Abstract
Quercetin is a dietary flavonoid which exerts vasodilator, antiplatelet and antiproliferative effects and reduces blood pressure, oxidative status and end-organ damage in humans and animal models of systemic hypertension. We hypothesized that oral quercetin treatment might be protective in a rat model of pulmonary arterial hypertension. Three weeks after injection of monocrotaline, quercetin (10 mg/kg/d per os) or vehicle was administered for 10 days to adult Wistar rats. Quercetin significantly reduced mortality. In surviving animals, quercetin decreased pulmonary arterial pressure, right ventricular hypertrophy and muscularization of small pulmonary arteries. Classic biomarkers of pulmonary arterial hypertension such as the downregulated expression of lung BMPR2, Kv1.5, Kv2.1, upregulated survivin, endothelial dysfunction and hyperresponsiveness to 5-HT were unaffected by quercetin. Quercetin significantly restored the decrease in Kv currents, the upregulation of 5-HT2A receptors and reduced the Akt and S6 phosphorylation. In vitro, quercetin induced pulmonary artery vasodilator effects, inhibited pulmonary artery smooth muscle cell proliferation and induced apoptosis. In conclusion, quercetin is partially protective in this rat model of PAH. It delayed mortality by lowering PAP, RVH and vascular remodeling. Quercetin exerted effective vasodilator effects in isolated PA, inhibited cell proliferation and induced apoptosis in PASMCs. These effects were associated with decreased 5-HT2A receptor expression and Akt and S6 phosphorylation and partially restored Kv currents. Therefore, quercetin could be useful in the treatment of PAH.
Introduction
Pulmonary arterial hypertension (PAH) is a rare disease characterized by elevated pulmonary arterial pressure (PAP) due to increased vasoconstriction, remodeling of the pulmonary microvasculature and thrombosis, leading to right ventricular hypertrophy (RVH) and premature death [1]. PAH exhibits a complex pathophysiology, unlikely to be explained by a single factor [2], [3]. Mutations in the bone morphogenetic protein receptor type 2 (BMPR2) are responsible for many heritable forms of PAH and downregulation of its expression underlie many idiopathic and secondary forms of PAH [4], [5]. BMPR2 dysfunction leads to increased transforming growth factor-β (TGF-β) signaling [6] leading to activation of proliferative pathways including the mitogen activated protein kinases (MAPKs) pathway, the phosphatidylinositide 3-kinases, serine/threonine kinase Akt and the mammalian target of rapamycin (PI3K/Akt/mTOR) pathway and the antiapoptotic protein survivin. Inactivation, downregulation or gene polymorphisms of voltage-gated potassium channels (KV) [7], hyperresponsiveness to 5-HT [8] and loss of NO bioavailability and the subsequent endothelial dysfunction have also been implicated in the pathophysiology of PAH [9]. Although no cure exists for PAH, the understanding of the pathophysiological mechanisms has led to the development of therapies which improve symptoms and slow the progression of the disease [10].
Quercetin is a natural flavonoid regularly consumed in the diet in the form of fruits, vegetables, nuts and derived products such as wine and chocolate. Prospective studies have shown an inverse correlation between dietary flavonoid intake and mortality from coronary heart disease [11]. Several studies using various animal models provide support for the observed protective effects of dietary flavonoids with respect to cardiovascular diseases [12]. Quercetin exerts systemic, coronary and pulmonary artery vasodilatation and antiaggregant effects in vitro [13], [14], [15], and reduces blood pressure, oxidative status and end-organ damage in animal models of hypertension [16].
We hypothesized that quercetin could be effective in reversing PAH. Therefore, we tested the efficacy of oral quercetin in a rat model of PAH generated by a single injection of the plant toxin monocrotaline. This model reproduces several key aspects of PAH, including elevated PAP, RVH, premature death, vascular remodeling, oxidative stress, endothelial dysfunction, and alteration in the BMPR2, KV and 5-HT pathways.
Methods
Ethics statement
The investigation conforms with the Directive 2010/63/EU of the European Parliament and the procedures were approved by our institutional Ethical Committee (Comité de Experimentación Animal de la Universidad Complutense de Madrid). All efforts were made to minimize suffering. Animals were monitored daily and eventually sacrificed by deep anesthesia followed by decapitation before the predefined duration of the treatment if death, due to right heart failure, could be anticipated based on immobility and general state of the animal.
Animals and treatments
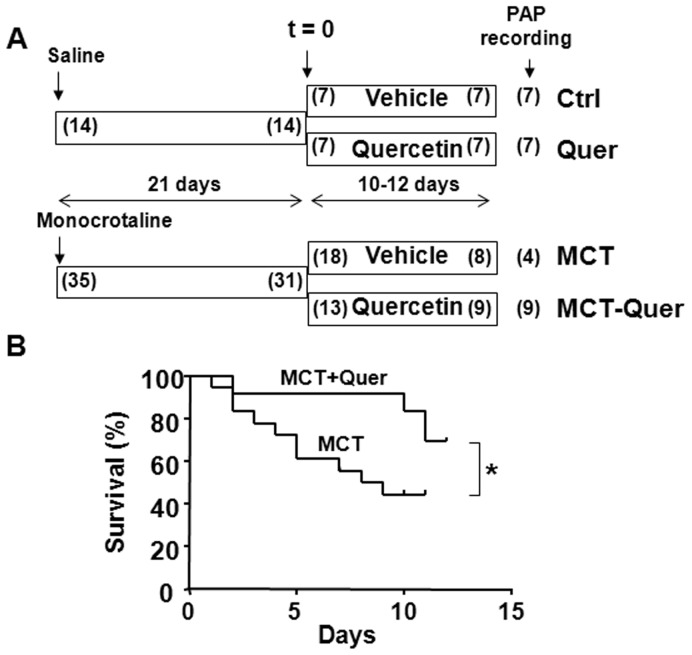
The study protocol is shown in Figure 1. Male Wistar rats of 225–250 g of body weight (BW) from Harlan Iberica (Barcelona, Spain) were maintained in the general animal facility of Universidad Complutense (ANUC), five per cage, at a constant temperature (24±1°C), with a 12-hour light/dark cycle, on a standard chow and water ad libitum. Animals were randomly divided into a control and a pulmonary hypertensive group. Pulmonary hypertension was induced by a single intraperitoneal (i.p.) injection of 60 mg kg−1 monocrotaline (control animals were injected with saline). Twenty one days after monocrotaline injection, i.e. after the animals have developed marked increases in PAP, rats from both groups were further randomly assigned to vehicle or quercetin treatment (10 mg kg−1 once daily 9∶00–11∶00 AM, dissolved in 1 mL of 1% methylcellulose) by gastric gavage for an additional period of 11 days. This dose has widely demonstrated to be effective in systemic hypertension in rats [16] and the treatment period was chosen because in the 4–6th week is the period of maximal worsening of the disease and higher mortality in this model. The study was run in three batches; all groups were followed in parallel in the first two and the two monocrotaline groups in the last one. The number of rats in each group was adjusted to reach a similar number of surviving rats in all groups at the end of the treatment based on the expected and actual mortality.
Figure 1. Quercetin increases survival.
(A) Study protocol. Numbers in parenthesis indicate the number of rats which started and finished each period. The numbers at the far right indicate the number of rats in which pulmonary arterial pressure could be recorded; the missing animals in the monocrotaline (MCT) group died during the anesthesia or surgery. (B) Kaplan-Meier analysis of survival in rats treated with monocrotaline and monocrotaline plus quercetin (Quer). * indicates P<0.05.
Pressure measurements
Twenty-four h after the last administration of quercetin or vehicle rats were anaesthetized i.p. with 80 mg kg−1 ketamine plus 8 mg kg−1 xylazine and ventilated with room air (tidal volume 9 ml kg−1, 60 breaths min−1, positive end-expiratory pressure 2 cm H2O). Right ventricular systolic and diastolic pressure (RVSP, RVDP) and systolic, diastolic and mean pulmonary arterial pressures (SPAP, DPAP and MPAP) were then measured in open-chest rats with a pressure transducer via a catheter advanced through the right ventricle into the PA. It should be noted that the pressure and heart rate values obtained in anaesthetized open-chest rats may represent an underestimation of the values in closed-chest conscious animals. At the end of the recordings, hearts were excised and the right ventricle (RV) and the left ventricle plus septum (LV+S) were carefully dissected and weighed. The ratio RV/BW and the Fulton Index [RV/(LV+S)] were calculated to assess the right ventricular hypertrophy.
Lung histology
The right lung was inflated in situ with formol saline through the right bronchus and embedded in paraffin. Lung sections were stained with haematoxylin and eosin and Masson trichrome techniques and examined by light microscopy, and elastin was visualized by its green autofluorescence. Small arteries (25–100 µm outer diameter) were analysed in a blinded fashion and categorized as muscular, partially muscular or nonmuscular as previously described [17].
Vascular reactivity
Intrapulmonary artery rings (2–3 mm long, ∼0.5–0.8 mm internal diameter) were dissected and mounted in Krebs solution bubbled with 21%O2 and 5% CO2 under 0.75 g of resting tension in organ chambers as previously described [18]. After equilibration, arterial rings from the four animal groups were firstly stimulated with KCl (80 mM). Thereafter, preparations were washed and after 45 min were contracted by 10−7M phenylephrine, and concentration–response curves to acetylcholine (10−9−3×10−4 M) were performed by cumulative addition. In different rings a concentration–response curves to serotonin (3×10−8−10−4 M) were performed by cumulative addition.
KV current recordings
For smooth muscle cells isolation, endothelium-denuded PA were cut into small segments (2×2 mm) and placed in Ca2+-free physiological salt solution containing (in mg/ml) 1 papain, 0.8 dithiothreitol and 0.7 albumin. Tissues were incubated in this solution at 4°C for 10 minutes and then agitated for 7 minutes at 37°C. Afterwards, tissues were washed in Ca2+-free physiological salt solution and disaggregated using a wide bore, smooth-tipped pipette. Cells were stored at 4°C and used within 8 h of isolation. Membrane currents were recorded using the whole-cell configuration of the patch clamp technique as previously described [19]. Membrane potential (Em) was measured under current clamp mode.
Real time RT-PCR
Total RNA was isolated and purified from whole lung homogenates using RNeasy Mini kit (Qiagen, Hilden, Germany). Real-time PCR was performed using a Taqman system (Roche Diagnostics, Mannheim, Germany) in the Genomic Unit of Universidad Complutense de Madrid. Specific primers were designed for Kv1.5 (sense 5′-GGAAGAACAAGGCAACCAGA-3′, antisense 5′-AGCTGACCTTCCGTTGACC-3′), iNOS (sense 5′-TTGGAGTTCACCCAGTTGTG-3′, antisense 5′-ACATCGAAGCGGCCATAG-3′), eNOS (sense 5′-GGTATTTGATGCTCGGGACT-3′, antisense 5′-TGTGGTTACAGATGTAGGTGAACA-3′), caveolin-1 (sense 5′-GGGCATGAAGGCAGGTTAT −3′, antisense 5′-AGTGAGGACAGCAACCAACTC −3′), BMPR2 (sense 5′-CGGGCAGGATAAATCAGGA-3′, antisense5’-CAGGAAAGTAAATTCGGGTGA-3′), 5HT2A (sense 5′-CTGCTCAATGTGTTTGTCTGG-3′, antisense 5′-GAACAACGTATATACCAGTGGATTGA-3′) and β-actin (sense 5′-GCCCTAGACTTCGAGCAAGA-3′, antisense 5′-TCAGGCAGCTCATAGCTCTTC-3′). Survivin, Kv 2.1, Kv 7.1 and Kv 7.5 expression was analyzed using commercial primers from Applied Biosystems. The data were corrected by the expression of β-actin and expressed as relative expression of target genes in control animals using the delta-delta Ct method (RQ).
Western blotting analysis
Lung homogenates were run on a sodium dodecyl sulphate-polyacrilamide electrophoresis. Proteins were transferred to polyvinylidene difluoride membranes, incubated with primary antibodies against survivin, ERK1/2, p38, Akt, S6 or the phosphorylated forms of ERK1/2, p38, Akt and S6 240–244 (all from Cell Signalling Technology, Danvers, MA, except the anti-survivin and the anti-p-p38 antibodies from Santa Cruz, Dallas) and then with the secondary peroxidase conjugated antibodies. Antibody binding was detected by an ECL system (Amersham Pharmacia Biotech, Amersham, UK) and densitometric analysis was performed using Scion Image-Release Beta 4.02 software Samples were re-probed for expression of smooth muscle β-actin.
PASMC and PA fibroblast proliferation
Pulmonary artery smooth muscle cells (PASMC) and fibroblasts were isolated from explants of PA from control and pulmonary hypertensive (21 days after monocrotaline treatment) rats and grown in Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal calf serum. PASMC, seeded at 30.000 cell/ml (approximately 50% confluence) in 96 well plates, were growth arrested by an exposure for 24 h in 0.1% fetal calf serum (FCS) medium, and then exposed to quercetin or vehicle (0.1% DMSO) in DMEM containing 10% FCS for 24 or 48 h. Medium in all experiments using quercetin or vehicle was supplemented with ascorbic acid (30 µM) to minimize quercetin oxidation. Cell viability and proliferation were measured using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) [20] and the 5-bromo-2′-deoxyuridine (BrdU) assays (according to the manufacturer’s protocol, Roche Applied Science), respectively. Nuclear morphology was assessed after 24 or 48 h of treatment by incubating the cells with the membrane permeable DNA dye Hoechst 33258 (4 µg/mL) and the membrane impermeable DNA dye propidium iodide (20 µg/mL) for 40 min at 37°C followed by observation using a fluorescence microscope.
Drugs and reagents
All drugs were from Sigma (Tres Cantos, Spain) except ketamine (Merial, Lyon, France) and xylazine (KVP Pharma und Veterinar-Produkte GmbH, Kiel, Germany).
Statistical analysis
Results are expressed as means ± SEM of n measurements where n identifies the number of animals. Statistical analysis was performed by one-way ANOVA followed by Bonferroni’s post hoc test except for mortality which was assessed using a Kaplan-Meier analysis and for the PA muscularization study using a χ2 test. A value of P<0.05 was considered statistically significant.
Results
Survival, pulmonary artery pressure and right ventricular hypertrophy
All animals were healthy at baseline. Four out of 35 rats treated with monocrotaline died within the first three weeks, i.e. before randomization to quercetin-vehicle (Figure 1A). Only 8 of the 18 monocrotaline-treated rats assigned to vehicle treatment survived until the end of the experiment and PAP could only be recorded in 4 of them because the rest died during the anesthesia or surgery. These latter rats were not included in the mortality analysis but their organs and tissues were immediately dissected for further use. Four out of 13 monocrotaline treated rats died during the treatment with quercetin. The Kaplan-Meier analysis (Figure 1B) shows that quercetin produced a significant increase in survival.
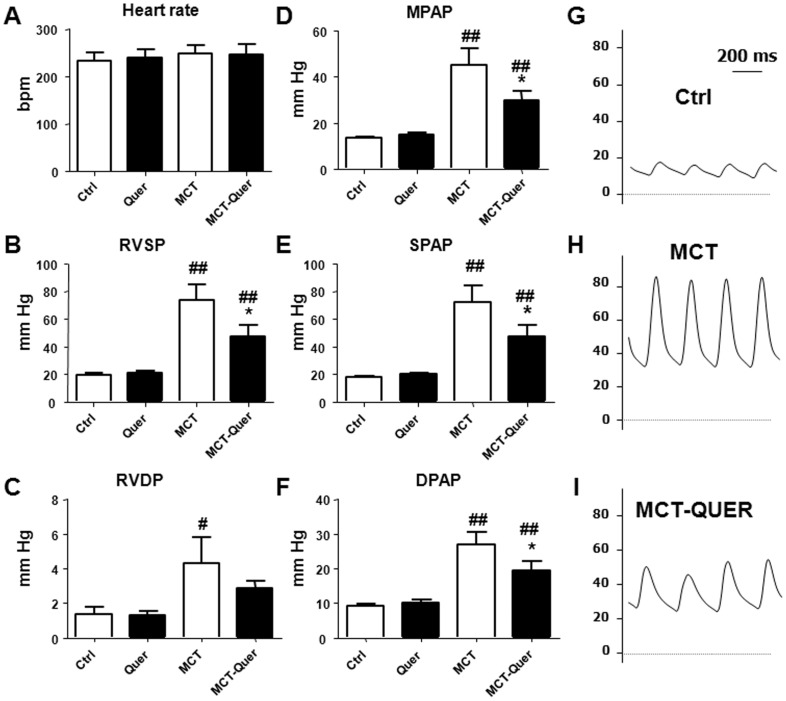
Monocrotaline produced significant increases in RVSP, RVDP, SPAP, DPAP and MPAP (Figure 2). Therefore, as expected, monocrotaline treated rats developed pulmonary hypertension, with MPAP values of 45.2±7.1 mmHg (vs. 13.6±0.6 mmHg in controls). An oral daily dose of quercetin starting three weeks after monocrotaline significantly reduced these parameters (except for RVDP) with MPAP values of 29.7±5.9 mmHg, which were still significantly above control values. The heart rate was similar in all experimental groups.
Figure 2. Quercetin reduces pulmonary artery pressure.
Heart rate (A), right systolic (B) and diastolic (C) ventricular pressure, mean (D), systolic (E) and diastolic (F) pulmonary arterial pressure. Panels G, H, I show pulmonary artery pressure recordings in the control (Ctrl), monocrotaline (MCT) and monocrotaline plus quercetin (Quer) group, respectively. Results are means ± SEM of 4–9 animals, * indicates P<0.05 versus MCT and #P<0.05, ## P<0.01 versus Ctrl.
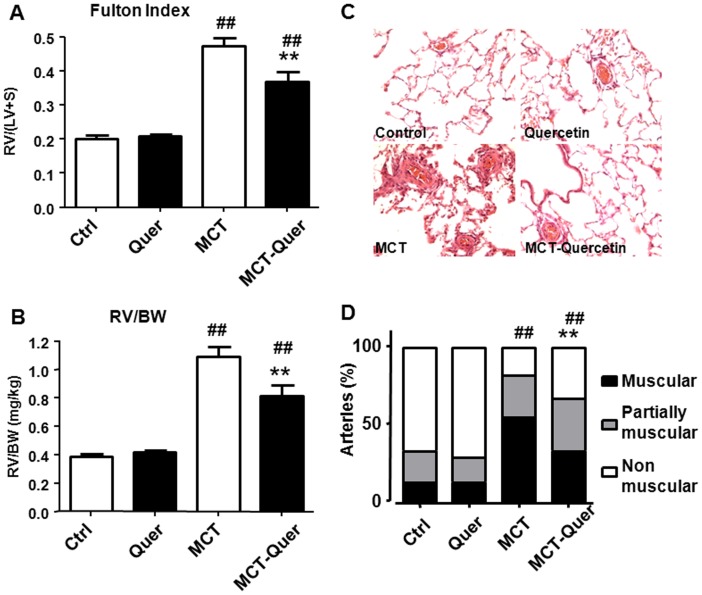
Rats treated with monocrotaline developed a marked right ventricular hypertrophy as shown by the increased RV weight referred either to the LV+S weight or to BW (Figure 3A and B). Quercetin significantly reduced both parameters but again they were still significantly above controls.
Figure 3. Quercetin reduces right ventricular hypertrophy and vascular remodeling.
(A) Fulton index [RV/(LV+S) ratio]. (B) Right ventricular weight relative to body weight (RV/BW). (C) Representative images of lung histology. (D) Percentage of muscular, partially muscular and non-muscular arteries in different groups. For panels A and B each column represents the mean ± SEM of 7–9 animals. ** For panel D between 26 and 110 arteries (25–100 µm) were analyzed in cross-sections of lungs (stained with haematoxylin and eosin) from at least four animals in each group. ** indicates P<0.01 versus monocrotaline (MCT) and ## indicates P<0.01 versus control (Ctrl).
Histological changes
Small PA (25–100 µm) observed in lung sections were classified as muscular, partially muscular and non-muscular arteries (Figures 3C and D). Monocrotaline increased the percentage of muscular arteries, with a corresponding decrease in partially muscular and non-muscular arteries. The treatment with quercetin resulted in a partial prevention of monocrotaline-induced vascular remodeling (P<0.01).
KV currents and membrane potential
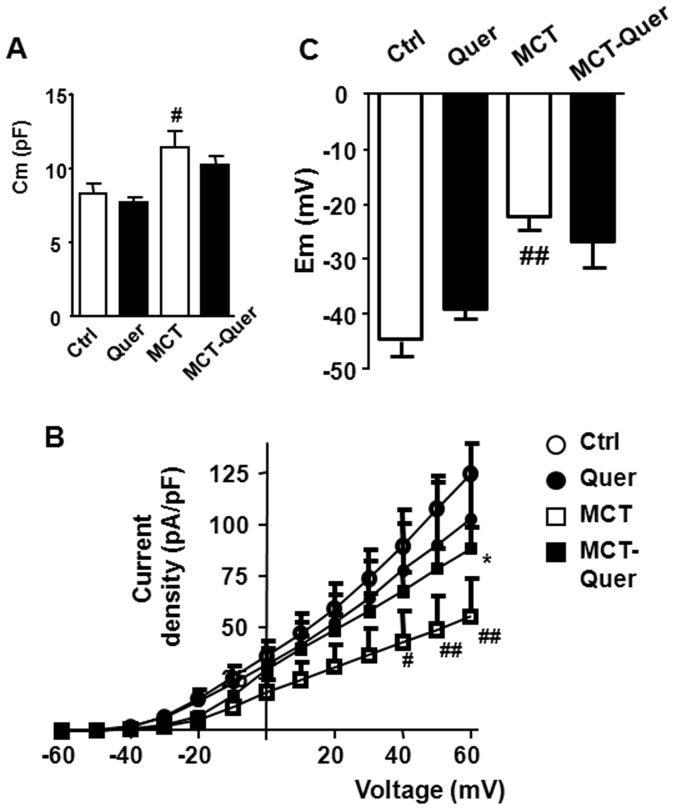
In freshly isolated PASMC, membrane capacitance, an estimate of membrane surface, was significantly increased in monocrotaline treated rats. Quercetin was not able to restore this increase (Figure 4A). Monocrotaline also induced a significant decrease in the amplitude of the KV currents and membrane depolarization. Quercetin significantly reversed the inhibition of the KV currents but it had no significant effect on membrane potential (Figure 4B and 4C).
Figure 4. Quercetin prevents the inhibition of KV currents.
(A) Cell capacitance (Cm). (B) Current–voltage relationships measured at the end of the pulse. (C) Membrane potential (Em). * indicates P<0.05 versus monocrotaline (MCT). # and ## indicate P<0.05 and P<0.01 versus control (Ctrl). Each column or symbol represents the mean value ± SEM (n = 4–6).
Gene expression
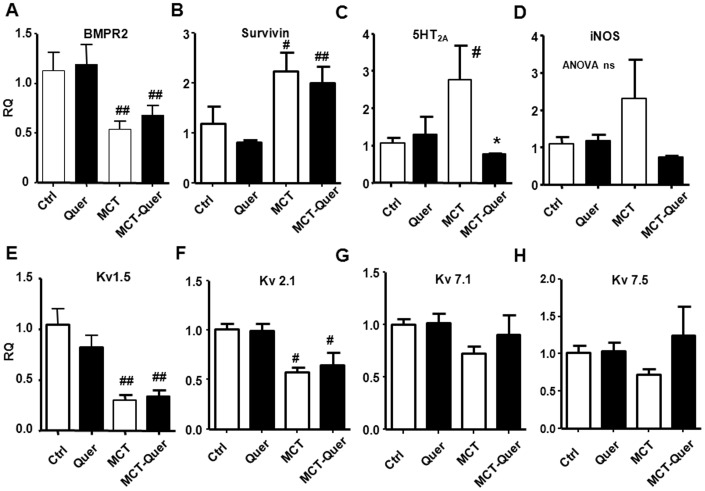
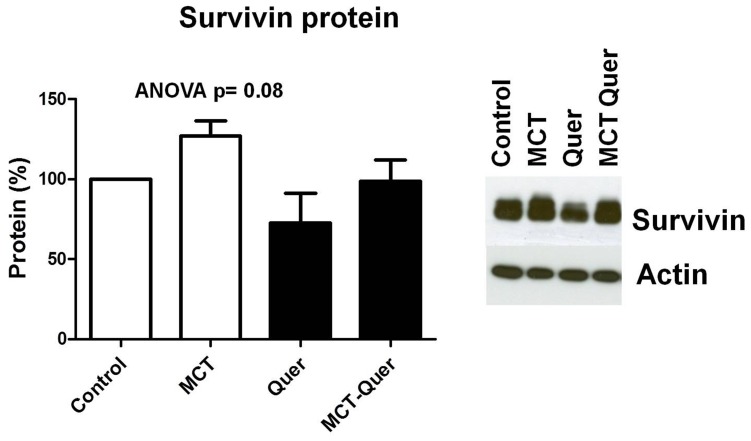
We examined the gene expression in whole lung homogenates of key proteins involved in PAH. BMPR2, Kv1.5 and Kv2.1 were strongly downregulated by monocrotaline (Figure 5). The difference in expression of these genes in the monocrotaline plus quercetin vs monocrotaline group was not statistically significant. The expression of survivin and 5HT2A mRNA was significantly increased by monocrotaline, and quercetin was able to reverse the latter change. However, changes in survivin protein (Figure 6) were smaller and not significant among groups. The mRNA expression of iNOS in whole lung was highly variable in the monocrotaline group, despite a trend for increased expression in the monocrotaline group the ANOVA did not yield significant differences. Thus, we analyzed the mRNA expression of iNOS in homogenates from isolated pulmonary arteries. In this tissue, iNOS mRNA was significantly increased by monocrotaline (2.4±0.7 fold) and this effect was inhibited by quercetin (1.02±0.6). Despite a positive trend, the changes in expression of Kv7.1 and Kv7.5 in whole lung were not statistically different among groups.
Figure 5. Changes in lung expression of (A) BMPRII, (B) Survivin, (C) 5HT2A, (D) iNOS, (E) Kv1.5, (F) Kv2.1, (G) Kv7.1 and (H) Kv7.5 mRNA by RT-PCR.
Results are means ± SEM of 4–8 animals normalized by the expression of β-actin. * indicates P<0.05 versus monocrotaline (MCT), # and ## P<0.05 and P<0.01 versus control (Ctrl).
Figure 6. Changes in the expression of survivin at the level of protein.
Lung homogenates were analyzed by Western blot. Results are means ± SEM of 5–8 animals. Protein levels were normalized by the expression of β-actin.
Vascular dysfunction
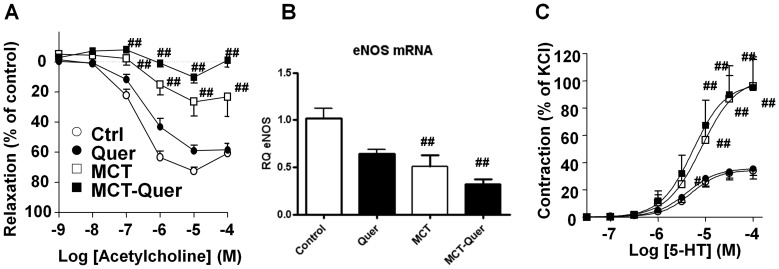
Monocrotaline significantly decreased the maximal relaxation evoked by acetylcholine in isolated rat pulmonary arteries (Figure 7A) and no changes were found in rats treated with quercetin. The endothelial dysfunction was accompanied by a reduction in the expression of eNOS which again was not reverted by quercetin (Figure 7B). Monocrotaline also produced a significant hyperresponsiveness of pulmonary arteries to 5-HT (Emax values of 102±3% of the response to KCl vs 35±1% in control) and quercetin again was not able to prevent it (98±1%) (Figure 7C).
Figure 7. Quercetin (Quer) does not prevent vascular dysfunction.
(A) Relaxant effects of pulmonary arteries to the endothelium-dependent vasodilator acetylcholine in pulmonary arteries stimulated with phenylephrine. (B) Expression of eNOS mRNA in pulmonary arteries homogenates analyzed by RT-PCR. (C) Contractile responses to 5-HT in pulmonary arteries expressed as a percent of a previous response to KCl. Results are means ± SEM of 4–9 experiments. Results are means ± SEM of 4–8 animals normalized by the expression of β-actin. * indicates P<0.05 versus monocrotaline (MCT), # and ## P<0.05 and P<0.01 versus control (Ctrl).
Expression of MAPK and Akt pathways
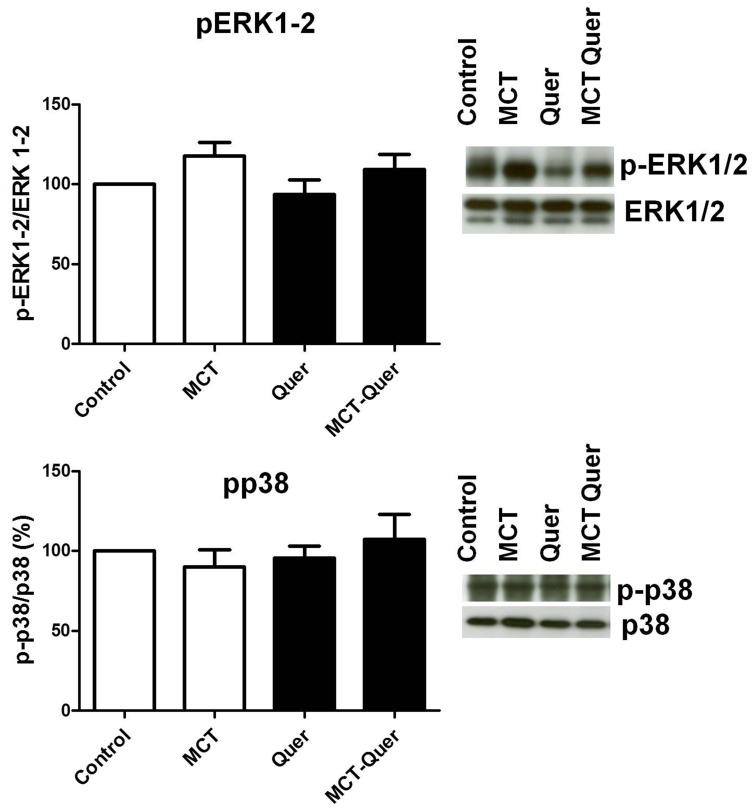
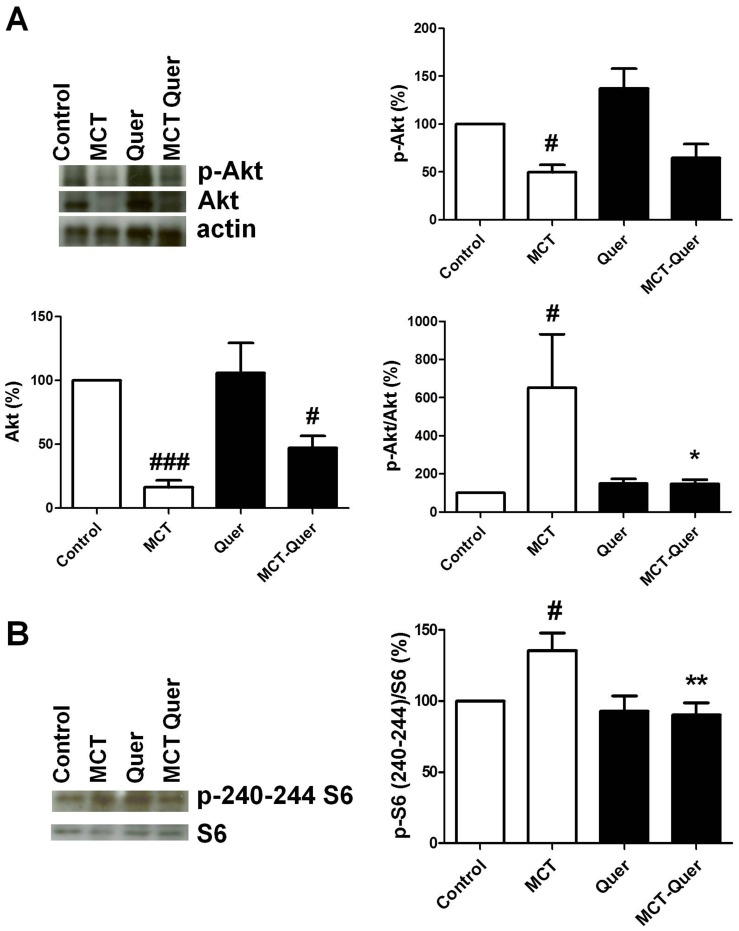
Despite an apparent increase in phosphoERK1/2 in some monocrotaline-treated rats, on average, the phosphorylation of MAP kinases ERK1/2 and p38 was not significantly different (Figure 8A,B). Pulmonary hypertensive animals showed a marked increased phosphorylation of Akt (p-Akt to total-Akt ratio), and this effect was not evident in monocrotaline-quercetin treated animals (Figure 9A). However, it should be noted that the increased p-Akt/total-Akt ratio was accompanied by a strong downregulation of Akt. Phosphorylation of S6 at residues 240–244 was significantly increased by monocrotaline and this effect was prevented by quercetin (Figure 9B).
Figure 8. Lack of changes in the activation of MAPKs: ERK1/2 and p38MAPK.
Lung homogenates were analyzed by Western blot and probed with the anti-phospho MAPK or the anti-total MAPK antibodies. Results expressed as phosphorylated forms normalized by the total protein, are means ± SEM of 5–8 animals.
Figure 9. Changes in the PI3K/Akt/mTOR/S6 signaling pathway.
Lung homogenates were analyzed by Western blot and probed with the anti-phospho Akt, the anti-total Akt or anti-β actin (A) or antiphospho S6 ribosomal protein (serines 240, 244) or total S6 (B) antibodies. Results expressed as phosphorylated forms normalized by the total protein, are means ± SEM of 5–8 animals. * indicates P<0.05 versus monocrotaline (MCT), #, ## indicate P<0.05, and P<0.0001 versus control (Ctrl).
Antiproliferative and pro-apoptotic effects in vitro
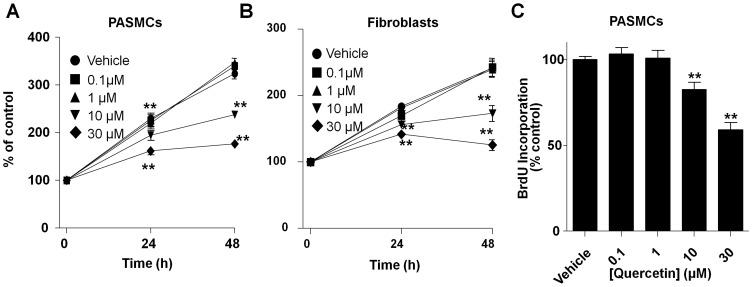
PASMC and fibroblasts from monocrotaline-treated rats cultured in the presence of 10% FCS grew at an exponential rate for 48 h. Addition of quercetin produced a concentration- and time-dependent decrease in the number of viable cells estimated by the MTT assay in both cell types (Figure 10A and 10B). We analyzed whether quercetin was reducing cell proliferation in PASMC from monocrotaline-treated rats by the analysis of BrdU incorporation. Quercetin produced significant inhibitory effects on BrdU incorporation at 24 h (Figure 10C).
Figure 10. Quercetin decreases PASMC and fibroblast proliferation.
(A) PASMC and (B) fibroblasts were isolated from monocrotaline-treated rats and grown in culture. Viable cells were estimated by the MTT test exposed to quercetin in culture for 24 or 48 h. (C) PASMC proliferation was analyzed by the BrdU incorporation after 24 h of treatment with increasing concentrations of quercetin. Results are means ± SEM of 3–4 experiments performed in triplicate. ** indicates P<0.01 versus vehicle (DMSO).
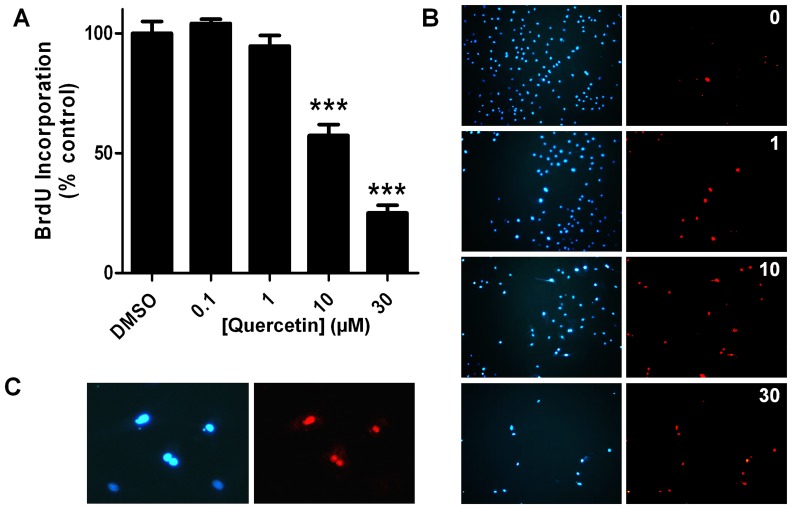
In PASMC from control rats, proliferation, measured by the MTT assay (not shown) or BrdU incorporation was also inhibited (Figure 11). It also strongly reduced the total number of cells (positive for the membrane permeable dye Hoescht 33258) and produced an increase in non viable cells (positive for the membrane impermeable dye propidium iodide, Figure 11). Non viable cells showed chromatin condensation and nuclear fragmentation characteristic of apoptotic cells.
Figure 11. Quercetin decreases proliferation (A) and induces apoptosis (B) in cultured PASMC from control rats.
(A) PASMC proliferation was analyzed by the BrdU incorporation after 24 h of treatment with increasing concentrations of quercetin. Results are means ± SEM of 4–5 experiments. (B) Photographs of PASMC after 48 h treatment with quercetin in culture stained with the membrane permeable blue dye Hoescht 33258 (left, viable and non-viable cells) and with the membrane impermeable red dye propidium iodide (right, non-viable cells). (C) Magnified photographs of cells treated with 10 µM quercetin showing three apoptotic cells (top and middle cells) with chromatin condensation and nuclear fragmentation and two healthy cells (bottom).
Discussion
PAH is characterized by increased pulmonary artery pressure as a result of increased pulmonary vascular resistance due to pulmonary vascular vasoconstriction, increased smooth muscle cell proliferation/apoptosis ratio and in situ thrombosis [3]. Quercetin has shown a number of vascular effects including vasodilator, antiplatelet, smooth muscle antiproliferative and pro-apoptotic effects in the systemic and coronary circulation. Herein we show that quercetin is also partially protective in a model of PAH by lowering PAP, RVH and vascular remodeling. In addition, in vitro quercetin exerted pulmonary vasodilator effects and inhibited cell proliferation in PASMCs.
PAH develops progressively and most PAH patients with earlier-stage disease such as New York Heart Association (NYHA) class I or II are asymptomatic and the condition is not identified until symptoms become severe [1]. Therefore, treatments for PAH must be effective once the symptoms have developed. Gao et al. have recently reported that a high dose of quercetin (10 fold the one we used) from day 2 to day 21 after monocrotaline and analyzing the results at day 41st, i.e. a preventive strategy, produced a reduction of PAP and RVH but they did not attempt to analyze the mechanisms involved [21]. We chose to start treatment with quercetin 21 days after monocrotaline. At this stage, the animals develop marked increases in PAP and the severity of the disease in our study was evidenced by 11% mortality. The final mortality in untreated animals was 56% (78% including animals which died during anesthesia or surgery). Moreover, surviving rats were in better hemodynamic conditions when treated by quercetin. As expected by the reduced right ventricular afterload, quercetin also decreased the right ventricular hypertrophy. The effectiveness of quercetin in this experiment with a very aggressive form of PAH suggests that it might prevent premature death in humans with PAH. It should be noted, however, that although all clinically useful drugs for PAH are effective in this animal model, not all drugs reversing monocrotaline-induced increased PAP are effective in clinical PAH [22].
Despite the increased survival and the hemodynamic, anatomical and histological improvement, most of the conventional markers of PAH such as membrane depolarization, endothelial dysfunction and downregulation of BMPR2 and KV1.5, were unaffected or modestly affected by quercetin. Endothelial dysfunction was accompanied by a reduced the expression of eNOS. This is consistent with previous studies showing that eNOS and other endothelial cell markers, such as caveolin-1 or CD31, are downregulated, suggesting that monocrotaline causes disruption of the integrity of the endothelial cells [23], [24]. Quercetin has demonstrated to improve endothelial function in several models of cardiovascular disease via decreased expression of NADPH oxidase subunits, restoring eNOS uncoupling, eNOS phosphorylation or scavenging superoxide [25]. However, quercetin was unable to restore eNOS downregulation which appears to be a major contributor to endothelial dysfunction in this model. In fact, a trend for reduced eNOS expression was found in quercetin-treated animals which is in agreement with in vitro data in human umbilical endothelial cells or in spontaneously hypertensive rats [26], [27]. Notably, quercetin inhibited the decrease in KV currents and the overexpression of 5-HT2A and iNOS induced by monocrotaline. Downregulated 5-HT2A receptors might contribute to reduce arterial remodeling [28] but it was not sufficient to decrease the vascular hyperresponsiveness to 5-HT. This later effect is probably related to changes in reactive oxygen species and cyclooxygenase activity as observed in other models of PAH as it can be normalized by cyclooxygenase inhibitors and antioxidants [29], [30], [31]. The fact that quercetin inhibits the changes in specific genes but does not affect other genes may reflect that changes in gene expression in this model of PAH operate through quercetin-sensitive and -insensitive signaling pathways.
Proliferation of PAMSC leading to increased muscularization of small PA and reduced vascular lumen is considered the hallmark of the most advanced and aggressive forms of PAH. Quercetin reduced the number of muscular arteries, an effect that is likely to contribute to the decrease in PAP. In order to confirm the in vivo findings, we tested the effects of quercetin on PASMC in vitro. We found that quercetin produced a concentration-dependent decrease in cell number of PASMC and fibroblasts, related to the inhibition of proliferation, as measured by the BrdU incorporation. However, quercetin also induces apoptosis in vascular smooth muscle [20] which may also marginally contribute to reduce cell viability in these in vitro experiments.
A large number of cell signaling pathways may be affected by quercetin. In fact, quercetin is a broad-spectrum protein kinase inhibitor being a competitive and reversible ligand for the ATP binding site [32]. However, few studies have confirmed it in its in vivo effects. Therefore, we analyzed in the rat lungs from the in vivo study the signaling pathways reported to be involved in the pathophysiology of PAH which could be potentially targeted by quercetin. These include those activated by platelet derived growth factor (PDGF) and transforming growth factor-β (TGF-β): MAPKs, the PI3K- Akt-mTor pathway, and the antiapoptotic protein survivin.
Quercetin is a canonical pan-inhibitor of MAPKs and the TGF-β pathway [33] and previous studies on the effects of quercetin on vascular smooth muscle cell viability, migration, and proliferation have related their findings to interactions with MAPKs [20], [34], [35]. MAPKs (e.g. ERK1/2, p38MAPK and JNK) play a key role in cell proliferation and this axis has been suggested as a potential therapeutic target in PAH [6]. However, their role in PAH induced by monocrotaline is controversial. In fact, increased [36], unchanged [37] or decreased [38] phosphorylation of p38MAPK and unchanged [36], [37] or increased [6] phosphorylation of ERK1/2 have been reported. We found no significant changes among groups in p38MAPK and ERK1/2 phosphorylation suggesting that MAPKs play little role in the PAH and in the effects of quercetin in this model.
Another potential pathway for PAMSC muscularization in PAH is via overexpression of the antiapoptotic protein survivin. In fact, genetic targeting of survivin has been shown to reduce PAH in the monocrotaline-treated rats [39]. We found that survivin mRNA was significantly overexpressed in the lungs of monocrotaline-treated rats but the protein increase did not achieve statistical significance. This difference might be due to post-transductional changes. Interestingly, quercetin has been shown to induce survivin downregulation in several cancer cells, via decreasing the survivin promoter activity [40]. Herein we show that chronic quercetin had no clear effect on survivin expression, suggesting that in vivo the downregulation of survivin is not a major mechanism for the antiproliferative effect. This is consistent with the high concentrations (50 µM) of quercetin required to downregulate survivin [40].
The intracellular signaling pathway involving the PI3K/Akt/mTOR/S6 plays an important role in cell proliferation in PAH and it is thought to be a promising therapeutic target for this syndrome [41], [42], [43]. Quercetin may interfere with the activity of both PI3K and mTOR [44], [45]. We found that Akt phosphorylation was increased by monocrotaline which was associated by a strong downregulation of Akt expression. As previously reported in other cell types [45], [46], the Akt phosphorylation was diminished by quercetin, suggesting that inhibition of this pathway may be involved in the decreased PA muscularization. This is consistent with the high potency of quercetin to inhibit PI3K (KD at submicromolar concentrations) [32]. Furthermore, we also found that phosphorylation of S6, the downstream target of mTOR, was higher in the group treated with monocrotaline and this increase was inhibited by quercetin.
Quercetin is also a well-known vasodilator [13], [18]. We also found that quercetin was similarly effective in isolated PA both from control and monocrotaline-treated rats stimulated by the thromboxane A2 mimetic U46619 (−logIC50 of 5.3 and 5.1, respectively, not shown). Thus, this vasodilator effect may be an additional contributor to the reduction in PA pressure in this model.
The pharmacokinetics of quercetin are complex. It is not present in the blood in its free form but circulates conjugated with glucuronide which is released in the tissues after deconjugation [47]. Because quercetin was not administered the day of the end-point measurements, it is likely that the effects observed reflect the chronic rather than the acute actions of quercetin and the reduction in PA pressure may be underestimated.
In conclusion, quercetin is partially protective in this rat model of PAH. It delayed mortality by lowering PAP, RVH and vascular remodeling. Quercetin exerted effective vasodilator effects in isolated PA, inhibited cell proliferation and induced apoptosis in PASMCs. These effects were associated with decreased 5-HT2A receptor expression and Akt and S6 phosphorylation and partially restored Kv currents. Therefore, quercetin could be useful in the treatment of PAH.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by grants and fellowships by the Spanish Ministerio de Economia y Competitividad (SAF2011-28150 to F.P-V, SAF2010-22066-C02-01 to JD, and −02 to AC); Instituto de Salud Carlos III Red HERACLES RD06/0009 to JD; Miguel Servet Program CP12/03304 to LM; predoctoral grants BES-2012-051904 to DMS, CM, JMS, and PG; and Junta de Andalucia (Proyecto de excelencia, P12-CTS-2722). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, et al. (2009) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 54:S43–54. [DOI] [PubMed] [Google Scholar]
- 2. Chan SY, Loscalzo J (2008) Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol 44:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rabinovitch M (2008) Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest 118:2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA 3rd, et al. (2000) Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet 26:81–84. [DOI] [PubMed] [Google Scholar]
- 5. Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, et al. (2002) Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 105:1672–1678. [DOI] [PubMed] [Google Scholar]
- 6. Nasim MT, Ogo T, Chowdhury HM, Zhao L, Chen CN, et al. (2012) BMPR-II deficiency elicits pro-proliferative and anti-apoptotic responses through the activation of TGFbeta-TAK1-MAPK pathways in PAH. Hum Mol Genet 21:2548–2558. [DOI] [PubMed] [Google Scholar]
- 7. Yuan JX, Aldinger AM, Juhaszova M, Wang J, Conte JV Jr, et al. (1998) Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation 98:1400–1406. [DOI] [PubMed] [Google Scholar]
- 8. Morecroft I, Loughlin L, Nilsen M, Colston J, Dempsie Y, et al. (2005) Functional interactions between 5-hydroxytryptamine receptors and the serotonin transporter in pulmonary arteries. J Pharmacol Exp Ther 313:539–548. [DOI] [PubMed] [Google Scholar]
- 9. Coggins MP, Bloch KD (2007) Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol 27:1877–1885. [DOI] [PubMed] [Google Scholar]
- 10. O’Callaghan DS, Savale L, Montani D, Jais X, Sitbon O, et al. (2011) Treatment of pulmonary arterial hypertension with targeted therapies. Nat Rev Cardiol 8:526–538. [DOI] [PubMed] [Google Scholar]
- 11. Huxley RR, Neil HA (2003) The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. Eur J Clin Nutr 57:904–908. [DOI] [PubMed] [Google Scholar]
- 12. Perez-Vizcaino F, Duarte J (2010) Flavonols and cardiovascular disease. Mol Aspects Med 31:478–494. [DOI] [PubMed] [Google Scholar]
- 13. Duarte J, Perez-Vizcaino F, Zarzuelo A, Jimenez J, Tamargo J (1993) Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur J Pharmacol 239:1–7. [DOI] [PubMed] [Google Scholar]
- 14. Gryglewski RJ, Korbut R, Robak J, Swies J (1987) On the Mechanism of Antithrombotic Action of Flavonoids. Biochemical Pharmacology 36:317–322. [DOI] [PubMed] [Google Scholar]
- 15. Menendez C, Jimenez R, Moreno L, Galindo P, Cogolludo A, et al. (2010) Lack of synergistic interaction between quercetin and catechin in systemic and pulmonary vascular smooth muscle. Br J Nutr 105:1287–1293. [DOI] [PubMed] [Google Scholar]
- 16. Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A (2009) Antihypertensive effects of the flavonoid quercetin. Pharmacological Reports 61:67–75. [DOI] [PubMed] [Google Scholar]
- 17. Moral-Sanz J, Lopez-Lopez JG, Menendez C, Moreno E, Barreira B, et al. (2012) Different patterns of pulmonary vascular disease induced by type 1 diabetes and moderate hypoxia in rats. Exp Physiol 97:676–686. [DOI] [PubMed] [Google Scholar]
- 18. Cogolludo A, Frazziano G, Briones AM, Cobeno L, Moreno L, et al. (2007) The dietary flavonoid quercetin activates BKCa currents in coronary arteries via production of H2O2. Role in vasodilatation. Cardiovascular Research 73:424–431. [DOI] [PubMed] [Google Scholar]
- 19. Cogolludo A, Moreno L, Lodi F, Frazziano G, Cobeno L, et al. (2006) Serotonin inhibits voltage-gated K+ currents in pulmonary artery smooth muscle cells: role of 5-HT2A receptors, caveolin-1, and KV1.5 channel internalization. Circ Res 98:931–938. [DOI] [PubMed] [Google Scholar]
- 20. Perez-Vizcaino F, Bishop-Bailley D, Lodi F, Duarte J, Cogolludo A, et al. (2006) The flavonoid quercetin induces apoptosis and inhibits JNK activation in intimal vascular smooth muscle cells. Biochem Biophys Res Commun 346:919–925. [DOI] [PubMed] [Google Scholar]
- 21. Gao H, Chen C, Huang S, Li B (2012) Quercetin attenuates the progression of monocrotaline-induced pulmonary hypertension in rats. J Biomed Res 26:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stenmark KR, Meyrick B, Galie N, Mooi WJ, McMurtry IF (2009) Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 297:L1013–1032. [DOI] [PubMed] [Google Scholar]
- 23. Mathew R, Huang J, Shah M, Patel K, Gewitz M, et al. (2004) Disruption of endothelial-cell caveolin-1alpha/raft scaffolding during development of monocrotaline-induced pulmonary hypertension. Circulation 110:1499–1506. [DOI] [PubMed] [Google Scholar]
- 24. Sahara M, Sata M, Morita T, Hirata Y, Nagai R (2012) Nicorandil attenuates monocrotaline-induced vascular endothelial damage and pulmonary arterial hypertension. Plos One 7:e33367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perez-Vizcaino F, Duarte J, Andriantsitohaina R (2006) Endothelial function and cardiovascular disease: Effects of quercetin and wine polyphenols. Free Radical Research 40:1054–1065. [DOI] [PubMed] [Google Scholar]
- 26. Tribolo S, Lodi F, Winterbone MS, Saha S, Needs PW, et al. (2013) Human metabolic transformation of quercetin blocks its capacity to decrease endothelial nitric oxide synthase (eNOS) expression and endothelin-1 secretion by human endothelial cells. J Agric Food Chem 61:8589–8596. [DOI] [PubMed] [Google Scholar]
- 27. Sanchez M, Galisteo M, Vera R, Villar IC, Zarzuelo A, et al. (2006) Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens 24:75–84. [DOI] [PubMed] [Google Scholar]
- 28. Liu Y, Tian H, Yan X, Fan F, Wang W, et al. (2013) Serotonin inhibits apoptosis of pulmonary artery smooth muscle cells through 5-HT2A receptors involved in the pulmonary artery remodeling of pulmonary artery hypertension. Exp Lung Res 39:70–79. [DOI] [PubMed] [Google Scholar]
- 29. Thomas BJ, Wanstall JC (2003) Alterations in pulmonary vascular function in rats exposed to intermittent hypoxia. Eur J Pharmacol 477:153–161. [DOI] [PubMed] [Google Scholar]
- 30. Sato K, Li J, Metais C, Bianchi C, Sellke F (2000) Increased pulmonary vascular contraction to serotonin after cardiopulmonary bypass: role of cyclooxygenase. J Surg Res 90:138–143. [DOI] [PubMed] [Google Scholar]
- 31. Lopez-Lopez JG, Moral-Sanz J, Frazziano G, Gomez-Villalobos MJ, Moreno L, et al. (2011) Type 1 diabetes-induced hyper-responsiveness to 5-hydroxytryptamine in rat pulmonary arteries via oxidative stress and induction of cyclooxygenase-2. J Pharmacol Exp Ther 338:400–407. [DOI] [PubMed] [Google Scholar]
- 32. Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, et al. (2000) Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 6:909–919. [DOI] [PubMed] [Google Scholar]
- 33. Nakamura T, Matsushima M, Hayashi Y, Shibasaki M, Imaizumi K, et al. (2011) Attenuation of transforming growth factor-beta-stimulated collagen production in fibroblasts by quercetin-induced heme oxygenase-1. Am J Respir Cell Mol Biol 44:614–620. [DOI] [PubMed] [Google Scholar]
- 34. Moon SK, Cho GO, Jung SY, Gal SW, Kwon TK, et al. (2003) Quercetin exerts multiple inhibitory effects on vascular smooth muscle cells: role of ERK1/2, cell-cycle regulation, and matrix metalloproteinase-9. Biochem Biophys Res Commun 301:1069–1078. [DOI] [PubMed] [Google Scholar]
- 35. Yoshizumi M, Tsuchiya K, Suzaki Y, Kirima K, Kyaw M, et al. (2002) Quercetin glucuronide prevents VSMC hypertrophy by angiotensin II via the inhibition of JNK and AP-1 signaling pathway. Biochem Biophys Res Commun 293:1458–1465. [DOI] [PubMed] [Google Scholar]
- 36. Henriques-Coelho T, Oliveira SM, Moura RS, Roncon-Albuquerque R Jr, Neves AL, et al. (2008) Thymulin inhibits monocrotaline-induced pulmonary hypertension modulating interleukin-6 expression and suppressing p38 pathway. Endocrinology 149:4367–4373. [DOI] [PubMed] [Google Scholar]
- 37. Morty RE, Nejman B, Kwapiszewska G, Hecker M, Zakrzewicz A, et al. (2007) Dysregulated bone morphogenetic protein signaling in monocrotaline-induced pulmonary arterial hypertension. Arterioscler Thromb Vasc Biol 27:1072–1078. [DOI] [PubMed] [Google Scholar]
- 38. Ramos M, Lame MW, Segall HJ, Wilson DW (2007) Monocrotaline pyrrole induces Smad nuclear accumulation and altered signaling expression in human pulmonary arterial endothelial cells. Vascul Pharmacol 46:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, et al. (2005) Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 115:1479–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim YH, Lee DH, Jeong JH, Guo ZS, Lee YJ (2008) Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem Pharmacol 75:1946–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goncharova EA (2013) mTOR and vascular remodeling in lung diseases: current challenges and therapeutic prospects. FASEB J 27:1796–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Houssaini A, Abid S, Mouraret N, Wan F, Rideau D, et al. (2013) Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am J Respir Cell Mol Biol 48:568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goncharov DA, Kudryashova TV, Ziai H, Ihida-Stansbury K, DeLisser H, et al. (2014) Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation 129:864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bruning A (2012) Inhibition of mTOR Signaling by Quercetin in Cancer Treatment and Prevention. Anticancer Agents Med Chem 13:1025–1031. [DOI] [PubMed] [Google Scholar]
- 45. Pratheeshkumar P, Budhraja A, Son YO, Wang X, Zhang Z, et al. (2012) Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling pathways. Plos One 7:e47516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yuan Z, Long C, Junming T, Qihuan L, Youshun Z, et al. (2012) Quercetin-induced apoptosis of HL-60 cells by reducing PI3K/Akt. Mol Biol Rep 39:7785–7793. [DOI] [PubMed] [Google Scholar]
- 47. Menendez C, Duenas M, Galindo P, Gonzalez-Manzano S, Jimenez R, et al. (2012) Vascular deconjugation of quercetin glucuronide: the flavonoid paradox revealed? Mol Nutr Food Res 55:1780–1790. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.