexpression of AQP9 in melanoma cells significantly increased the resistance to arsenite-induced apoptosis [41]. These reports prompted us to determine the levels of AQP9 mRNA by semi-quantitative RT-PCR in EL-4, BW5147, L1210, Jurkat and HPB-ALL T-cell lines, and in APL-derived NB4 cells. However, we did not find any correlation between the expression levels of AQP9 mRNA and the As2O3 sensitivity in the T-cell lines (unpublished data). Therefore, our data strongly suggest the existence of additional factors determining the sensitivity of T cells to As2O3 cytotoxicity. Herein, we have hypothesized that phosphatase B220 could be such a factor, since we have shown previously that treatment with As2O3 of autoimmune MRL/lpr mice selectively eliminates pathogenic B220-expressing T cells in vivo [13, 14].
Normal effector T cells entering apoptosis after repeated activation by their antigen express the tyrosine phosphatase B220 on their surface [15, 16, 42], suggesting a role for B220 induction in the transition from activation to apoptosis. On the other hand, B220 is the isoform of CD45 predominantly expressed on pathogenic DN T cells from patients and mice with a deficiency in the death receptor Fas, or its ligand FasL. FasL-deficient mice (gld mutation) with only one functional CD45 allele (gld/gld, CD45+/-) display a strong reduction in the pathogenic DN T-cell population [43], suggesting that CD45 is an important regulator of T-cell apoptosis or a survival factor for T cells. Whether and how B220 expression on T cells regulates signaling for death or survival remains unknown. The induction of B220 and FasL (in EL-4 cells) as well as the activation of initiator caspase-8 in As2O3-treated leukemic T-cell lines were reminiscent of apoptosis in normal effector T cells triggered by repeated antigenic stimulation. Therefore, leukemic T-cell lines were activated with calcium ionophore A23187, a well-known trigger for T-cell activation and death. Importantly, we found that As2O3 and calcium ionophore A23187 have opposite efficiencies on the T-cell lines, evidenced by the induction of B220, activation marker CD69 and membrane-bound HSP70 expression, and cell death (data summarized in Table 1). While both B220 expression and cell death were massively induced in EL-4 cells after treatment with As2O3, they were only slightly induced after treatment with calcium ionophore A23187. The complete reverse situation was observed in HPB-ALL cells, indicating that A23187 and As2O3 had opposite effects on B220 expression and cell death on the same leukemic T-cell panel. Moreover, treatment with A23187, but not As2O3, induced the activation marker CD69 on T-cell lines before B220 expression and cell death, indicating that A23187, but not As2O3, kills the T-cell lines by an activation-induced cell death mechanism.
HSP70 is overexpressed in various cancer cells [44]. HSP70 inhibits apoptosis by modulating multiple events within apoptotic pathways, which might promote cancer development [45, 46]. However, a tumor-specific plasma membrane form of HSP70 has been described [44, 47], which facilitates tumor rejection by the immune system [27, 47–49]. In the present study, we found a strong upregulation of membrane-bound HSP70 by As2O3 treatment, but not by the calcium ionophore A23187. This upregulation of HSP70 by As2O3 strictly paralleled the induction of B220 on EL-4, BW5147, Jurkat and HPB-ALL T-cell lines (Table 1). Consequently, As2O3-sensitive EL-4 cells expressed both high levels of HSP70 and B220, whereas low expression levels of HSP70 and B220 were found on As2O3-resistant HPB-ALL cells. Likewise, constitutive expression of B220 on As2O3-resistant L1210 cells was associated with low expression levels of membrane-bound HSP70. In vivo, the direct cytotoxic effects of As2O3 could be amplified by the upregulation of membrane-bound HSP70 on tumor cells, which might facilitate tumor immune rejection.
CD45 is known to positively regulate antigen-receptor signaling during activation of normal T and B cells via dephosphorylation of src kinases, and to negatively regulate cytokine receptor signaling via dephosphorylation of JAK kinases [17, 18]. In this study, we show that the modulation of B220 cell surface expression plays an important role in determining the sensitivity of leukemic T cells to As2O3 and calcium ionophore A23187 cytotoxicity. In addition, we found that As2O3 treatment represses nuclear translocation of NF-κB p50 in a dose dependent manner. Moreover nuclear translocation of NF-κB p50 was increased in CD45-deficient Jurkat T cell line (clone J45.01) after treatment with As2O3, suggesting a link between B220 and NF-κB signaling pathways.
In conclusion, on a panel of mouse and human leukemic T-cell lines, we have presented evidence for a tight correlation between the induction of B220 membrane expression and their sensitivity to cell death induced by As2O3 or A23187. Our data strongly support the hypothesis that B220 plays a checkpoint role in death pathways. This could provide additional tools to potentiate As2O3 therapy against leukemic T cells.
Conclusions
In contrast to As2O3-treated APL cells, GSH content and O2- production do not play a significant role in As2O3 sensitivity of leukemic T cells, suggesting the existence of additional factors determining the sensitivity of T cells to As2O3 cytotoxicity. The B220 isoform of transmembrane tyrosine phosphatase CD45 may be one such factor. Indeed, we show that As2O3 treatment induces B220 plasma membrane expression and cell death in leukemic T-cell lines in a dose and time dependent manner. The levels of B220 induction on the T-cell lines strictly correlate with both the extent and form of cell death. Leukemic T cells died by an apoptotic form of cell death when high levels of B220 membrane expression were achieved with low doses of As2O3. Taken together, our data suggest that transmembrane tyrosine phosphatase B220 plays a checkpoint role in apoptotic pathways since its expression is markedly and rapidly induced on the surface of T cells undergoing apoptosis.
Materials and methods
Reagents
Arsenic trioxide (As2O3), phorbol 12-myristate 13-acetate (PMA) and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO), and Calcium ionophore A23187 was from Calbiochem (EMD Biosciences Inc, San Diego, CA). As2O3 was dissolved in 1 M NaOH, and stored as a 330 mM stock solution, which was further diluted to 5 mM with phosphate-buffered saline (PBS).
Cell culture and cell treatment
Leukemic cell lines used in this study included mouse T-cell lines EL-4, BW5147 and L1210, human T-cell lines HPB-ALL, Jurkat (clone E6-1) and CD45 deficient variant of the E6-1 clone of Jurkat (clone J45.01) (European Collection of Cell Cultures), and human acute promyelocytic leukemia cell line NB4. Leukemic T cell lines (EL-4, BW5147, L1210, Jurkat (clone E6-1), HPB-ALL), and APL derived cell line NB4 were kindly provided by Dr Colette Kanellopoulos-Langevin (Centre for Inflammation Research, INSERM, Hôpital Bichat, Paris, France) and Dr Jacqueline Robert-Lézénès (Inserm U940, Hôpital Saint-Louis, Paris, France), respectively. All cells were grown in RPMI 1640 containing Glutamax (Invitrogen, Cergy Pontoise, France) and supplemented with 10% heat-inactivated fetal calf serum, 50 U/ml penicillin and 50 μg/ml streptomycin at 37°C in a humidified 5% CO2 atmosphere. This culture medium will be referred to as complete medium. To avoid possible effects of cell density on cell growth and survival, cells were maintained at less than 5 × 105 cells/ml with daily adjusting cell density through the addition of fresh medium. Cell viability was estimated by the 4% Trypan-blue dye exclusion assay.
Leukemic T cells were seeded in 12-well plates at a density of 1 × 105 cells /ml and incubated in complete medium alone or in the presence of different concentrations of As2O3 or calcium ionophore A23187 at 37°C for 12, 24 and 48 h depending on the experiment.
Flow cytometry and imaging flow cytometry
The expression levels of cell surface markers on untreated and As2O3- or A23187-treated leukemic T-cell lines was analyzed by flow cytometry using either fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, allophycocyanin (APC)- or biotin-conjugated monoclonal antibodies (mAb): rat anti-mouse Thy-1.2/CD90.2 (clone 53–2.1), anti-CD45 (clone 2D1), anti-B220/CD45R (clone RA3-6B2), anti-mouse and human CD69 (clone H1.2 F3 and FN50), anti-mouse and human Fas (clone Jo2 and DX2), anti-mouse and human FasL (clone MFL3 and NOK-1) and anti-HSP70 (clone SMC-103A) (all from eBioscience, CliniSciences, Montrouge, France), and rat IgG2a, mouse IgG, mouse IgG1, and Armenian hamster IgG1 as the isotype control (eBiosciences). Use of mAb to mouse and human Fcγ receptor (PharMingen, BD Bioscience, San Jose, CA) avoided non-specific antibody binding.
The subcellular localization of B220/CD45R molecules was determined by imaging flow cytometry, following the protocol supplied by the manufacturer (Amnis Corp., Seattle, WA). Briefly, cells (1 × 106) stained with PE-conjugated anti-B220/CD45R mAbs and DAPI were run on an ImageStream apparatus (ImagoSeine, Institut Jacques Monod, CNRS-Université Paris Diderot, France). At least 10,000 images were collected per sample at 40× or 60× magnification, and analyzed using IDEAS image-analysis software (Amnis Corp.).
Cell proliferation, cell death and caspase activation assay
Total cell numbers in untreated and As2O3-treated groups were determined by flow cytometry by acquiring events for a fixed time period of 1 min. As2O3- and Calcium ionophore-induced cell death was analyzed by propidium iodide (PI) (Invitrogen) staining, and flow cytometry. Among PI+ cells, to discriminate between apoptotic and necrotic cells, the cells were stained using either FITC-conjugated Annexin V (PharMingen) or CaspaTag Caspase 8 or Caspase 9 In situ Assay Kit, Fluorescein according to the manufacturer's instructions (Chemicon, Temecula, CA). Annexin V staining and the levels of active caspase-8 and caspase-9 were measured by flow cytometry.
Analysis of reduced glutathione content and O2- production
GSH content and O2- production in T-cell lines treated or not with As2O3 were measured by flow cytometry using 100 nM CellTracker probe CMFDA and 5 μM DHE probes, respectively, following the manufacturer’s instructions (Molecular Probes, Eugene, OR).
B220/CD45R mRNA quantification by RT-PCR
Total RNA was extracted from 5 × 106 leukemic T cells treated or not with As2O3 using the RNeasy Plus Mini kit (Qiagen, Courtaboeuf, France) following the manufacturer’s instructions and was used to generate cDNA utilizing oligo(dT) primer and SuperScript II Reverse Transcriptase (Invitrogen). PCR were conducted for 25 cycles with the following primer pairs: B220/CD45R forward primer (5'-CAC ATA TCA TCC AGG TGT GTT ATC C-3') and reverse primer (5'-GTC CTC TCC CCT GGC ACA CCT G-3'); β-actin forward primer (5'-ATC GTG GGC CGC CCT AGG CAC-3') and reverse primer (5'-TGG CCT TAG GGT TCA GAG GGG C-3'). Semi-quantitative determination (ImageJ densitometric analysis software program) of B220 cDNA, present in each of the various samples, was normalized with respect to the concentration of internal control cDNA (β-actin) detected in the same sample, and B220/ β-actin cDNA ratios were calculated.
Quantitative measurement of NF-κBactivation
Nuclear extracts were prepared from 8 × 106 leukemic T cells treated or not with As2O3. The protein from nuclear extracts was quantified by the Bradford method (Bio-Rad, France). An equal amount of nuclear extract (5 μg) was assayed for NF-κB p50 activity using a TransAm NFκB p50 Transcription Factor Assay Kit according to the manufacturer’s recommendations (ActiveMotif, Rixensart, Belgium).
Statistical analyses
Data are reported as fluorescence means ± SE. Significant differences between sample means were determined using the Student t test. Statistical significance was accepted at P ≤0.05.
Electronic supplementary material
Additional file 1: Figure S1: (A) Cell morphology of As2O3-treated T-cell lines. APL-derived NB4 cells as well as EL-4, BW5147, L1210, Jurkat, CD45-deficient Jurkat variant (J45.01) and HPB-ALL T-cell lines were treated for 24 h with As2O3 in doses ranging from 1 to 20 μM. Cells were then analyzed by flow cytometry with respect to size (FSC) and granulosity (SSC). Regions R1 and R2 identified on a FSC vs. SSC dot plot encompassed cells with FSCint/lowSSChigh and FSChighSSClow, respectively. At least 20,000 events were analyzed for each sample. FSC vs. SSC dot plots on murine EL-4 cells and human Jurkat cells are representative of more than 10 independent experiments. (B) Basal level of CD45 plasma membrane expression. Jurkat (■) and CD45-deficient Jurkat variant (J45.01) (□) cells were stained with PE-conjugated anti-CD45 mAb (clone 2D1) or PE-conjugated rat IgG2a isotype control, and then analyzed by flow cytometry. (C) B220 mRNA expression in As2O3-treated T-cell lines. RT-PCR analysis were performed to assess the levels of B220 mRNA in EL-4 and BW5147 T cells cultured in the presence or absence of 1, 2 and 4 μM As2O3 for 3, 6 and 9 h. Results are representative of two other experiments. (TIFF 2 MB)
Additional file 2: Figure S2: (A) Constitutive B220/CD45R cell surface expression on CD90+ L1210 T cells. Cells were labeled with APC-conjugated anti-CD90 and PE-conjugated anti-B220/CD45R mAbs, or fluorescent isotype control, and then analyzed by flow cytometry. (B) B220 expression on As2O3-treated cells. L1210 T cells were treated without or with As2O3 for 24 h in doses ranging from 1 to 20 μM. L1210 cells were then stained with PE-conjugated anti-B220/CD45R mAb or PE-conjugated rat IgG2a isotype control, and further analyzed by flow cytometry with respect to size (FSC) versus granulosity (SSC) and B220 expression. FSC vs. SSC dot plots were used to define gates R1 and R2 with FSCint/lowSSChigh and FSChighSSClow, respectively. B220 histograms were then gated in R1 and R2 to determine the percentages of cells expressing B220 (n =10 independent experiments). At least 20,000 events were analyzed for each sample. (C) HSP70 induction on As2O3-treated cells. L1210 T cells stained with anti-B220 and anti-HSP70 antibodies were analyzed by flow cytometry as described in Figure 3B. (D) FasL induction on Ca2+ ionophore treated cells. Histograms obtained with PE-conjugated Armenian hamster (clone MFL3) anti-FasL mAb (open histogram) are overlaid on histograms obtained with PE-conjugated Armenian hamster isotype control (shaded histogram) (n = 3 independent experiments). At least 20,000 events were analyzed for each sample. (TIFF 2 MB)
Additional file 3: Figure S3: Duration of B220/CD45R membrane expression upon As2O3 treatment. EL-4 and Jurkat T cells were cultured in the absence or in the presence of 1, 2, 4 or 8 μM As2O3 for 24 h. Then, cells were extensively washed with PBS to eliminate all traces of As2O3, and cultured for 9 additional days. Expression of B220 was measured by flow cytometry at the time of As2O3 removal (referred to as day 0) and 1 to 9 days after As2O3 was removed. At least 20,000 events were analyzed for each sample. Dot plots of FSC vs. SSC on 8 μM As2O3-treated EL-4 and Jurkat cells are representative of more than 3 independent experiments. Graphs report the percentages of B220+ EL-4 or B220+ Jurkat cells at the indicated time-points and concentrations of As2O3, with the same isotype control labelling as in Figure 3. (TIFF 2 MB)
Acknowledgments
We thank Dr Colette Kanellopoulos-Langevin (Centre for Inflammation Research, INSERM U699, Hôpital Bichat, Paris, France) for helpful discussions and for critical review of the manuscript. This work was funded by the Centre National de la Recherche Scientifique (CNRS), and grants from Agence Nationale de la Recherche (ANR-07BLAN0089-02).
Abbreviations
- APL
Acute promyelocytic leukemia
- AQP9
Aquaglyceroporin-9
- As2O3
Arsenic trioxide
- DN
Double negative
- FasL
Fas ligand
- FSC
Forward scatter
- EC50
Half maximal effective concentration
- IC50
Half maximal inhibitory concentration
- HSP70
Heat shock protein-70
- mAb
Monoclonal antibody
- NF-κB
Nuclear factor-kappa B
- PI
Propidium iodide
- ROS
Reactive oxygen species
- GSH
Reduced glutathione
- SSC
Side scatter
- O2-
Superoxide anion radical
- MFI
Mean fluorescence intensity.
Footnotes
Competing interests
The authors declare they have no competing interests.
Authors’ contributions
PB and MB conceived and designed the experiments; MB and AM performed the experiments; MB, AM and PB analyzed the data; PB wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Mohcine Benbijja, Email: mohcine.benbijja@gmail.com.
Amine Mellouk, Email: amine.mellouk@u-psud.fr.
Pierre Bobé, Email: pierre.bobe@u-psud.fr.
References
- 1.Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 2.Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP., Jr Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 3.Niu C, Yan H, Yu T, Sun HP, Liu JX, Li XS, Wu W, Zhang FQ, Chen Y, Zhou L, Li JM, Zeng XY, Yang RR, Yuan MM, Ren MY, Gu FY, Cao Q, Gu BW, Su XY, Chen GQ, Xiong SM, Zhang TD, Waxman S, Wang ZY, Chen Z, Hu J, Shen ZX, Chen SJ. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94:3315–3324. [PubMed] [Google Scholar]
- 4.Lallemand-Breitenbach V, Zhu J, Chen Z, de The H. Curing APL through PML/RARA degradation by As2O3. Trends Mol Med. 2012;18:36–42. doi: 10.1016/j.molmed.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Miller WH, Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–3903. [PubMed] [Google Scholar]
- 6.Lallemand-Breitenbach V, Jeanne M, Benhenda S, Nasr R, Lei M, Peres L, Zhou J, Zhu J, Raught B, de The H. Arsenic degrades PML or PML-RARalpha through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat Cell Biol. 2008;10:547–555. doi: 10.1038/ncb1717. [DOI] [PubMed] [Google Scholar]
- 7.Tatham MH, Geoffroy MC, Shen L, Plechanovova A, Hattersley N, Jaffray EG, Palvimo JJ, Hay RT. RNF4 is a poly-SUMO-specific E3 ubiquitin ligase required for arsenic-induced PML degradation. Nat Cell Biol. 2008;10:538–546. doi: 10.1038/ncb1716. [DOI] [PubMed] [Google Scholar]
- 8.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang P, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, de The H, Chen SJ, Chen Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 9.Yang CH, Kuo ML, Chen JC, Chen YC. Arsenic trioxide sensitivity is associated with low level of glutathione in cancer cells. Br J Cancer. 1999;81:796–799. doi: 10.1038/sj.bjc.6690766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–277. [PubMed] [Google Scholar]
- 11.Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, Dang CV. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc Natl Acad Sci U S A. 2004;101:4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dilda PJ, Hogg PJ. Arsenical-based cancer drugs. Cancer Treat Rev. 2007;33:542–564. doi: 10.1016/j.ctrv.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Bobé P, Bonardelle D, Benihoud K, Opolon P, Chelbi-Alix MK. Arsenic trioxide: a promising novel therapeutic agent for lymphoproliferative and autoimmune syndromes in MRL/lpr mice. Blood. 2006;108:3967–3975. doi: 10.1182/blood-2006-04-020610. [DOI] [PubMed] [Google Scholar]
- 14.Le Gall SM, Legrand J, Benbijja M, Safya H, Benihoud K, Kanellopoulos JM, Bobé P. Loss of P2X7 receptor plasma membrane expression and function in pathogenic B220+ double-negative T lymphocytes of autoimmune MRL/lpr mice. PLoS One. 2012;7:e52161. doi: 10.1371/journal.pone.0052161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renno T, Attinger A, Rimoldi D, Hahne M, Tschopp J, MacDonald HR. Expression of B220 on activated T cell blasts precedes apoptosis. Eur J Immunol. 1998;28:540–547. doi: 10.1002/(SICI)1521-4141(199802)28:02<540::AID-IMMU540>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 16.Bleesing JJ, Morrow MR, Uzel G, Fleisher TA. Human T cell activation induces the expression of a novel CD45 isoform that is analogous to murine B220 and is associated with altered O-glycan synthesis and onset of apoptosis. Cell Immunol. 2001;213:72–81. doi: 10.1006/cimm.2001.1865. [DOI] [PubMed] [Google Scholar]
- 17.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 18.Holmes N. CD45: all is not yet crystal clear. Immunology. 2006;117:145–155. doi: 10.1111/j.1365-2567.2005.02265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee I, Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]
- 20.Klaus SJ, Sidorenko SP, Clark EA. CD45 ligation induces programmed cell death in T and B lymphocytes. J Immunol. 1996;156:2743–2753. [PubMed] [Google Scholar]
- 21.Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, Baum LG. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 22.Clark MC, Baum LG. T cells modulate glycans on CD43 and CD45 during development and activation, signal regulation, and survival. Ann N Y Acad Sci. 2012;1253:58–67. doi: 10.1111/j.1749-6632.2011.06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davison K, Mann KK, Waxman S, Miller WH., Jr JNK activation is a mediator of arsenic trioxide-induced apoptosis in acute promyelocytic leukemia cells. Blood. 2004;103:3496–3502. doi: 10.1182/blood-2003-05-1412. [DOI] [PubMed] [Google Scholar]
- 24.Pelicano H, Feng L, Zhou Y, Carew JS, Hileman EO, Plunkett W, Keating MJ, Huang P. Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem. 2003;278:37832–37839. doi: 10.1074/jbc.M301546200. [DOI] [PubMed] [Google Scholar]
- 25.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Multhoff G, Botzler C, Wiesnet M, Muller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61:272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- 27.Stangl S, Gehrmann M, Riegger J, Kuhs K, Riederer I, Sievert W, Hube K, Mocikat R, Dressel R, Kremmer E, Pockley AG, Friedrich L, Vigh L, Skerra A, Multhoff G. Targeting membrane heat-shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc Natl Acad Sci U S A. 2011;108:733–738. doi: 10.1073/pnas.1016065108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 29.McCafferty-Grad J, Bahlis NJ, Krett N, Aguilar TM, Reis I, Lee KP, Boise LH. Arsenic trioxide uses caspase-dependent and caspase-independent death pathways in myeloma cells. Mol Cancer Ther. 2003;2:1155–1164. [PubMed] [Google Scholar]
- 30.Scholz C, Wieder T, Starck L, Essmann F, Schulze-Osthoff K, Dorken B, Daniel PT. Arsenic trioxide triggers a regulated form of caspase-independent necrotic cell death via the mitochondrial death pathway. Oncogene. 2005;24:1904–1913. doi: 10.1038/sj.onc.1208233. [DOI] [PubMed] [Google Scholar]
- 31.Perkins ND. The diverse and complex roles of NF-kappaB subunits in cancer. Nat Rev Cancer. 2012;12:121–132. doi: 10.1038/nrc3204. [DOI] [PubMed] [Google Scholar]
- 32.El-Sabban ME, Nasr R, Dbaibo G, Hermine O, Abboushi N, Quignon F, Ameisen JC, Bex F, de The H, Bazarbachi A. Arsenic-interferon-alpha-triggered apoptosis in HTLV-I transformed cells is associated with tax down-regulation and reversal of NF-kappa B activation. Blood. 2000;96:2849–2855. [PubMed] [Google Scholar]
- 33.Bornhauser BC, Bonapace L, Lindholm D, Martinez R, Cario G, Schrappe M, Niggli FK, Schafer BW, Bourquin JP. Low-dose arsenic trioxide sensitizes glucocorticoid-resistant acute lymphoblastic leukemia cells to dexamethasone via an Akt-dependent pathway. Blood. 2007;110:2084–2091. doi: 10.1182/blood-2006-12-060970. [DOI] [PubMed] [Google Scholar]
- 34.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Le Gall SM, Bobé P, Reiss K, Horiuchi K, Niu XD, Lundell D, Gibb DR, Conrad D, Saftig P, Blobel CP. ADAMs 10 and 17 represent differentially regulated components of a general shedding machinery for membrane proteins such as transforming growth factor alpha, L-Selectin, and tumor necrosis factor alpha. Mol Biol Cell. 2009;20:1785–1794. doi: 10.1091/mbc.E08-11-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu XH, Shen YL, Jing YK, Cai X, Jia PM, Huang Y, Tang W, Shi GY, Sun YP, Dai J, Wang ZY, Chen SJ, Zhang TD, Waxman S, Chen Z, Chen GQ. Apoptosis and growth inhibition in malignant lymphocytes after treatment with arsenic trioxide at clinically achievable concentrations. J Natl Cancer Inst. 1999;91:772–778. doi: 10.1093/jnci/91.9.772. [DOI] [PubMed] [Google Scholar]
- 37.Michel L, Dupuy A, Jean-Louis F, Sors A, Poupon J, Viguier M, Musette P, Dubertret L, Degos L, Dombret H, Bachelez H. Arsenic trioxide induces apoptosis of cutaneous T cell lymphoma cells: evidence for a partially caspase-independent pathway and potentiation by ascorbic acid (vitamin C) J Invest Dermatol. 2003;121:881–893. doi: 10.1046/j.1523-1747.2003.12479.x. [DOI] [PubMed] [Google Scholar]
- 38.Bazarbachi A, El-Sabban ME, Nasr R, Quignon F, Awaraji C, Kersual J, Dianoux L, Zermati Y, Haidar JH, Hermine O, de The H. Arsenic trioxide and interferon-alpha synergize to induce cell cycle arrest and apoptosis in human T-cell lymphotropic virus type I-transformed cells. Blood. 1999;93:278–283. [PubMed] [Google Scholar]
- 39.Chang J, Voorhees P, Kolesar J, Ahuja H, Sanchez F, Rodriguez G, Kim K, Werndli J, Bailey H, Kahl B. Phase II study of arsenic trioxide and ascorbic acid for relapsed or refractory lymphoid malignancies: a Wisconsin Oncology Network study. Hematol Oncol. 2009;27:11–16. doi: 10.1002/hon.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung J, Pang A, Yuen WH, Kwong YL, Tse EW. Relationship of expression of aquaglyceroporin 9 with arsenic uptake and sensitivity in leukemia cells. Blood. 2007;109:740–746. doi: 10.1182/blood-2006-04-019588. [DOI] [PubMed] [Google Scholar]
- 41.Gao L, Gao Y, Li X, Howell P, Kumar R, Su X, Vlassov AV, Piazza GA, Riker AI, Sun D, Xi Y. Aquaporins mediate the chemoresistance of human melanoma cells to arsenite. Mol Oncol. 2012;6:81–87. doi: 10.1016/j.molonc.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renno T, Hahne M, Tschopp J, MacDonald HR. Peripheral T cells undergoing superantigen-induced apoptosis in vivo express B220 and upregulate Fas and Fas ligand. J Exp Med. 1996;183:431–437. doi: 10.1084/jem.183.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks WP, Lynes MA. Effects of hemizygous CD45 expression in the autoimmune Fasl(gld/gld) syndrome. Cell Immunol. 2001;212:24–34. doi: 10.1006/cimm.2001.1845. [DOI] [PubMed] [Google Scholar]
- 44.Sherman M, Multhoff G. Heat shock proteins in cancer. Ann N Y Acad Sci. 2007;1113:192–201. doi: 10.1196/annals.1391.030. [DOI] [PubMed] [Google Scholar]
- 45.Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003;22:9041–9047. doi: 10.1038/sj.onc.1207114. [DOI] [PubMed] [Google Scholar]
- 46.Beere HM. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci. 2004;117:2641–2651. doi: 10.1242/jcs.01284. [DOI] [PubMed] [Google Scholar]
- 47.Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 49.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo GC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1: (A) Cell morphology of As2O3-treated T-cell lines. APL-derived NB4 cells as well as EL-4, BW5147, L1210, Jurkat, CD45-deficient Jurkat variant (J45.01) and HPB-ALL T-cell lines were treated for 24 h with As2O3 in doses ranging from 1 to 20 μM. Cells were then analyzed by flow cytometry with respect to size (FSC) and granulosity (SSC). Regions R1 and R2 identified on a FSC vs. SSC dot plot encompassed cells with FSCint/lowSSChigh and FSChighSSClow, respectively. At least 20,000 events were analyzed for each sample. FSC vs. SSC dot plots on murine EL-4 cells and human Jurkat cells are representative of more than 10 independent experiments. (B) Basal level of CD45 plasma membrane expression. Jurkat (■) and CD45-deficient Jurkat variant (J45.01) (□) cells were stained with PE-conjugated anti-CD45 mAb (clone 2D1) or PE-conjugated rat IgG2a isotype control, and then analyzed by flow cytometry. (C) B220 mRNA expression in As2O3-treated T-cell lines. RT-PCR analysis were performed to assess the levels of B220 mRNA in EL-4 and BW5147 T cells cultured in the presence or absence of 1, 2 and 4 μM As2O3 for 3, 6 and 9 h. Results are representative of two other experiments. (TIFF 2 MB)
Additional file 2: Figure S2: (A) Constitutive B220/CD45R cell surface expression on CD90+ L1210 T cells. Cells were labeled with APC-conjugated anti-CD90 and PE-conjugated anti-B220/CD45R mAbs, or fluorescent isotype control, and then analyzed by flow cytometry. (B) B220 expression on As2O3-treated cells. L1210 T cells were treated without or with As2O3 for 24 h in doses ranging from 1 to 20 μM. L1210 cells were then stained with PE-conjugated anti-B220/CD45R mAb or PE-conjugated rat IgG2a isotype control, and further analyzed by flow cytometry with respect to size (FSC) versus granulosity (SSC) and B220 expression. FSC vs. SSC dot plots were used to define gates R1 and R2 with FSCint/lowSSChigh and FSChighSSClow, respectively. B220 histograms were then gated in R1 and R2 to determine the percentages of cells expressing B220 (n =10 independent experiments). At least 20,000 events were analyzed for each sample. (C) HSP70 induction on As2O3-treated cells. L1210 T cells stained with anti-B220 and anti-HSP70 antibodies were analyzed by flow cytometry as described in Figure 3B. (D) FasL induction on Ca2+ ionophore treated cells. Histograms obtained with PE-conjugated Armenian hamster (clone MFL3) anti-FasL mAb (open histogram) are overlaid on histograms obtained with PE-conjugated Armenian hamster isotype control (shaded histogram) (n = 3 independent experiments). At least 20,000 events were analyzed for each sample. (TIFF 2 MB)
Additional file 3: Figure S3: Duration of B220/CD45R membrane expression upon As2O3 treatment. EL-4 and Jurkat T cells were cultured in the absence or in the presence of 1, 2, 4 or 8 μM As2O3 for 24 h. Then, cells were extensively washed with PBS to eliminate all traces of As2O3, and cultured for 9 additional days. Expression of B220 was measured by flow cytometry at the time of As2O3 removal (referred to as day 0) and 1 to 9 days after As2O3 was removed. At least 20,000 events were analyzed for each sample. Dot plots of FSC vs. SSC on 8 μM As2O3-treated EL-4 and Jurkat cells are representative of more than 3 independent experiments. Graphs report the percentages of B220+ EL-4 or B220+ Jurkat cells at the indicated time-points and concentrations of As2O3, with the same isotype control labelling as in Figure 3. (TIFF 2 MB)


