Abstract
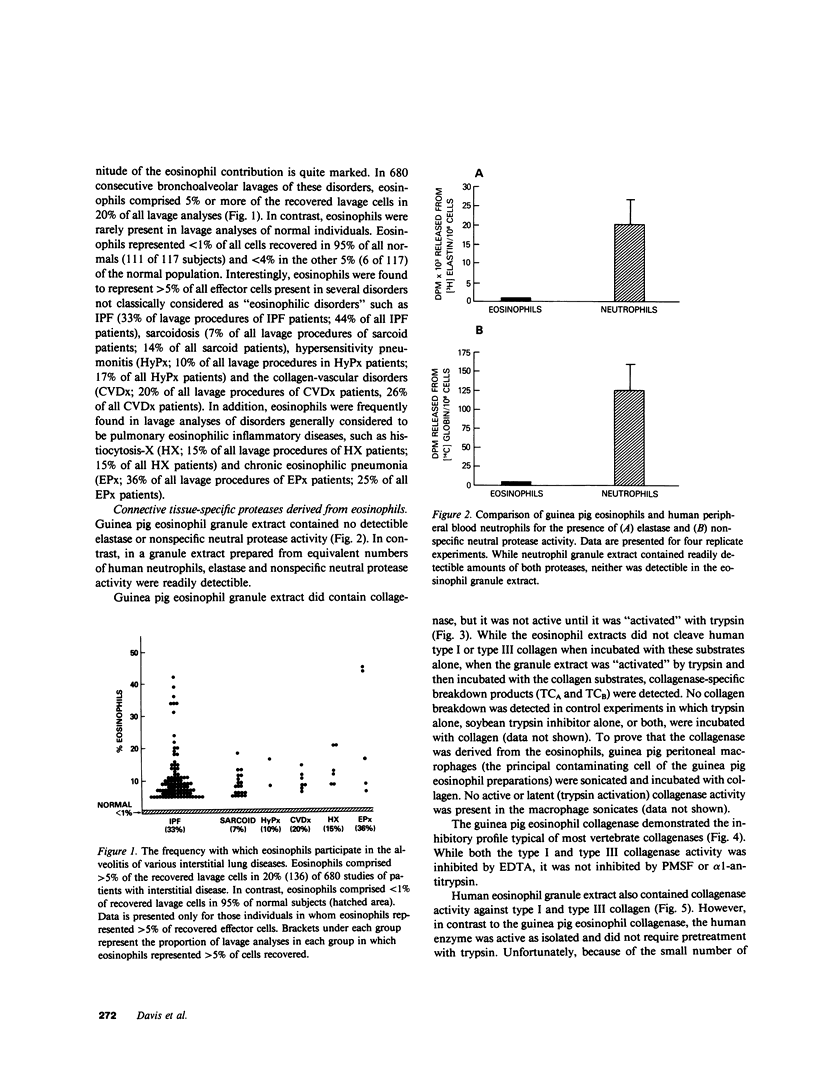
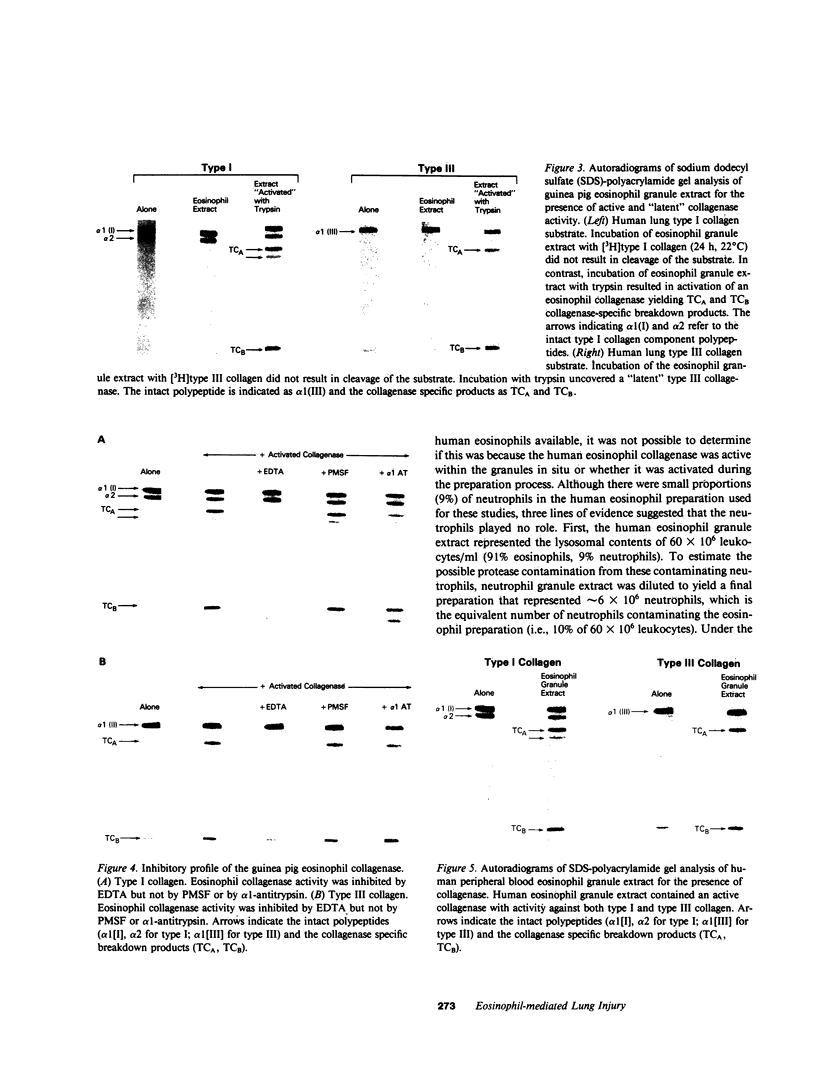
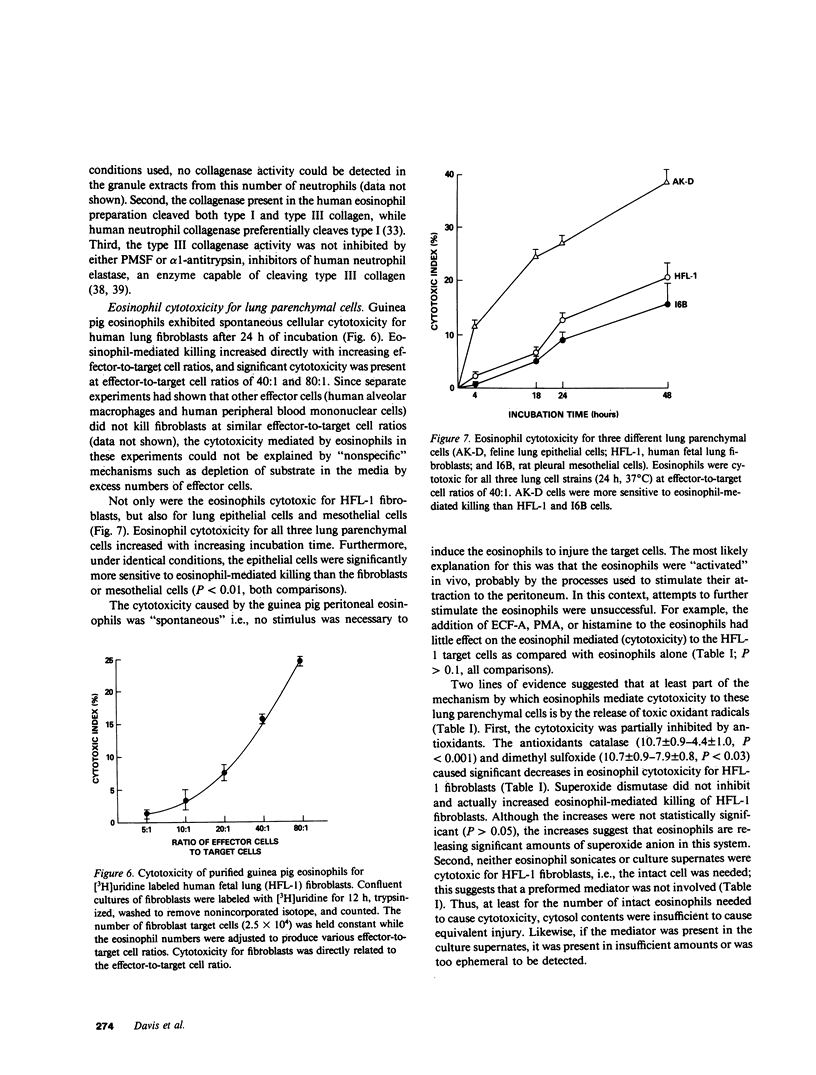
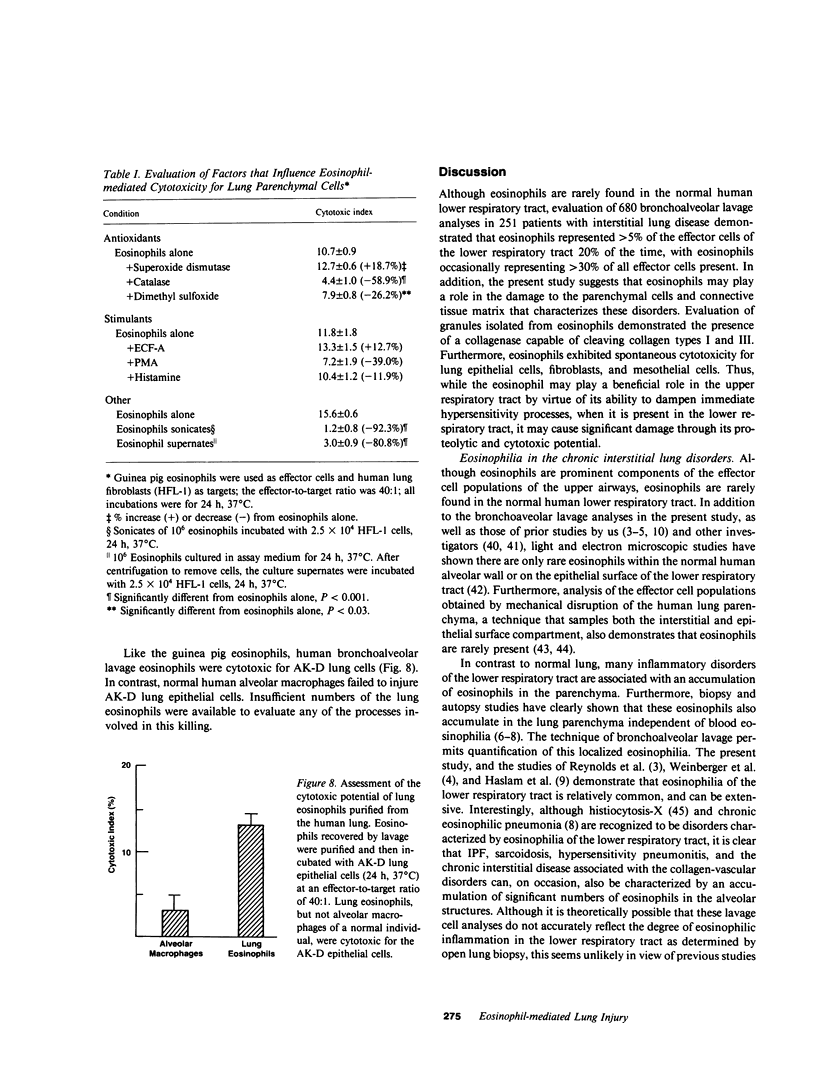
Eosinophils are a common component of the inflammation of the lower respiratory tract that characterizes the interstitial lung disorders. Bronchoalveolar lavage analyses (n = 680) of 251 patients with interstitial lung disease demonstrated that eosinophils represented greater than 5% of the effector cells comprising the alveolitis in 20% of all lavages. In contrast, lavage of normal individuals (n = 117) showed that eosinophils were never greater than 5% of the total effector cells recovered. To evaluate a possible role for eosinophils in mediating some of the cellular and connective tissue matrix derangements of the lung parenchyma found in interstitial disease, eosinophils were evaluated for the presence of proteases capable of cleaving connective tissue proteins found in the lung and for the ability to mediate cytotoxicity to lung parenchymal cells. Evaluation of guinea pig and human eosinophils demonstrated that eosinophil granules contained a collagenase that specifically cleaved human collagen types I and III, the two major connective tissue components of the human lung parenchyma. In contrast, the eosinophil did not contain an elastase or a nonspecific neutral protease. The eosinophil collagenase appeared to be a metalloprotease, as it was inhibited by ethylenediaminetetraacetate but not by phenylmethanesulfonyl-fluoride or alpha 1-antitrypsin. The eosinophil also has the capacity to injure lung parenchymal cells. Without further stimulation, eosinophils purified from peritoneal exudates of guinea pigs demonstrated spontaneous cytotoxicity for human lung fibroblasts (HFL-1), cat lung epithelial cells (AK-D) and rat lung mesothelial cells (I6B). Under identical conditions, the epithelial cells were more sensitive to eosinophil-mediated cytotoxicity than the fibroblasts or mesothelial cells (P less than 0.01), consistent with the clinical observation that in the interstitial disorders, the alveolar epithelial cells are damaged more commonly than fibroblasts or pleural cells. The eosinophil-mediated cytotoxicity could be partially inhibited by the antioxidants catalase and dimethylsulfoxide suggesting that toxic oxygen radicals play a role in mediating the cellular damage. Importantly, eosinophils purified from bronchoalveolar lavage of human interstitial lung disease also demonstrated spontaneous cytotoxicity for lung epithelial cells. These observations demonstrate that eosinophils are frequent participants of the alveolitis of the interstitial lung disorders and suggest that these cells have the potential to damage the parenchymal cells and collagen matrix of the lower respiratory tract.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basset F., Corrin B., Spencer H., Lacronique J., Roth C., Soler P., Battesti J. P., Georges R., Chrétien J. Pulmonary histiocytosis X. Am Rev Respir Dis. 1978 Nov;118(5):811–820. doi: 10.1164/arrd.1978.118.5.811. [DOI] [PubMed] [Google Scholar]
- Bassett E. G., Baker J. R., BAKER P. A., MYERS D. B. Comparison of collagenase activity in eosinophil and neutrophil fractions from rat peritoneal exudates. Aust J Exp Biol Med Sci. 1976 Oct;54(5):459–465. doi: 10.1038/icb.1976.46. [DOI] [PubMed] [Google Scholar]
- Bell D. Y., Haseman J. A., Spock A., McLennan G., Hook G. E. Plasma proteins of the bronchoalveolar surface of the lungs of smokers and nonsmokers. Am Rev Respir Dis. 1981 Jul;124(1):72–79. doi: 10.1164/arrd.1981.124.1.72. [DOI] [PubMed] [Google Scholar]
- Bielefeld D. R., Senior R. M., Yu S. Y. A new method for determination of elastolytic activity using (14C) labeled elastin and its application to leukocytic elastase. Biochem Biophys Res Commun. 1975 Dec 15;67(4):1553–1559. doi: 10.1016/0006-291x(75)90203-x. [DOI] [PubMed] [Google Scholar]
- Boswell R. N., Austen K. F., Goetzl E. J. Intermediate molecular weight eosinophil chemotactic factors in rat peritoneal mast cells: immunologic release, granule association, and demostration of structura heterogeneity. J Immunol. 1978 Jan;120(1):15–20. [PubMed] [Google Scholar]
- Bradley K. H., Kawanami O., Ferrans V. J., Crystal R. G. The fibroblast of human lung alveolar structures: a differentiated cell with a major role in lung structure and function. Methods Cell Biol. 1980;21A:37–64. doi: 10.1016/s0091-679x(08)60757-8. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., Remold H. G., Houba V., David J. R., Franks D., David P. H., Sturrock R. F. Antibody-dependent eosinophil-mediated damage to 51Cr-labeled schistosomula of Schistosoma mansoni: mediation by IgG, and inhibition by antigen-antibody complexes. J Immunol. 1977 Jun;118(6):2230–2236. [PubMed] [Google Scholar]
- Butterworth A. E., Sturrock R. F., Houba V., Rees P. H. Antibody-dependent cell-mediated damage to schistosomula in vitro. Nature. 1974 Dec 6;252(5483):503–505. doi: 10.1038/252503a0. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- CROFTON J. W., LIVINGSTONE J. L., OSWALD N. C., ROBERTS A. T. M. Pulmonary eosinophilia. Thorax. 1952 Mar;7(1):1–35. doi: 10.1136/thx.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington C. B., Addington W. W., Goff A. M., Madoff I. M., Marks A., Schwaber J. R., Gaensler E. A. Chronic eosinophilic pneumonia. N Engl J Med. 1969 Apr 10;280(15):787–798. doi: 10.1056/NEJM196904102801501. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Gallin J. I., Kaplan A. P. The selective eosinophil chemotactic activity of histamine. J Exp Med. 1975 Dec 1;142(6):1462–1476. doi: 10.1084/jem.142.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Gadek J. E., Ferrans V. J., Fulmer J. D., Line B. R., Hunninghake G. W. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981 Mar;70(3):542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- Dechatelet L. R., Migler R. A., Shirley P. S., Bass D. A., McCall C. E. Enzymes of oxidative metabolism in the human eosinophil. Proc Soc Exp Biol Med. 1978 Sep;158(4):537–541. doi: 10.3181/00379727-158-40241. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Harley J. B., Roberts W. C., Ferrans V. J., Gralnick H. R., Bjornson B. H. NIH conference. The idiopathic hypereosinophilic syndrome. Clinical, pathophysiologic, and therapeutic considerations. Ann Intern Med. 1982 Jul;97(1):78–92. doi: 10.7326/0003-4819-97-1-78. [DOI] [PubMed] [Google Scholar]
- Frigas E., Loegering D. A., Gleich G. J. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest. 1980 Jan;42(1):35–43. [PubMed] [Google Scholar]
- Frigas E., Loegering D. A., Solley G. O., Farrow G. M., Gleich G. J. Elevated levels of the eosinophil granule major basic protein in the sputum of patients with bronchial asthma. Mayo Clin Proc. 1981 Jun;56(6):345–353. [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Wright D. G., Crystal R. G. Human neutrophil elastase functions as a type III collagen "collagenase". Biochem Biophys Res Commun. 1980 Aug 29;95(4):1815–1822. doi: 10.1016/s0006-291x(80)80110-0. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Kelman J. A., Fells G., Weinberger S. E., Horwitz A. L., Reynolds H. Y., Fulmer J. D., Crystal R. G. Collagenase in the lower respiratory tract of patients with idiopathic pulmonary fibrosis. N Engl J Med. 1979 Oct 4;301(14):737–742. doi: 10.1056/NEJM197910043011401. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Frigas E., Loegering D. A., Wassom D. L., Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979 Dec;123(6):2925–2927. [PubMed] [Google Scholar]
- Gleich G. J., Loegering D. A., Kueppers F., Bajaj S. P., Mann K. G. Physiochemical and biological properties of the major basic protein from guinea pig eosinophil granules. J Exp Med. 1974 Aug 1;140(2):313–332. doi: 10.1084/jem.140.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich G. J., Loegering D. A., Maldonado J. E. Identification of a major basic protein in guinea pig eosinophil granules. J Exp Med. 1973 Jun 1;137(6):1459–1471. doi: 10.1084/jem.137.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich G. J., Loegering D. Selective stimulation and purification of eosinophils and neutrophils from guinea pig peritoneal fluids. J Lab Clin Med. 1973 Sep;82(3):522–528. [PubMed] [Google Scholar]
- Goetzl E. J., Weller P. F., Sun F. F. The regulation of human eosinophil function by endogenous mono-hydroxy-eicosatetraenoic acids (HETEs). J Immunol. 1980 Feb;124(2):926–933. [PubMed] [Google Scholar]
- Goetzl E. J., Woods J. M., Gorman R. R. Stimulation of human eosinophil and neutrophil polymorphonuclear leukocyte chemotaxis and random migration by 12-L-hydroxy-5,8,10,14-eicosatetraenoic acid. J Clin Invest. 1977 Jan;59(1):179–183. doi: 10.1172/JCI108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Nagai Y. Specific degradation of the collagen molecule by tadpole collagenolytic enzyme. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1197–1204. doi: 10.1073/pnas.54.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam P. L., Cromwell O., Dewar A., Turner-Warwick M. Evidence of increased histamine levels of lung lavage fluids from patients with cryptogenic fibrosing alveolitis. Clin Exp Immunol. 1981 Jun;44(3):587–593. [PMC free article] [PubMed] [Google Scholar]
- Haslam P. L., Turton C. W., Heard B., Lukoszek A., Collins J. V., Salsbury A. J., Turner-Warwick M. Bronchoalveolar lavage in pulmonary fibrosis: comparison of cells obtained with lung biopsy and clinical features. Thorax. 1980 Jan;35(1):9–18. doi: 10.1136/thx.35.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam P. L., Turton C. W., Lukoszek A., Salsbury A. J., Dewar A., Collins J. V., Turner-Warwick M. Bronchoalveolar lavage fluid cell counts in cryptogenic fibrosing alveolitis and their relation to therapy. Thorax. 1980 May;35(5):328–339. doi: 10.1136/thx.35.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y., Klebanoff S. J. Eosinophil peroxidase-induced mast cell secretion. J Exp Med. 1980 Aug 1;152(2):265–279. doi: 10.1084/jem.152.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Zanolari B., Schwartzman N. A., Hong S. R. Intracellular control of human neutrophil secretion. I. C5a-induced stimulus-specific desensitization and the effects of cytochalasin B. J Immunol. 1978 Sep;121(3):851–855. [PubMed] [Google Scholar]
- Hibbs M. S., Mainardi C. L., Kang A. H. Type-specific collagen degradation by eosinophils. Biochem J. 1982 Dec 1;207(3):621–624. doi: 10.1042/bj2070621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., LeMarbe P. A., Perri R., Repine J. E. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. doi: 10.1164/arrd.1981.123.1.85. [DOI] [PubMed] [Google Scholar]
- Horwitz A. L., Hance A. J., Crystal R. G. Granulocyte collagenase: selective digestion of type I relative to type III collagen. Proc Natl Acad Sci U S A. 1977 Mar;74(3):897–901. doi: 10.1073/pnas.74.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A. L., Kelman J. A., Crystal R. G. Activation of alveolar macrophage collagenase by a neutral protease secreted by the same cell. Nature. 1976 Dec 23;264(5588):772–774. doi: 10.1038/264772a0. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Fales H. M., Crystal R. G. Human alveolar macrophage-derived chemotactic factor for neutrophils. Stimuli and partial characterization. J Clin Invest. 1980 Sep;66(3):473–483. doi: 10.1172/JCI109878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Lawley T. J., Crystal R. G. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1981 Jul;68(1):259–269. doi: 10.1172/JCI110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Kawanami O., Ferrans V. J., Young R. C., Jr, Roberts W. C., Crystal R. G. Characterization of the inflammatory and immune effector cells in the lung parenchyma of patients with interstitial lung disease. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):407–412. doi: 10.1164/arrd.1981.123.4.407. [DOI] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Fulmer J. D., Crystal R. G. Ultrastructure of pulmonary mast cells in patients with fibrotic lung disorders. Lab Invest. 1979 Jun;40(6):717–734. [PubMed] [Google Scholar]
- Kay A. B., Austen K. F. The IgE-mediated release of an eosinophil leukocyte chemotactic factor from human lung. J Immunol. 1971 Sep;107(3):899–902. [PubMed] [Google Scholar]
- Kay A. B., Shin H. S., Austen K. F. Selective attraction of eosinophils and synergism between eosinophil chemotactic factor of anaphylaxis (ECF-A) and a fragment cleaved from the fifth component of complement (C5a). Immunology. 1973 Jun;24(6):969–976. [PMC free article] [PubMed] [Google Scholar]
- Kay A. B., Stechschulte D. J., Austen K. F. An eosinophil leukocyte chemotactic factor of anaphylaxis. J Exp Med. 1971 Mar 1;133(3):602–619. doi: 10.1084/jem.133.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazura J. W., Grove D. I. Stage-specific antibody-dependent eosinophil-mediated destruction of Trichinella spiralis. Nature. 1978 Aug 10;274(5671):588–589. doi: 10.1038/274588a0. [DOI] [PubMed] [Google Scholar]
- Liebow A. A., Carrington C. B. The eosinophilic pneumonias. Medicine (Baltimore) 1969 Jul;48(4):251–285. doi: 10.1097/00005792-196907000-00001. [DOI] [PubMed] [Google Scholar]
- Mainardi C. L., Hasty D. L., Seyer J. M., Kang A. H. Specific cleavage of human type III collagen by human polymorphonuclear leukocyte elastase. J Biol Chem. 1980 Dec 25;255(24):12006–12010. [PubMed] [Google Scholar]
- Parrillo J. E., Fauci A. S. Human eosinophils. Purification and cytotoxic capability of eosinophils from patients with the hypereosinophilic syndrome. Blood. 1978 Mar;51(3):457–473. [PubMed] [Google Scholar]
- Pincus S. H. Production of eosinophil-rich guinea pig peritoneal exudates. Blood. 1978 Jul;52(1):127–134. [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Thomas J. A. A comparison of the cytotoxic activity of eosinophils and other cells by 51 chromium release and time lapse microcinematography. Immunology. 1978 Apr;34(4):771–780. [PMC free article] [PubMed] [Google Scholar]
- Taylor J. C., Crawford I. P. Purification and preliminary characterization of human leukocyte elastasel. Arch Biochem Biophys. 1975 Jul;169(1):91–101. doi: 10.1016/0003-9861(75)90320-3. [DOI] [PubMed] [Google Scholar]
- Thiollet J., Jaurand M. C., Kaplan H., Bignon J., Hollande E. Culture procedure of mesothelial cells from the rat parietal pleura. Biomedicine. 1978 Apr;29(2):69–73. [PubMed] [Google Scholar]
- Weinberger S. E., Kelman J. A., Elson N. A., Young R. C., Jr, Reynolds H. Y., Fulmer J. D., Crystal R. G. Bronchoalveolar lavage in interstitial lung disease. Ann Intern Med. 1978 Oct;89(4):459–466. doi: 10.7326/0003-4819-89-4-459. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Jeffrey J. J., Eisen A. Z. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 1981 Sep 25;256(18):9511–9515. [PubMed] [Google Scholar]
- Weller P. F., Goetzl E. J. The human eosinophil: roles in host defense and tissue injury. Am J Pathol. 1980 Sep;100(3):791–820. [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr Endogenous activiation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N Engl J Med. 1977 May 5;296(18):1017–1023. doi: 10.1056/NEJM197705052961801. [DOI] [PubMed] [Google Scholar]
- Zeijlemaker W. P., Roos M. T., Schellekens P. T., Eijsvoogel V. P. Antibody-dependent human lymphocytotoxicity: a micro assay system. Eur J Immunol. 1975 Aug;5(8):579–584. doi: 10.1002/eji.1830050815. [DOI] [PubMed] [Google Scholar]























