Abstract
Background:
Sublesional declines in hip and knee region bone mass are a well-established consequence of motor complete spinal cord injury (SCI), placing individuals with SCI at risk for fragility fracture, hospitalization, and fracture-related morbidity and mortality.
Objectives:
To describe the 1-year incidence of fracture and osteoporosis prevalence in a community cohort of Canadians with chronic SCI.
Methods:
As part of the SCI Community Survey, consenting adult participants with chronic SCI completed an online or telephone survey regarding their self-reported medical comorbidities, including fracture and osteoporosis, in the 12 months prior to survey conduct. Survey elements included sociodemographic and impairment descriptors and 4 identified risk factors for lower extremity fragility fracture: injury duration ≥ 10 years, motor complete and sensory complete (AIS A or A-B) paraplegia, and female gender.
Results:
Consenting participants included 1,137 adults, 70.9% were male, mean (SD) age was 48.3 (13.3) years, and mean (SD) time post injury was 18.5 (13.1) years. Eighty-four participants (7.4%) reported a fracture in the previous 12 months and 244 (21.5%) reported having osteoporosis in the same time period, with corresponding treatment rates of 84.5% and 64.8%, respectively. The variables most strongly associated with fracture were osteoporosis (odds ratio [OR], 4.3; 95% CI, 2.72-6.89) and having a sensory-complete injury (OR, 2.2; 95% CI, 1.38-3.50) or a motor complete injury (OR, 1.7; 95% CI, 1.10-2.72).
Conclusions:
The discordance between fracture occurrence and treatment and the strength of the association between osteoporosis diagnosis and incident fractures necessitates improved bone health screening and treatment programs, particularly among persons with complete SCI.
Key words: fracture, health services, health surveys, osteoporosis, spinal cord injuries
There are significant decreases in hip, distal femur, and proximal tibia region bone mass to 30% to 50% of matched able-bodied controls in the first year following motor complete spinal cord injury (SCI). This excessive resorption occurs at a rate of approximately 4% per month in the subacute phase of SCI and results in adverse changes in bone architecture and an increased propensity for fragility fracture. Although a positive relationship between time post injury and declining bone mineral density has been reported,1,2 some authors have described a bone mass steady state that is achieved in the chronic phase of injury.3 Lower extremity bone mineral density is closely related to fracture risk, with a threshold for increased lower extremity fracture risk occurring when bone mineral density has declined by 36%.4
Fragility fractures among individuals with SCI often occur in the absence of trauma and are caused by routine activities of daily living such as transferring to the car from a wheelchair, low velocity falls on a flexed knee, torsion of the distal extremity, or bumping unseen objects.5,6 The presence of a fracture introduces a myriad of potential medical complications; 54% of patients with SCI who sustain a fracture have postfracture complications such as respiratory illness, pressure ulcers, cellulitis, urinary tract infections, and depression.7,8 This increased risk of complications persists for 12 months post fracture and results in hospitalization rates 7 times longer than those that are not fracture related.7,9 Risk of mortality also increases following lower extremity fracture in men with SCI and adjusted hazard ratios (aHR) of 1.38 (95% CI, 1.15-1.61) have been reported, with heightened risk among men over 50 years of age (aHR, 3.42; 95% CI, 2.75-4.25).10
As many as 50% of individuals with SCI will experience a fracture during their lifetime.11 Previous studies have identified 1-year incident fracture rates of 2.8% in a cohort of men with chronic SCI living in the United States and 2.2% in a cohort of men with chronic or acute paraplegia living in Switzerland.2,7,10 Several factors limit the inferences that can be made from these studies, including sample size and the inclusion of predominantly male participants with traumatic motor complete paraplegia, which limits their applicability to persons in the SCI community with alternate gender, heritage, or impairment.
Previously identified risk factors for fracture after SCI are female gender,4 family history of fragility fracture,12 and SCI-specific characteristics, including age younger than 16 years old at injury onset,13 injury duration greater than or equal to 10 years,4,8 motor complete injuries,5,8,9 and paraplegia.14 Modifiable risk factors for fracture include alcohol intake greater than 5 servings per day,9 body mass index (BMI) less than 19,4 prescription opioid use,15 and anticonvulsant therapy.16
The purpose of this study was to report the 1-year incidence of fracture and describe the prevalence of osteoporosis in a large community cohort of Canadians with chronic, traumatic SCI. In addition, we sought to report the associated rates of treatment and limitations of fragility fracture and osteoporosis on daily activities. A third objective was to enable fracture risk stratification in the absence of medical imaging (ie, dual-energy x-ray absorptiometry [DXA]) based on 4 previously identified fragility fracture risk factors: duration of injury, sex, completeness of injury (motor or sensory), and level of injury (paraplegia vs tetraplegia).
Methods
This investigation was part of a community survey to identify current health care needs and utilization of Canadians living in the community with SCI. The survey was completed in a Webbased or telephone format, in English or French, based on participant preference. The detailed survey methodology is published elsewhere.17,18
Participants
Eligible participants were adults older than 18 years of age with a traumatic SCI living in the community for at least 1 year. Participants were recruited through national and local media campaigns, and information was provided to relevant community stakeholder groups for distribution.
SCI Health Questionnaire: Comorbidities
The SCI Health Questionnaire (SCI-HQ) was created as a comprehensive community follow-up questionnaire and includes 13 items referring to the most common comorbidities that may negatively influence health post SCI. The reliability and construct validity of this measure has been established in the SCI population.17
Two comorbidities related to bone health are fracture and osteoporosis. For each question, participants were asked to indicate whether they had experienced fracture or osteoporosis in the previous 12 months and, if so, whether they had received some form of treatment and to what extent the problem limited their activities, measured on a scale from not at all to completely. Fracture incidence and osteoporosis prevalence were defined through self-report and a positive response and were not verified by medical imaging. Previous literature in the able-bodied population has validated the use of this approach for large public health surveys.19
Ethical approval was obtained from a national review board (Institutional Research Board Services) in addition to relevant institutionspecific boards. Informed consent was obtained either via online form and electronic signature or verbally, if the survey was completed over the telephone.
Data analysis
Data from the survey questions are presented as percentages (counts) or mean ± SD. Fracture and osteoporosis cohorts were compared using a test for the equality of proportion (R software, version 3.0.0) for categorical variables and a 2-way analysis of variance (ANOVA; Fracture x Osteoporosis) for continuous variables. Bivariate associations between fracture incidence, osteoporosis prevalence, and covariates (risk factors) were assessed using chi-square tests. Effect sizes are expressed as odds ratios (OR) with their 95% confidence intervals. We considered P < .05 to be statistically significant.
Results
Participants
A total of 1,137 participants (age 48.3 ± 13.3 years) with chronic (18.0 ± 13.1 years post injury) traumatic SCI (C1-T12; American Spinal Injury Association Impairment Scale [AIS] A-D) completed the survey (Table 1). Gender distribution, impairment characteristics, and age of the reported sample are consistent with national averages.20 Based on interpretation of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI), we classified individuals according to the AIS and assumed those reporting a sensory-complete injury as AIS A and those reporting motor complete injury as AIS A or B. The majority of the sample (60%) lived in an urban environment with a population greater than 100,000 and were English speaking (77%).
Table 1. Characteristics of the survey respondents.
| Variable | Entire cohort | Fracture cohort | Osteoporosis cohort | Pa |
| n | 1,137 | 84 (7.4%) | 244 (21.5%) | |
| Gender | ||||
| Male | 806 (71%) | 57 (68%) | 139 (57%) | .1039 |
| Female | 331 (29%) | 27 (32%) | 105 (43%) | |
| Age, years | 48.3 (13.3) | 47.8 (11.4) | 49.6 (11.7) | .0514 |
| Years post injury | 18.5 (13.1) | 19.6 (12.6) | 23.4(13.1) | .2356 |
| First language | ||||
| English | 877 (77%) | 65 (77%) | 209 (86%) | .111 |
| French | 260 (23%) | 19 (23%) | 35 (14%) | |
| Location of residence (population) | ||||
| >100,000 | 673 (60%) | 48 (60%) | 157 (66%) | .2959 |
| 10,000-100,000 | 196 (18%) | 13 (16%) | 31 (13%) | .6475 |
| <10,000 | 244 (22%) | 19 (24%) | 50 (21%) | .7969 |
| Location of residence (Canadian region) | ||||
| Atlantic | 116 (10%) | 9 (11%) | 28 (11%) | 1.000 |
| Quebec | 275 (24%) | 22 (26%) | 35 (14%) | .0212 |
| Ontario | 245 (22%) | 20 (24%) | 71 (29%) | .4281 |
| Prairies | 274 (24%) | 21 (25%) | 53 (22%) | .6393 |
| British Columbia | 227 (20%) | 12 (14%) | 57 (23%) | .1085 |
| Type of lesion | ||||
| Complete tetraplegia | 139 (12%) | 21 (25%) | 52 (21%) | .5831 |
| Incomplete tetraplegia | 426 (37%) | 25 (30%) | 79 (32%) | .7578 |
| Complete paraplegia | 305 (27%) | 28 (33%) | 71 (29%) | .5542 |
| Incomplete paraplegia | 267 (23%) | 10 (12%) | 42 (17%) | .3292 |
Note: Values are frequency (%) or mean (SD) for age and year postinjury variables.
Calculated from test for the equality of proportion or group interaction (Fracture x Osteoporosis) of analysis of variance (for age and years postinjury variables).
Fracture incidence and osteoporosis prevalence
Overall, 7.4% and 21.5% of participants reported experiencing a fracture or having osteoporosis, respectively, over the previous 12 months (Table 1). Corresponding rates of treatment were 84.5% for those with fracture and 64.8% for those with osteoporosis. Concerning the effects of the problem, 38.1% of respondents indicated activity limitations “to a great extent” following fracture, whereas the most frequent response (41.0%) was “not at all” among individuals reporting osteoporosis (Table 2).
Table 2. Self-reported frequency of receiving treatment for fracture or osteoporosis and associated activity limitations.
| Item | Fracture frequency (%) | Osteoporosis frequency (%) |
| Have experienced in last 12 months | 84 (7.4) | 244 (21.5) |
| Did you receive treatment for this problem? | ||
| Yes | 71 (84.5) | 158 (64.8) |
| No | 13 (15.5) | 86 (35.2) |
| To what extent did it limit your activities? | ||
| Not at all | 3 (3.6) | 100 (41.0) |
| Very little | 16 (19.0) | 79 (32.4) |
| To some extent | 18 (21.4) | 49 (20.1) |
| To a great extent | 32 (38.1) | 11 (4.5) |
| Completely | 15 (17.9) | 5 (2.0) |
Fracture risk profiles
Table 3 displays the associations between fracture incidence and osteoporosis prevalence and previously identified risk factors. Osteoporosis was the most strongly associated with fracture incidence (OR, 4.3; 95% CI, 2.72-6.89; P < .00), followed by having a sensory (AIS A) and motor (AIS A or B) complete injury.
Table 3. Relationships between fracture and osteoporosis frequency and identified fracture risk factors.
| Variable | Fracture OR (95% CI) | P | Osteoporosis OR (95% CI) | P |
| Osteoporosis | 4.3 (2.72 – 6.89) | < .001 | – | – |
| Female gender | 1.2 (0.72 – 1.88) | .533 | 2.4 (1.74 – 3.17) | <.001 |
| Paraplegia | 0.8 (0.51 – 1.25) | .330 | 0.8 (0.62 – 1.09) | .178 |
| Having 3 or more risk factorsa | 1.4 (0.90 – 2.23) | .128 | 2.4 (1.78 – 3.18) | <.001 |
| > 10 years post injury | 1.2 (0.76 – 1.99) | .397 | 3.0 (2.10 – 4.23) | < .001 |
| Motor complete injury (AIS A or B) | 1.7 (1.10 – 2.72) | .017 | 1.9 (1.42 – 2.55) | < .001 |
| Sensory complete injury (AIS A) | 2.2 (1.38 – 3.50) | .001 | 2.0 (1.47 – 2.63) | < .001 |
Note: AIS = American Spinal Injury Association Impairment Scale.
Age at injury, duration of injury, motor complete injury, sensory complete injury, paraplegia, female.
Discussion
In this large community cohort of 1,137 adults with chronic, traumatic SCI, approximately 1 in 10 (7.4%) individuals reported experiencing a fracture in the past 12 months and nearly 1 in 4 participants reported having osteoporosis (21.5%). Consistent with previous reports establishing the relationship between bone mineral density and fracture risk, the results of the present study identify osteoporosis as a strong correlate of fracture among adults with chronic SCI.21 The known risk factors for fragility fracture among men with motor complete SCI, including duration ≥ 10 years and having motor or sensory complete injury impairment, were also noted correlates of self-reported osteoporosis among the population sample with gender, culture, and impairment heterogeneity. These findings suggest that these fracture risk factors can help guide clinical decision making in the absence of or in conjunction with diagnostic imaging, specifically DXA, for diagnosing osteoporosis.
Osteoporosis is largely asymptomatic; in the absence of proper screening practices, it is often undetected until a lower extremity fracture occurs. In the SCI population, there is an increased necessity for routine osteoporosis and fracture screening due to the observed high fracture-related morbidity and mortality.7,10 Further, many individuals with complete injuries may not be aware of the occurrence of a fracture due to decreased or impaired cutaneous and deep tissue sensation.
Despite self-reported bone health screening practices between 54% and 82% 22,23 among health care providers, a recent retrospective review of postfracture care across 4 Veterans Affairs Medical Centers in the United States revealed that less than 5% of individuals with SCI had a DXA scan in the year before or after fracture.6 The lack of proper screening practices and awareness among health care providers suggests that current practices are likely failing to identify persons at high risk of fracture, thereby limiting the ability to prevent fracture occurrence.
The frequency of fracture observed in the current SCI survey is higher than what has been reported in the general Canadian population (1.3% in 1 year),24 postmenopausal women (4.6% in 4 years),25 and similar populations with neurological disease, including multiple sclerosis (2.4% in 4 years for all fractures; 1.6% in 4 years for fragility fractures).26 Economic burden of incidence and prevalence fractures among Canadians older than 50 years has been estimated at $9.2 billion, with a cost ratio that is similar to episodes of coronary heart disease and stroke.24 In the SCI population, we would estimate the per-case cost to be much higher due to the increased risk of secondary complications, hospitalization, disability, and mortality.7,9,10 Thus, we believe bone health awareness has become an urgent and important public health issue for the SCI population.
The discordance between participants who reported having osteoporosis and the percentage that reported receiving treatment, a finding that has been reported previously,8 identifies a gap in service provision for this high-risk population and likely indicates that the true prevalence of osteoporosis and incidence of fracture in the SCI population is underestimated by the current investigation. In a recent prospective cohort study completed by our research team, participants in Ontario who participated in regular bone health screening protocols and who reported adherence to therapy reported rates of fracture that were much higher at 26% (0.14 fractures/person-year) over a 2-year period, and only 39% reported osteoporosis diagnosis post fracture.27
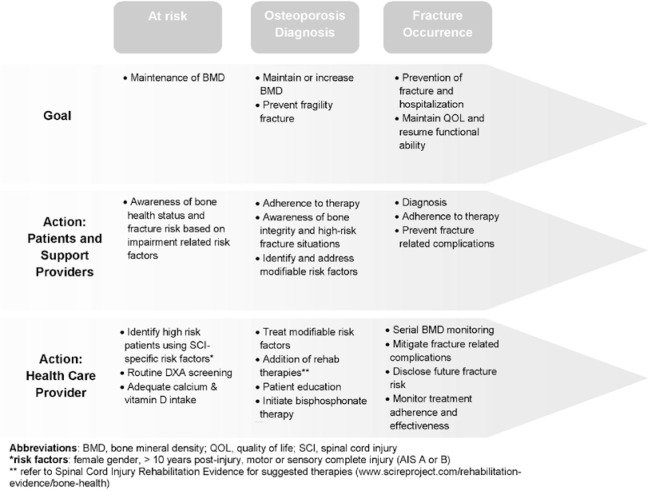
Although we have previously published clinical paradigms for diagnosis and management of sublesional osteoporosis to assist clinician decision making,28 we propose the REACT framework as a population health campaign and a necessary strategy to engage the individuals with SCI, their family, members of their support network, and relevant health care providers in prioritizing bone health (Figure 1). This model identifies a continuum of fracture prevention for persons at risk, persons with an osteoporosis diagnosis, and persons with a fracture. Health care providers should Recognize bone health status, Educate patients on the importance of maintaining good bone health, obtain an Annual assessment of bone status (including SCI-specific fracture risk assessment tools and DXA), Classify those who are at high risk, and provide therapy as indicated with routine assessment of Therapy adherence and effectiveness using Spinal Cord Injury Rehabilitation Evidence (SCIRE) as a guide (www.scireproject.com/rehabilitation-evidence/bone-health).29
Figure 1. Model of care for the prevention of sublesional osteoporosis and fragility fracture in the spinal cord injury population according to the principles outlined in the REACT framework.
Study limitations
A detailed discussion of general limitations of the SCI Community Survey is published elsewhere.18 The most relevant issue relating to this article is the self-reported nature of the data, specifically demographic-related correlates, recall of osteoporosis diagnosis, and fracture occurrence. Further, the timeline for self-report was 12 months, and the presence of osteoporosis is likely to span multiple years, with possible resolution and/or recurrence of fractures, information that was not captured. Similarly, it is not possible to clearly define the timeline for osteoporosis diagnosis and fracture occurrence, and it is possible that the strong relationship observed between osteoporosis and fracture is due to the diagnosis of osteoporosis concurrent with or after fracture onset. In a sample of able-bodied Canadians, Cadarette et al have reported that the self-report of a clinical diagnosis of osteoporosis was better among persons with a fragility or low trauma fracture, suggesting that the observed estimate of osteoporosis prevalence is likely an underestimate.19 Further, the survey method also precludes us from reporting true fracture incidence, as we did not verify fracture occurrence or the timeline for fracture occurrence. Further, the inability of the current routine practice of measuring lumbar spine and hip region bone mineral density and estimating fracture risk based on fracture risk assessment (FRAX) criteria may result in underdiagnosis, as the FRAX tool does not apply to men or premenopausal women under 50 years of age and may not adequately predict lower extremity fractures following SCI.30 Thus, we believe the present SCI Community Health Survey data represent an underestimation of the current prevalence of osteoporosis and the annual 1-year fracture incidence among the Canadian SCI population. It is also not possible for us to describe what treatment was received for either fracture or osteoporosis and whether it was effective.
Conclusions
The high incidence of self-reported fracture and prevalence of osteoporosis combined with a low frequency of routine bone health assessment and/ or treatment in the SCI community identifies an urgent need for screening and prevention strategies to combat the progression of declining sublesional hip and knee region bone mass, osteoporosis onset, and subsequent fragility fracture. Our findings indicate that osteoporosis occurred more frequently than fracture; however, treatment rates were higher and activity limitations were greater among those reporting a fracture. Diagnosis of osteoporosis at time of fracture is unacceptable; health service reforms that include primary and secondary prevention strategies are required. Given that osteoporosis or low bone mass was the variable most strongly associated with fracture, there is the theoretical potential to reduce fragility fracture in this population, with maintenance of sublesional bone mass.
Acknowledgments
The study was supported by the Rick Hansen Institute (grant 2010-03) and the Ontario Neurotrauma Foundation (grant 2010-RHI-SURVEY-812 and 2011-ONF-REPAR 2-885). Dr. Craven reports grants from Rick Hansen Institute, other honoraria from the Rick Hansen Institute, and Allergan, outside the submitted work. Dr. Giangregorio reports grants from Amgen, grants from Merck Canada Inc, and personal fees from Eli Lilly, outside the submitted work. The other authors have no conflict to disclose. The authors are indebted to all participants who took the survey and to the members of the Community Integration Practice Network (RHI) for their invaluable comments during the initial phase of the survey design and development. We particularly thank Ms. Cathy McGuiness and Lydia Cartar from RHI for their support throughout the survey development phases.
References
- 1.Eser P, Schiessl H, Willnecker J.Bone loss and steady state after spinal cord injury: A cross-sectional study using pQCT. J Musculoskel Neuron Interact. 2004;4(2):197–198. [PubMed] [Google Scholar]
- 2.Zehnder Y, Luthi M, Michel D, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: A cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15(3):180–189. [DOI] [PubMed] [Google Scholar]
- 3.Frotzler A, Berger M, Knecht H, Eser P.Bone steadystate is established at reduced bone strength after spinal cord injury: A longitudinal study using peripheral quantitative computed tomography (pQCT). Bone. 2008;43(3):549–555. [DOI] [PubMed] [Google Scholar]
- 4.Garland DE, Adkins RH, Stewart CA.Fracture threshold and risk for osteoporosis and pathologic fractures in individuals with spinal cord injury. Top Spinal Cord Inj Rehabil. 2005;11(1):61–69. [Google Scholar]
- 5.Lala D, Craven BC, Thabane L, et al. Exploring the determinants of fracture risk among individuals with spinal cord injury. Osteoporos Int. 2014;25(1):177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akhigbe T, Chin AS, Svircev JN, et al. A retrospective review of lower extremity fracture care in patients with spinal cord injury [published online ahead of print 2013]. J Spinal Cord Med. doi: http://dx.doi.org/10.1179/2045772313Y.0000000156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbone LD, Chin AS, Burns SP, et al. Morbidity following lower extremity fractures in men with spinal cord injury. Osteoporos Int. 2013;24(8):2261–2267. [DOI] [PubMed] [Google Scholar]
- 8.Gifre L, Vidal J, Carrasco J, et al. Incidence of skeletal fractures after traumatic spinal cord injury: A 10-year follow-up study. Clin Rehabil. 2014;28(4):361–369. [DOI] [PubMed] [Google Scholar]
- 9.Morse LR, Battaglino RA, Stolzmann KL, et al. Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int. 2009;20(3):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbone LD, Chin AS, Burns SP, et al. Mortality after lower extremity fractures in men with spinal cord injury. J Bone Miner Res. 2014;29(2):432–439. [DOI] [PubMed] [Google Scholar]
- 11.Szollar SM, Martin EME, Sartoris DJ, Parthemore JG, Deftos LJ.Bone mineral density and indexes of bone metabolism in spinal cord injury. Am J Phys Med Rehabil. 1998;77:28–35. [DOI] [PubMed] [Google Scholar]
- 12.Vestergaard P, Krogh K, Rejnmark L, Mosekilde L.Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36:790–796. [DOI] [PubMed] [Google Scholar]
- 13.Parsons K, Lammertse D.Epidemiology, prevention and system of care of spinal cord disorders. Arch Phys Med Rehabil. 1991;72(Suppl 4):S293–294. [PubMed] [Google Scholar]
- 14.Freehafer AA.Limb fractures in patients with spinal cord injury. Arch Phys Med Rehabil. 1981;76(9):823–827. [DOI] [PubMed] [Google Scholar]
- 15.Carbone LD, Chin AS, Lee TA, et al. The association of opioid use with incident lower extremity fractures in spinal cord injury. J Spinal Cord Med. 2013;36(2):91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carbone L, Chin AS, Lee TA, et al. The association of anticonvulsant use with fractures in spinal cord injury. Am J Phys Med Rehabil. 2013;92(12):1037–1046. [DOI] [PubMed] [Google Scholar]
- 17.Noreau L, Cobb J, Belanger LM, Dvorak MF, Leblond J, Noonan VK.Development and assessment of a community follow-up questionnaire for the Rick Hansen spinal cord injury registry. Arch Phys Med Rehabil. 2013;94(9):1753–1765. [DOI] [PubMed] [Google Scholar]
- 18.Noreau L, Noonan VK, Cobb J, Leblond J, Dumont FS.Spinal Cord Injury Community Survey: A national, comprehensive study to portray the lives of Canadians with spinal cord injury. Top Spinal Cord Inj Rehabil. 2014;20(4):249–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cadarette SM, Beaton DE, Gignac MA, Jaglal SB, Dickson L, Hawker GA.Minimal error in self-report of having had DXA, but self-report of its results was poor. J Clin Epidemiol. 2007;60(12):1306–1311. [DOI] [PubMed] [Google Scholar]
- 20.Pickett GE, Campos-Benitez M, Keller JL, Duggal N.Epidemiology of traumatic spinal cord injury in Canada. Spine. 2006;31(7):799–805. [DOI] [PubMed] [Google Scholar]
- 21.Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M.Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39:208–214. [DOI] [PubMed] [Google Scholar]
- 22.Morse LR, Giangregorio L, Battaglino RA, et al. VA-based survey of osteoporosis management in spinal cord injury. PM R. 2009;1(3):240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashe MC, Eng JJ, Krassioukov A.Physiatrists’ opinions and practice patterns for bone health after SCI. Spinal Cord. 2009;47(3):242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hopkins RB, Tarride JE, Leslie WD, et al. Estimating the excess costs for patients with incident fractures, prevalent fractures, and nonfracture osteoporosis. Osteoporos Int. 2013;24(2):581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD.Low bone mineral density and fracture burden in postmenopausal women. Can Med Assoc J. 2007;177(6):575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bazelier MT, van Staa TP, Uitdehaag BMJ, et al. Risk of fractures in patients with multiple sclerosis: A population-based cohort study. Neurology. 2012;78:1967–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lynch CL, Giangregorio L, Adachi JD, et al. Fragility fractures after spinal cord injury: Insights from the bone quality in individuals with chronic SCI study. J Spinal Cord Med. In press. [Google Scholar]
- 28.Craven BC, Robertson LA, McGillivray CF, Adachi JD.Detection and treatment of sublesional osteoporosis among patients with chronic spinal cord injury. Top Spinal Cord Inj Rehabil. 2009;14(4):1–22. [Google Scholar]
- 29.Craven BC, Lynch CL, Eng JJ.Bone health following spinal cord injury. In: Eng JJ, Teasell RW, Miller WC, et al. , eds. Spinal Cord Injury Rehabilitation Evidence (SCIRE). Vol 5.0.2014:1–37. [Google Scholar]
- 30.Silverman SL, Calderon AD.The utility and limitations of FRAX: A US perspective. Curr Osteoporos Rep. 2010;8(4):192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]