Abstract
This protocol describes a cryopreservation procedure using an enzyme-free dissociation method to harvest cells and preserve cells in albumin-free chemically defined E8 medium for human pluripotent stem cells (hPSCs). The dissociation by EDTA/PBS produces small cell aggregates that allow high survival efficiency in passaging and cryopreservation. The preservation in E8 medium eliminates serum or other animal products, and is suitable for the increasing demand for high quality hPSCs in translational research. In combination with the special feature of EDTA/PBS dissociation, this protocol allows efficient cryopreservation in more time-saving manner.
Keywords: Pluripotent stem cells, induced pluripotent stem cells, embryonic stem cells, dissociation, cell culture, cryopreservation
INTRODUCTION
Human pluripotent stem cells (hPSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) can generate specific cell types in all three germ layers (Thomson et al. 1998; Takahashi et al. 2007; Yu et al. 2009). Their great potentials in disease modeling, drug screening and cell therapy have garnered a lot of interest, and also demand a high standard to maintain their quality for future applications. Cryopreservation is one of the most crucial steps that affect hPSC quality in the stem cell culture cycle after derivation and expansion. This unit describes the protocol to efficiently cryopreserve hPSCs in animal-free defined conditions. This protocol is suitable for basic research and future translational research.
Basic Protocol 1 describes in detail the procedures to prepare hPSCs for cryopreservation. Basic Protocol 2 describes the procedures to harvest and preserve hPSCs. Basic protocol 3 describes the steps to recover the cells after thawing. In companion with basic protocols, we also described alternative protocols to preserve and recover cells in small scale during colony expansion.
Note: All the hPSC related work should follow the Institutional Regulation and Guideline. The patient iPSC studies should be performed after the Institutional Review Board approval and proper patient consent. The human ESC studies should follow the NIH guideline, and the federal funding can only be used on ESCs in the NIH registry.
Note: All Culture incubations are performed in a humidified 37°C, 5% CO2 incubator. 5% O2 is desired for higher recovery after cryopreservation.
Note: All protocols are adapted for six-well plates unless otherwise indicated. Volumes can be increased proportionally for larger culture when needed.
Note: Considering the wide use of Matrigel in the field, Matrigel is described in this protocol as the main coating material. However, vitronectin can be used in place of Matrigel in this protocol to make fully defined culture conditions.
BASIC PROTOCOL 1. PASSAGING OF HUMAN ESCs/iPSCs WITH EDTA DISSOCIATION – PREPARING CELLS 2–3 DAYS BEFORE CRYOPRESERVATION
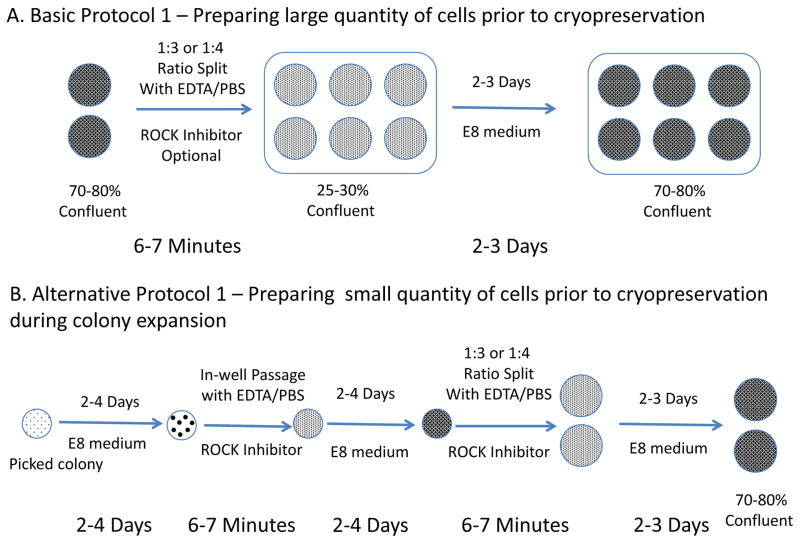
This protocol describes the passaging of human ESCs/iPSCs using EDTA dissociation method in E8 medium (Beers et al. 2012). The process takes 6–7 min per plate. (Figure 1A)
Figure 1.
Flow charts for preparing hPSCs before cryopreservation. A. Basic Protocol 1 - Passage cells from six-well plates to freeze more than 10 vials of cells. B. Alternative Protocol 1 - Passage colonies in-well to freeze 4 to 8 vials of cells during colonal expansion.
Note: This step is a critical, but often ignored step that affects cryopreservation.
Note: For single line large-scale expansion and cryopreservation, Protocol 1 should be used.
Note: For colony expansion for many lines and small scale preservation, Alternative Protocol 1 should be used.
Materials
Human ESCs: H1 cells, WA01 (US National Institutes of Health (NIH), human ESC registry No. 0043)
Human iPSCs from fibroblasts: ND2 (NIH control iPSC line)
-
Matrigel, growth factor reduced (BD Biosciences, cat. no. 354230)
Note: Always store in a non-antifreeze freezer, preferably −80°C freezer.
Note: Recombinant vitronectin can be used to replace Matrigel in all the protocols. 1 mg vitronecitn can be resuspended in PBS or DMEM/F12 to coat ten 6-well or 12-well plates. It is recommended to optimize the dose for first time users. (Life Technologies Inc., cat. no. A14701SA; Stem Cell Technologies Inc., cat. no. 07180)
EDTA dissociation solution (see recipe)
Full E8 medium. E8 medium can be prepared as previously described, and is also available as Essential 8 media from Life Technologies (cat. no. A14666SA) or from Stem Cell Technologies as TeSR-E8 (cat. no. 05840).
Rho-associated protein kinase (ROCK) inhibitor (optional): Y-27632 (Tocris, 1254). Dissolve the Y-27632 in sterile H2O or sterile DMSO with a final concentration of 10 mM (1000x), then aliquot and store it at −80°C. The soluti on is stable for at least 1 year. Y-27632 is the ROCK inhibitor that we commonly use. Other ROCK inhibitors may also be used.
Laminar flow hood with vacuum
Cell culture incubator (37°C, 95% humidity, 5% CO2 and 5%O2 atmosphere, Heracell, Thermo Scientific)
Inverted phase contrast microscope (x4 and x10 objectives, Zeiss or equivalent)
Six-well Nunclon Delta Surface tissue culture dishes (Thermo Scientific cat. no. 140675 or equivalent)
Pipet-aid and sterile 5- and 10-ml plastic disposable pipettes
P1000 and P20 pipetman and sterile tips with filter
Sterile filter units, 500 ml (Millipore, Stericup, cat. no. SCGPU05RE), optional if E8 medium is home-made.
Protocol Steps
hPSCs are cultured in E8 medium on matrigel-coated plate till 70–80% confluence. These cells are under daily medium change and 4- or 5-day splitting schedule.
Resuspend 1 mg frozen Matrigel with 6 ml 4°C DMEM/F12, and aliquot 1 ml into each well of 6-well plate. Incubate at room temperature (RT, 20–25°C) for at least 30 minutes or at 4°C overnight. Place one new Matrigel-coated six-well plate in the tissue culture hood and warm to RT. Label the plate. Warm the EDTA dissociation solution to RT.
-
Aspirate the Matrigel from the plate and replace with 1.5–2 ml full E8 medium per well.
E8 medium plus 10μM ROCK inhibitor can be used instead of E8 medium if desired. -
Take one six-well plate of ESCs or iPSCs that are about to be passaged. Aspirate the media from the cells. Rinse the wells with 1 ml of EDTA dissociation solution per well. Aspirate the solution off immediately without incubating.
This step removes the magnesium and calcium from the medium.Add EDTA to the walls of the wells slowly to avoid washing the colonies off the plate too early. Wash the cells with EDTA solution for a second time as described in step 4.
-
Add 1 ml per well of EDTA solution to the cells. Let sit for 2–5 minutes at RT within the biosafety cabinet.
The longer the cells sit in the EDTA, the smaller the resulting colonies will be.Minimize movement of the plate during this step to avoid lifting the colonies completely. -
Carefully aspirate the EDTA solution without dislocating the cells. Rapidly add 4 ml of E8 medium to wash the colonies off the plate and disperse the colonies as quickly as possible.
Do not break apart the colonies too much by excess pipetting.E8 medium plus ROCK inhibitor can be used instead of E8 medium if desired.Any remaining EDTA will be neutralized by the calcium and magnesium in the medium.Perform this and the next step quickly as cells will start to reattach to the plate rapidly once calcium and magnesium are added. Ensure the cell suspension is well mixed. Add the desired amount of cells to each well in the newly prepared plate from step 3.
Shake the plate back and forth and side to side to distribute the cells. Place the plate in a 37°C incubator. Let it sit overnight for maximum attachment.
-
On the next day, remove the medium from the cells, and add 2 ml of E8 medium to each well.
E8 medium may be warmed to RT in advance if desired.No ROCK inhibitor is necessary. -
Change medium and monitor the cell growth daily. When cells reach ~80% confluence (usually between 2–3 days after seeding at 1:4 splitting ratio), move forward to cryopreservation.
Passage cells for the desired number of passages. We recommend measurement of pluripotency markers by FACS and karyotyping after five passages.
Alternative Protocol 1 – iPSC Clonal Expansion before Cryopreservation
This protocol describes the passaging of human ESCs/iPSCs when large numbers of lines are involved. The process takes 6–7 min per line for each passage, usually around 40 minutes for 24 lines. (Figure 1B)
Materials
See Basic Protocol 1.
Protocol Steps
-
Individual hPSC colonies are picked by 20μl pipette (see procedure below) into matrigel-coated 12-well plate wells in E8 medium with 10μM ROCK inhibitor.
Although Rock inhibitor is described as optional in the EDTA passaging method, ROCK inhibitor will greatly increase the survival chances of newly picked colonies. Follow the following steps to pick colonies using a P20 pipette:- Spray 70% (vol/vol) ethanol on a microscope and on the surrounding area, as well as on a pipette and box of tips. (We pick the colonies on a benchtop in the laboratory using a normal inverted light microscope. If space allows, a microscope can be placed inside a laminar flow hood or a bench top PCR clean hood to allow for a sterile field while picking colonies. If preferred, a dissection microscope can be used to find and manually passage the colonies.)
- While wearing a facemask, find iPSC colonies under the microscope with the x4 objective. Using a P20 pipette with a tip, circle around the colony until it is loosened from surrounding cells.
- While still using a pipette tip, cross-hatch the colony so that it will come off the plate in smaller pieces.
- Next, use the pipette to push the colony off the plate and suck it into a pipette tip. Transfer the colony pieces into one well of the 12-well plate.
After 24 hours, change E8 medium daily without ROCK inhibitor till the confluence reach 10–20%.
Aspirate the spent medium, and wash with 0.5 ml EDTA/PBS once.
Add 0.5ml EDTA/PBS onto plate and incubate for 3 minutes.
Carefully aspirate the EDTA solution without dislocating the cells. Rapidly add 0.5ml of E8 medium with 10μM ROCK inhibitor to wash the colonies off the well, break the colonies by pipetting 2–3 times, and put the cell suspension back to the same well.
After 24 hours, change E8 medium daily without ROCK inhibitor till the confluence reach 70–80%.
Aspirate the spent medium, and wash with 0.5 ml EDTA/PBS once.
Add 0.5ml EDTA/PBS onto plate to incubate for 3 minutes.
Carefully aspirate the EDTA solution without dislocating the cells. Rapidly add 0.5ml of E8 medium with 10μM ROCK inhibitor to wash the colonies off the well, break the colonies by pipetting 2–3 times, and dilute the cells in 4ml E8 medium.
Plate 2 ml cell suspension each into two pre-coated 6-well plate wells. After 24 hours, switch to E8 medium without ROCK inhibitor.
Change medium and monitor the cell growth daily. When cells reach ~80% confluence (usually between 2–3 days after seeding at around 1:4 splitting ratio), move forward to cryopreservation.
BASIC PROTOCOL 2. CRYOPRESERVATION OF HUMAN ESCs/iPSCs WITH EDTA DISSOCIATION
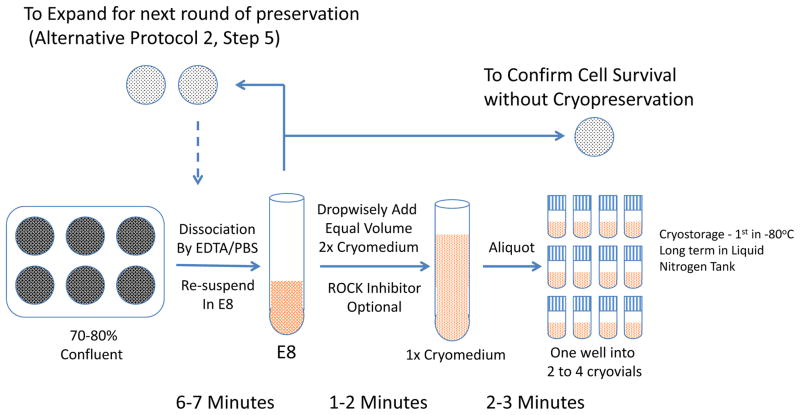
This protocol describes the procedures for cryopreservation of human ESCs/iPSCs that are prepared in Protocol 1. The process takes 6–10 min per line. (Figure 2)
Figure 2.
Flow charts for cryopreserving hPSCs. Basic Protocol 2 and alternative protocol 2.
Materials
Human ESCs: H1 cells, WA01 (US National Institutes of Health (NIH), human ESC registry No. 0043)
Human iPSCs from fibroblasts: ND2 (NIH control iPSC line)
EDTA dissociation solution (see recipe)
Full E8 medium. E8 medium can be prepared as previously described, and is also available as Essential 8 media from Life Technologies (cat. no. A14666SA) or from Stem Cell Technologies as TeSR-E8 (cat. no. 05840).
ROCK inhibitor (optional): Y-27632 (Tocris, 1254). Dissolve the Y-27632 in sterile H2O or sterile DMSO with a final concentration of 10 mM (1000x), then aliquot and store it at −80°C. The soluti on is stable for at least 1 year. Y-27632 is the ROCK inhibitor that we commonly use. Other ROCK inhibitors may also be used.
2x cryopreservation medium (20% (vol/vol) DMSO in full E8 medium, Optional – 20 μM ROCK inhibitor)
Laminar flow hood with vacuum
Inverted phase contrast microscope (x4 and x10 objectives, Zeiss or equivalent)
Six-well Nunclon Delta Surface tissue culture dishes (Thermo Scientific cat. no. 140675 or equivalent)
Pipet-aid and sterile 5- and 10-ml plastic disposable pipettes
P1000 and P20 pipetman and sterile tips with filter
Cryovials, 1.2 ml (USA Scientific, cat. no. 1412-9100 or equivalent)
Isopropanol Cell-Freezing Container
Freezer −80°C (Thermal Scientific)
Liquid nitrogen Tank
Four-way Flipper Rack (Thermal Scientific)
Protocol steps
Print cryovial labels with essential cell line information (Line name, cell type, passage number, date, initial of the researcher). In a biosafety cabinet, put the labels onto cryovials, and loosen the caps.
-
Prepare 2x cryopreservation medium (20% DMSO in E8 medium) at RT.
10 μM ROCK inhibitors can be added into cryopreservation medium if desired. Dissociate cells with EDTA dissociation solution, as described in Basic Protocol 1 (steps 4–6).
After 2–5 min incubation, carefully aspirate the EDTA solution from the cells. Quickly add 1 ml of full E8 medium per well of a six-well plate to harvest the cells.
In a drop-wise manner, add an equal volume (1 ml per well) of 2x cryopreservation medium into the harvested cell suspension. This yields a final concentration of 10% (vol/vol) DMSO.
-
Aliquot 500–1000 μl of cells into each labeled cryovial.
From one well of a six-well plate with 70–80% confluence, it is possible to freeze two to eight vials. We recommend newcomers to start with two vials/well.If iPSCs are sparse (e.g. in the early phase of expanding lines or clones when colonies can be few and sparse), it is more common to freeze two vials from one well.For clonal expansion, if large numbers of colonies need to be cryopreserved and later thawed out, cells can be resuspended in 0.5 ml E8 medium plus 0.5 ml 2x cryomedium, and frozen as 250 μl aliquots. In this way, cells can be directly diluted before plating to bypass centrifugation (Alternative Protocol 2 and 3). Place cryovials at −80°C in an isopropanol freezing container for at least 2 hours.
-
Transfer cells to labeled cryobox, and store it in liquid nitrogen tank for long-term storage.
Cells can be stored in liquid nitrogen for more than 5 years. Record the vial location and line information in a cell line database.
Alternative Protocol 2 – iPSC Clonal Expansion while Cryopreservation
This protocol describes the passaging of human ESCs/iPSCs when large numbers of colonies are involved. The process takes 6–7 min per line for each passage, usually around 40 minutes for 24 lines. (Figure 2)
Materials
See Basic Protocol 2.
Protocol Steps
Steps 1–4. Identical to Basic Protocol 2 Steps 1–4.
-
5
2 ml cell suspension is collected from two wells of 6-well plate. Use 0.5ml suspension each to passage onto two fresh matrigel-coated wells as suggested in Alternative Protocol 1 Steps 9–11.
-
6
In a drop-wise manner, add an equal volume (1.5ml) of 2x cryopreservation medium into the rest of harvested cell suspension. This yields a final concentration of 10% (vol/vol) DMSO.
-
7
Aliquot 250 – 500 μl of cells into each labeled cryovial.
-
8
Place cryovials at −80°C in an isopropanol freezing container for at least 2 hours.
-
9
Transfer cells to labeled cryobox, and store it in liquid nitrogen tank for long-term storage.
-
10
Record the vial location and line information in a cell line database.
-
11
Expand the cells from Step 5, and repeat the cryopreservation again.
If the resources are limited, the alternative protocol can be performed for initial preservation set around Passage 3, and again around Passage 10. When the cells reach around Passage 15, Basic Protocols 1 and 2 should be used for large scale preservation.
BASIC PROTOCOL 3. RECOVERY OF HUMAN ESC/iPSC COLONIES AFTER CRYOPRESERVATION
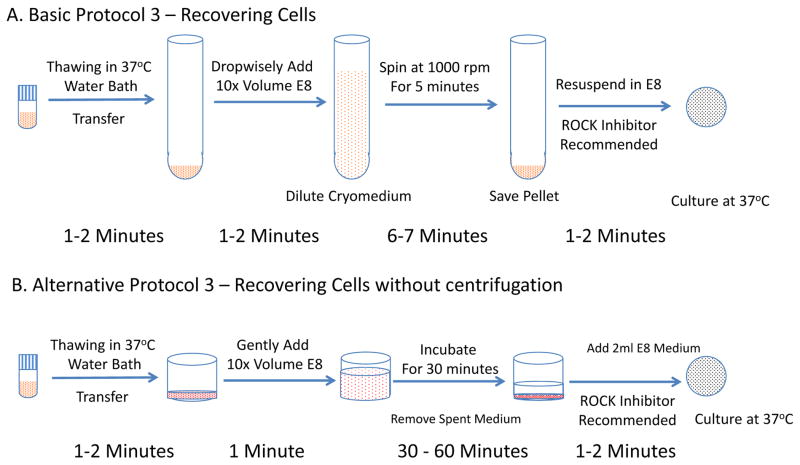
This protocol describes the recovery of ESC/iPSC colonies after cryopreservation with EDTA method. (Figure 3A)
Figure 3.
Flow charts for recovering hPSCs after cryopreservation. A. Basic Protocol 3 - Recover less than 6 vials of cells in a set. B. Alternative Protocol 3 - Recover more than 6 vials in one thaw.
Materials
Human ESCs: H1 cells, WA01 (US National Institutes of Health (NIH), human ESC registry No. 0043)
Human iPSCs from fibroblasts: ND2 (NIH control iPSC line)
Full E8 medium. E8 medium can be prepared as previously described, and is also available as Essential 8 media from Life Technologies (cat. no. A14666SA) or from Stem Cell Technologies as TeSR-E8 (cat. no. 05840).
ROCK inhibitor (optional): Y-27632 (Tocris, 1254). Dissolve the Y-27632 in sterile H2O or sterile DMSO with a final concentration of 10 mM (1000x), then aliquot and store it at −80°C. The soluti on is stable for at least 1 year. Y-27632 is the ROCK inhibitor that we commonly use. Other ROCK inhibitors may also be used.
Matrigel, growth factor reduced (BD Biosciences, cat. no. 354230)
Water bath, 37°C
Laminar flow hood with vacuum
Conical tubes, 15 ml (Falcon, cat. nos. 352097 or equivalent)
Pipet-aid and sterile 5- and 10-ml plastic disposable pipettes
P1000 and P20 pipetman and sterile tips with filter
Centrifuge (Beckman Coulter Allegra X15-R with SX4750 swinging bucket rotor or equivalent)
Six-well Nunclon Delta Surface tissue culture dishes (Thermo Scientific cat. no. 140675 or equivalent)
Cell Culture Incubator (37°C, 95% humidity, 5% CO2 and 20% O2 atmosphere, Heracell, Thermo Scientific). To improve recovery rate, 5% O2 incubator can be used.
Protocol steps
Find the location of specific cell line in liquid nitrogen tank in the cell line database, and record the position number from which the lines are being taken.
Take the cryovial(s) out of liquid nitrogen, move vials to the tissue culture room on dry ice, and directly place into 37°C water bath.
Gently stir the water with the cryovial, and check closely for the disappearance of ice.
When only a small ice particle is left floating, spray the cryovial thoroughly with 70% (vol/vol) ethanol, wipe dry and transfer the cryovial into a biosafety cabinet.
Gently transfer the cells into an empty 15 ml conical tube.
Add 10 ml of E8 medium in a drop-wise manner while continuously mixing the solution in the conical tube.
Centrifuge at 1000 rpm for 5 minutes at 4°C.
Carefully aspirate and discard the supernatant and keep the cell pellet.
-
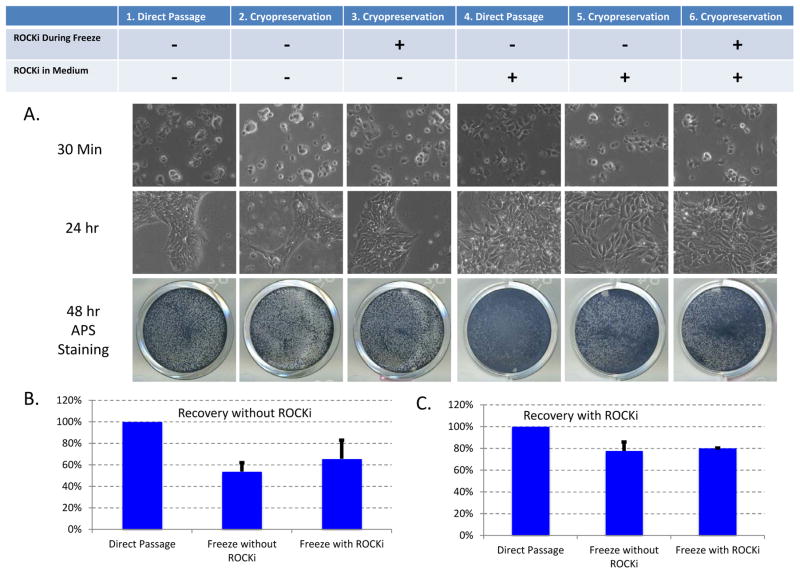
Resuspend cell pellet in E8 medium (2 ml per well) and plate cells into 1–3 wells of a Matrigel-coated six-well plate. Cells usually attach in 30 minutes after plating. (Figure 4)
10 μM ROCK inhibitors can be added into E8 medium if desired.To further improve cell survival, plates can be incubated in 5% O2 for the first two days.
Figure 4.
Comparison between regular passaging and cell recovery after cryopreservation. Six wells of H1 ESCs were dissociated by EDTA/PBS, harvested in E8 medium, and split into 24 equal portions. The cells were treated in triplicates as the table indicated. 1, Cells were directly plated into E8; 4. Cells were directly plated with ROCK inhibitor. 2 and 3, Cells were cryopreserved in E8 medium with 10% DMSO without (2) or with (3) ROCK inhibitor, and later recovered in E8 medium without ROCK inhibitor. 5 and 6, Cells were cryopreserved in E8 medium with 10% DMSO without (5) or with (6) ROCK inhibitor, and later recovered in E8 medium with ROCK inhibitor. A. Images of cells on matrigel-coated plate after different treatments. APS Staining was performed 48 hours after plating. B. Cryopreservation efficiency for cells recovered without ROCK inhibitor in E8 medium (Conditions 1, 2 and 3). The cell survival was analyzed 24 hours after plating, and normalized by the data obtained in Condition 1. C. Cryopreservation efficiency for cells recovered with ROCK inhibitor in E8 medium (Conditions 4, 5 and 6). The cell survival was analyzed 24 hours after plating, and normalized by the data obtained in Condition 4.
Alternative Protocol 3 – iPSC Recovery after Cryopreservation
This protocol describes the alternative approach to thaw large numbers of colonies. The process takes 6–7 min per line for each passage, usually around 40 minutes for 24 lines. (Figure 3B)
Materials
See Basic Protocol 3.
Protocol Steps
Find the location of specific cell lines in liquid nitrogen tank in the cell line database, and record the position number from which the lines are being taken. Take all the lines and rest them on dry-ice.
Place 6 to 12 tubes on Four-way Flipper Rack, and directly place the rack into 37°C water bath.
Gently stir the water with the rack, and check closely for the disappearance of ice.
When only a small ice particle is left floating in multiple tubes, spray the cryovials and rack thoroughly with 70% (vol/vol) ethanol, wipe dry and transfer the cryovials into a biosafety cabinet.
Gently add around 500ul E8 medium into each vial.
-
Gently transfer the cells into one well of 6-well plate that is previously coated with Matrigel.
If more than one million cells were frozen in each vial, cells can be split into two wells. Add 5 ml of E8 medium with 10 μM ROCK inhibitor into each well, and incubate the plate at 37°C for 30 minutes to 1 hour. Most live cells should have attached to the plate at this time.
Carefully aspirate and discard the supernatant.
-
Add fresh E8 medium with 10 μM ROCK inhibitor, and follow routine maintenance procedure.
To further improve cell survival, plates can be incubated in 5% O2 for the first two days.
REAGENTS AND SOLUTIONS
EDTA dissociation solution
Add 500 μl 0.5 M EDTA stock solution (pH 8.0, K&D Medical cat. no. RGF-3130 or equivalent) into 500 ml calcium/magnesium-free PBS (Invitrogen, cat. no. 14190-250 or equivalent). Add 0.9g of NaCl (Sigma S5886 or equivalent) and adjust the osmolarity to 340 mOsm. Sterilize the solution by filtration, and store it at 4°C for up to 6 months.
Note: The osmolarity of EDTA solution is designed to be the same as that of E8 medium to achieve the least disturbance of cells during dissociation. The osmolarity of PBS is around 280 mOsm, and addition of 0.9g NaCl will bring it up to 340 mOsm. The osmolarity can be measured using an osmometer (we used Advanced® Model 3320 Micro-Osmometer). In regular lab, it is not necessary to measure the number, because slight difference does not affect the cell culture significantly.
COMMENTARY
Background Information
Human PSCs can expand without limit, and can generate all cell types. They thus have gathered tremendous interests for their scientific and practical values. Human PSCs such as ESCs only transiently exist in vivo during embryogenesis, and their existence in cell culture is mainly controlled by their environment in a dish. So the quality of the hPSCs is deeply affected by various culture conditions and culture techniques. Cryopreservation has long been a major obstacle in hPSC research, and the low cryopreservation efficiency often leads to the arising of abnormal cells. The technical improvements in cryopreservation focus on three major aspects as discussed below.
First, the improvement of handling methods. hPSCs don’t survive well after individualization, so hPSCs are traditionally harvested as large aggregates using Dispase or collagenase, and such aggregates survive well in passaging but not in cryopreservation. The utilization of ROCK inhibitor in hPSC culture allows the individualized cells to survive efficiently in both passaging and cryopreservation (Li et al. 2008; Martin-Ibanez et al. 2008; Claassen et al. 2009; Heng 2009; Li et al. 2009; Martin-Ibanez et al. 2009; Mollamohammadi et al. 2009; Baharvand et al. 2010).
Second, the improvement of cell culture conditions. Even with improved cryopreservation methods, many people are still using serum products or complicated formula to preserve the cells(Cohen et al. 2014; Miyazaki et al. 2014). Such practice could introduce complication if the cells will later be used in potential clinical applications. With more and more hPSC lines being derived in defined media (Ludwig et al. 2006; Chen et al. 2011; Beers et al. 2012), it would be desirable to preserve the cells also in xeno-free defined medium.
Third, the development of specialized equipment and procedures. Sophisticated freezing apparatus were often required to achieve better preservation (Reubinoff et al. 2001; Ji et al. 2004; Ware et al. 2005; Beier et al. 2013; Lin et al. 2013). However, such equipment is not readily available to most research labs.
In our studies on cell survival and cell culture composition, we found two important facts: 1. EDTA dissociation allows good survival in both passaging and cryopreservation; 2. Base medium DMEM/F12 with 10% DMSO is sufficient for cryopreservation when cells were dissociated by EDTA. The cryopreservation protocol is thus developed for defined cell culture with enzyme-free method.
Critical Parameters
In order to maximize the cryopreservation efficiency, the following guideline should be followed. Make sure that all medium materials are fresh within two weeks after preparation. Cryopreservation medium with DMSO should be prepared on the day of the experiment. Cells should never be overgrown, because this affects cell viability and severely increases potential abnormality. For cell health and consistency, cells need to be fed daily before cryopreservation.
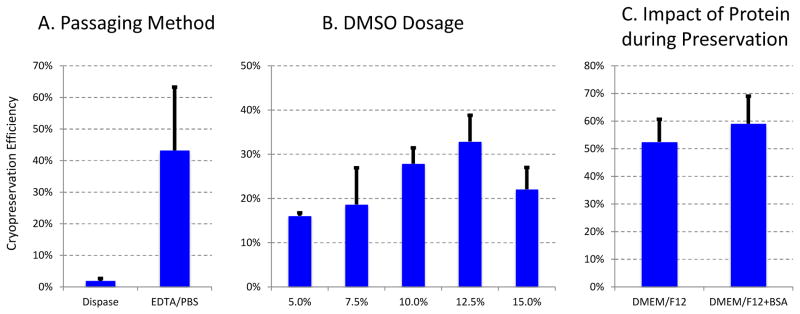
We explored the critical factors that could significantly affect cryopreservation efficiency. First we found that dissociation methods make one of the biggest impacts on cryopreservation. EDTA dissociated cells survive much better than Dispase dissociated cells after cryopreservation (Figure 5A). It is consistent with previous report that EDTA/EGTA-mediated dissociation benefits cell survival(Li et al. 2009).
Figure 5.
Important Factors affecting cryopreservation efficiency. The cryopreservation efficiency was calculated by using cell survival after cryopreservation normalized by the survival without cryopreservation. A. Dissociation method is critical for success of cryopreservation. H1 ESCs were dissociated by Dispase or EDTA/PBS, frozen in 10% DMSO/EDTA, and then recovered in E8. The cell survival was analyzed after 24 hours. B. DMSO dosage is important for cryopreservation. H1 ESCs were dissociated by EDTA/PBS, frozen in different concentration of DMSO in E8, and then recovered in E8. The cell survival was analyzed after 24 hours. C. Protein content is not critical for efficiency of cryopreservation. H1 ESCs were dissociated by EDTA/PBS, frozen in Base Medium DMEM/F12 with or without 1% Bovine Serum Albumin (BSA), and then recovered in E8. The cell survival was analyzed after 24 hours.
Secondly, cryopreservant DMSO concentration also plays an important role. We found that 10–12% DMSO in E8 provides best cryopreservation efficiency (Figure 5B). We also found that protein-rich medium is not necessary for efficient cryopreservation. Many researchers preserve hPSCs with richest possible cryomedium such as serum or KOSR based medium. Such practice is widely utilized, largely because of the poor cryopreservation efficiency in feeder culture or dispase/collagenase dissociated cells. However, serum- or KOSR-containing medium is not ideal for translational research because of their animal origin and undefined composition. With improved cryopreservation by EDTA or Accutase/TryPLE/ROCKi, cells could achieve efficient survival in defined culture even without the addition of growth factors or protein source such as albumin. This allows us to preserve hPSCs in simple medium such as E8 or DMEM/F12 without much concern of significant reduction in cryopreservation.
We also notice that ROCK inhibitor is not necessary for efficient cryopreservation. With EDTA/PBS dissociation, cells can survive well without the presence of ROCK inhibitor in cryomedium or during recovery medium. However, because of potential benefit with ROCK inhibitor treatment, we always recommend the addition of ROCK inhibitor in cryopreservation medium and recovery step as insurance for the routine practice to ensure efficient cryopreservation even under suboptimal conditions that often come up when many sample are involved or when low passage hPSCs are being preserved after reprogramming.
Troubleshooting
This protocol has been very efficient in our hands by following the passaging schedule and cryopreservation procedures. We noticed that poor cryopreservation usually comes from cells that were passaged more than 4 days before cryopreservation. People traditionally tend to grow cells to highest possible density before cryopreservation, which means that cells were often in static culture without passaging for more than four days. Such cells usually do not have good survival without ROCK inhibitors, and cannot be preserved at ideal efficiency. We found that freshly passaged (2–3 days after passaging) hPSCs have the best cell survival after dissociation and cryopreservation. At the same time, cells passaged 5–6 days before often survive poorly. It is much more efficient to expand culture for cryopreservation through frequent passaging than static waiting without intermediate dissociations. In order to quickly tell poor cryopreservation from poor survival after passaging, during cryopreservation, we recommend always passage some cells to a fresh plate to check the survival without freezing. Good survival after regular passaging is often consistent with good cryopreservation. It is also important to thaw one vial of frozen cells to confirm the efficiency of cryopreservation. We often preserve multiple batches of the same cell lines as stock.
Anticipated Results
The cryopreservation efficiency for hPSCs could range from 30% to 80% depending on the cell lines and cell culture conditions. Cells usually attach to the plate in 30 minutes after thawing (Figure 4). Generally, newly derived iPSCs have lower survival rate after passaging or cryopreservation. ROCK inhibitors are highly recommended during those processes for inexperience users.
Time Considerations
The preparation for final cryopreservation takes 3 to 4 days. Cryopreservation step takes about 30 minutes. Thawing and recovery takes about 30 minutes.
Acknowledgments
This work was supported by the NHLBI Intramural Program and University of Macau Faculty of Health Sciences.
LITERATURE CITED
- Baharvand H, Salekdeh GH, Taei A, Mollamohammadi S. An efficient and easy-to-use cryopreservation protocol for human ES and iPS cells. Nat Protoc. 2010;5:588–594. doi: 10.1038/nprot.2009.247. [DOI] [PubMed] [Google Scholar]
- Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen GK. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nature Protocols. 2012;7:2029–2040. doi: 10.1038/nprot.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier AF, Schulz JC, Zimmermann H. Cryopreservation with a twist - towards a sterile, serum-free surface-based vitrification of hESCs. Cryobiology. 2013;66:8–16. doi: 10.1016/j.cryobiol.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Chen GK, Gulbranson DR, Hou ZG, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, et al. Chemically defined conditions for human iPSC derivation and culture. Nature Methods. 2011;8:424–U476. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen DA, Desler MM, Rizzino A. ROCK inhibition enhances the recovery and growth of cryopreserved human embryonic stem cells and human induced pluripotent stem cells. Mol Reprod Dev. 2009;76:722–732. doi: 10.1002/mrd.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RI, Thompson ML, Schryver B, Ehrhardt RO. Standardized cryopreservation of pluripotent stem cells. Curr Protoc Stem Cell Biol. 2014;28(Unit 1C):14. doi: 10.1002/9780470151808.sc01c14s28. [DOI] [PubMed] [Google Scholar]
- Heng BC. Effect of Rho-associated kinase (ROCK) inhibitor Y-27632 on the post-thaw viability of cryopreserved human bone marrow-derived mesenchymal stem cells. Tissue Cell. 2009;41:376–380. doi: 10.1016/j.tice.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Ji L, de Pablo JJ, Palecek SP. Cryopreservation of adherent human embryonic stem cells. Biotechnol Bioeng. 2004;88:299–312. doi: 10.1002/bit.20243. [DOI] [PubMed] [Google Scholar]
- Li X, Krawetz R, Liu S, Meng G, Rancourt DE. ROCK inhibitor improves survival of cryopreserved serum/feeder-free single human embryonic stem cells. Hum Reprod. 2009;24:580–589. doi: 10.1093/humrep/den404. [DOI] [PubMed] [Google Scholar]
- Li X, Meng G, Krawetz R, Liu S, Rancourt DE. The ROCK inhibitor Y-27632 enhances the survival rate of human embryonic stem cells following cryopreservation. Stem Cells Dev. 2008;17:1079–1085. doi: 10.1089/scd.2007.0247. [DOI] [PubMed] [Google Scholar]
- Lin PY, Yang YC, Hung SH, Lee SY, Lee MS, Chu IM, Hwang SM. Cryopreservation of human embryonic stem cells by a programmed freezer with an oscillating magnetic field. Cryobiology. 2013;66:256–260. doi: 10.1016/j.cryobiol.2013.02.061. [DOI] [PubMed] [Google Scholar]
- Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Martin-Ibanez R, Stromberg AM, Hovatta O, Canals JM. Cryopreservation of dissociated human embryonic stem cells in the presence of ROCK inhibitor. Curr Protoc Stem Cell Biol. 2009;Chapter 1(Unit 1C):8. doi: 10.1002/9780470151808.sc01c08s10. [DOI] [PubMed] [Google Scholar]
- Martin-Ibanez R, Unger C, Stromberg A, Baker D, Canals JM, Hovatta O. Novel cryopreservation method for dissociated human embryonic stem cells in the presence of a ROCK inhibitor. Hum Reprod. 2008;23:2744–2754. doi: 10.1093/humrep/den316. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Nakatsuji N, Suemori H. Optimization of slow cooling cryopreservation for human pluripotent stem cells. Genesis. 2014;52:49–55. doi: 10.1002/dvg.22725. [DOI] [PubMed] [Google Scholar]
- Mollamohammadi S, Taei A, Pakzad M, Totonchi M, Seifinejad A, Masoudi N, Baharvand H. A simple and efficient cryopreservation method for feeder-free dissociated human induced pluripotent stem cells and human embryonic stem cells. Hum Reprod. 2009;24:2468–2476. doi: 10.1093/humrep/dep244. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16:2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Ware CB, Nelson AM, Blau CA. Controlled-rate freezing of human ES cells. Biotechniques. 2005;38:879–880. 882–873. doi: 10.2144/05386ST01. [DOI] [PubMed] [Google Scholar]
- Yu JY, Hu KJ, Smuga-Otto K, Tian SL, Stewart R, Slukvin II, Thomson JA. Human Induced Pluripotent Stem Cells Free of Vector and Transgene Sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]