Abstract
Introduction:
Chronic lower back pain (CLBP) is problematic in older veterans. Spinal manipulative therapy (SMT) is commonly utilized for CLBP in older adults, yet there are few randomized placebo-controlled trials evaluating SMT.
Methods:
The purpose of the study was to compare the effectiveness of SMT to a sham intervention on pain (Visual Analogue Scale, SF-36 pain subscale), disability (Oswestry Disability Index), and physical function (SF-36 subscale, Timed Up and Go) by performing a randomized placebo-controlled trial at 2 Veteran Affairs Clinics.
Results:
Older veterans (≥ 65 years of age) who were naive to chiropractic were recruited. A total of 136 were included in the study with 69 being randomly assigned to SMT and 67 to sham intervention. Patients were treated 2 times per week for 4 weeks assessing outcomes at baseline, 5, and 12 weeks postbaseline. Both groups demonstrated significant decrease in pain and disability at 5 and 12 weeks. At 12 weeks, there was no significant difference in pain and a statistically significant decline in disability scores in the SMT group when compared to the sham intervention group. There were no significant differences in adverse events between the groups.
Conclusions:
The SMT did not result in greater improvement in pain when compared to our sham intervention; however, SMT did demonstrate a slightly greater improvement in disability at 12 weeks. The fact that patients in both groups showed improvements suggests the presence of a nonspecific therapeutic effect.
Keywords: older adults, veterans, spinal manipulative therapy, randomized placebo controlled trial, chronic lower back pain
Introduction
Epidemiological data suggest that between 2010 and 2030, the size of the population of those 65 years of age and older will increase more than 70%.1,2 Approximately 88% of people aged 65 and older have one or more chronic conditions, and more than 75% of all US health expenditures are related to the treatment of chronic conditions.3 Lower back pain (LBP) is a significant health problem in the older adult population,1 especially older veterans served by the Veterans Health Administration (VHA).4 Chronic LBP (CLBP) is one the most common musculoskeletal complaints among veterans with a prevalence in excess of 40%.5 Unmanaged LBP may contribute to depression, functional disability, compromised quality of life, and increased analgesic medication usage.6
The identification of alternative safe and effective interventions for CLBP in the elderly individuals is critical in view of its high prevalence, negative impact on quality of life, and the treatment risks associated with chronic medication use.7 Published guidelines from the American Geriatric Society listed chiropractic management among the nonpharmacologic strategies for treating chronic pain symptoms in older adults.8 Patient’s undergoing chiropractic care also reported greater satisfaction when compared to standard medical care.9–11 Despite the general clinical acceptance of chiropractic care and high levels of satisfaction with chiropractic services, evidence on the potential benefit and safety of chiropractic management of CLBP in older adults is lacking,12 lending justification to further investigation of the role of chiropractic care in older adults.
The most common method of treatment utilized by chiropractors is spinal manipulative therapy (SMT).12 Recent systematic reviews report that SMT has effectiveness similar to several other treatment interventions such as exercise, injections, and nonsteroidal anti-inflammatory drugs.13 Critical in these therapeutic effectiveness studies is disentangling the specific therapeutic effect produced by the treatment from the generic nonspecific therapeutic effect that is associated with the doctor–patient encounter.14 To investigate the efficacy of SMT in older adults with CLBP, there is a need to utilize a sham intervention that not only involves a doctor–patient interaction but also utilizes a treatment that is believable yet does not produce a specific treatment effect. There have been a few sham-controlled trials involving SMT,15,16 but those trials did not focus on older adult patients and had difficulties associated with defining a believable yet inert sham intervention. The role of SMT for CLBP in the older adult has yet to be evaluated in a controlled trial that uses a sham intervention.17
The study purpose was to evaluate the effectiveness of SMT in older veterans having CLBP in a randomized control trial (RCT) using a sham intervention. Our primary hypothesis was that SMT would produce a significant reduction in pain, our primary outcome, from baseline to 5- and 12-week follow-up compared to patients receiving a sham intervention. Our secondary hypothesis was that SMT would produce a significant reduction in disability and improvement in function from baseline to 5- and 12-week follow-up.
Methods
Trial Design
The study was a product of a Department of Veterans Affairs (VA) Clinic Science and Research and Development grant. The principal investigator is a full-time employee of the VA. No conflicts of interest were identified with any of the authors. The study was a prospective RCT utilizing a sham intervention. Older adults, ≥65 years old, having CLBP and naive to chiropractic were recruited at 2 different VA Medical Centers (VAMCs). Qualified patients were then randomized to receive either SMT or sham intervention. Patients were treated 2 times per week for 4 weeks. The protocol received institutional review board approval through the Syracuse/Canandaigua VAMC and through the VA Western New York Healthcare System (VA WNYHS). This trial was registered at Clinicaltrials.gov under the title: “Chiropractic Management of Chronic Lower Back Pain in Older Adults”; registration number: NCT00475787.
Participants
Inclusion criteria were based on previously defined parameters for chronic “mechanical” nonspecific LBP.18 These included LBP pain ≥3 months in duration, localized pain to the lumbosacral and gluteal regions and no focal radicular symptoms, pain elicited upon deep palpation of the lumbar erector spinae musculature, and pain exacerbated or relieved by varying body position. In addition, they could never have undergone chiropractic care and they also agreed to refrain from any additional treatment for CLBP other than that provided in the treatment group for the 12-week duration of the study, unless urgent care was required. Patients were allowed to continue medications that they were taking at the initiation of the study.
Exclusion criteria included any radiographic or examination evidence of cauda equina syndrome, spinal neoplasia or metastatic disease, destructive joint pathology such as rheumatoid arthritis, urinary retention/incontinence associated with cauda equine syndrome, peripheral neuropathy or focal lumbosacral radiculopathy, progressive myelopathy or neurogenic claudication, spinal surgery within the past 6 months, a history of fragility fracture (defined as a fracture that resulted from a fall from a standing height or less) or radiographic evidence of lumbar compression fracture, and any previous chiropractic care. We also excluded moderately cognitively impaired older adults, as indicated by their previous medical history and Mini-Mental Status Examination scores of 22 or less.
Study Settings
This multi-site prospective RCT was conducted at 2 separate VAMCs in Upstate New York. The study interventions were provided to veterans at the VA WNYHS in Buffalo, New York, and the VA Rochester Outpatient Clinic (VA ROPC) in Rochester, New York. The interventions were delivered by the staff chiropractors at each facility; each were experienced clinicians with 7 and 17 years of experience, respectively, at the beginning of the trial.
Interventions
Spinal manipulative therapy was performed as defined previously in the literature.18,19 The SMT included high-velocity, low-amplitude (HVLA) spinal manipulation, and/or flexion distraction therapy and/or mobilization.18–20 The decision to use high-velocity, low-amplitude spinal manipulation, or flexion distraction therapy, alone or in combination, was based on the clinician’s judgment. The decision on management strategy was influenced by clinical examination, risk factors, and patient preference; however, there was not a specific algorithm that determined the specific strategy, improving the generalizability of the study. No other physical treatments were permitted, but the clinicians could make reference to the Arthritis Foundation brochure that contained stretching and strengthening exercises. The brochure was given to both the groups.
The sham intervention consisted of “detuned ultrasound” applied over the lumbar spine for 11 minutes. This type of intervention is chosen to purposefully simulate a treatment that is believable by the patient and one that minimizes patient bias.21,22 The patient lay prone on the treatment table. The clinician applied ultrasound gel over the patient’s lumbosacral region. The clinician then turned on the machine and set the intensity at “0” w/cm2, a timer started, and the fan of the machine was on. The clinician then utilized the ultrasound wand and gently ran the wand over the patient’s back for the 11 minutes of intervention. The clinician asked the patient during this time whether there was any discomfort. At the end of the 11 minutes, the timer went off to let the patient know that the treatment was over and the patient was queried as to how they felt after the treatment.
Patients in both groups of the study received an educational pamphlet from the Arthritis Foundation entitled “Back Pain.” This brochure contained “an explanation of the different types of back pain, causes, and factors that worsen back pain” (http://www.arthritis.org/resources/brochures-and-other-resources). This brochure also contained stretching and strengthening exercises that could be utilized by the patient for the management of their CLBP. At both sites, the treatment environments were standardized to limit variations in the patient experiences. Furthermore, the clinicians were given a standardized script to memorize and follow to define the doctor–patient interaction at the beginning of the visit to assure consistency between the groups. Treatment was performed 2 times per week for 4 weeks. The choice of 2 times per week for 4 weeks was a pragmatic decision. The one study available at the time of initiation of this study that did evaluate the effect of treatment frequency utilized 4 weeks of intervention and did not identify an ideal treatment frequency.23
Outcomes
Our primary outcome was reduction in pain. The pain outcome was operationalized by the scores on the Visual Analog Scale (VAS)24 and the pain subscale of the SF-36.25 The secondary outcomes of improvement in disability and function were operationalized by the scores of the Oswestry Disability Index (ODI),23 the physical function subscale of the SF-36,25 and the Timed Up and Go (TUG)26 test, a performance measure of function. The original TUG was measured using pressure sensors in the chair and at the different data points (at 1.5 m, 3 m, and 4.5 m). We discovered problems with the sensors after about one-third of the patients were tested. We then switched to a method using laser beams at each of the data points to record the time of the test. The patient sat in a chair and a loud “beep” went off and the patient stood and ambulated 3 m out to a cone and then 3 m back; the patient did a “practice run” and then performed the test 3 times. For the TUG analyses, we summed together the times across the 3 trials at baseline, 5-, and 12-week follow-up. Outcome measures were collected at baseline, 5, and 12 weeks postbaseline. Adverse event data were collected at each treatment visit and at the 5- and 12-week follow-up. In addition, we measured patients’ expectations regarding the treatment at the end of the first treatment session in both the SMT and the sham intervention groups. These measures served as “manipulation checks” to determine the credibility of the sham intervention treatment. First, at the end of the first treatment session (after randomization), we asked patients to rate on a 10-point, face valid scale how confident they were that the treatment they will be receiving will be successful in reducing CLBP. Second, we administered the 4-item modified Borkovec and Nau Scale (mBNS)28 at the end of the first treatment. The mBNS asks patients to rate on 5-point Likert-type scale the extent to which they were confident in the treatment, confident in recommending the treatment to a friend, felt the treatment was logical, and felt the treatment would be successful in alleviating other complaints. We calculated a mean treatment expectation score by computing the mean of the responses on the 4 items, with the higher the score, the more favorable the expectation. The scale was reliable (Cronbach α = .85).
Randomization
According to data from recently published study,30 a clinically significant difference in the VAS is 20. Assuming a power (1-β) of .80 and the usual α of P < .05, we would have sufficient power to detect a difference of at least 20 on the VAS in improvement from baseline between the 2 groups with at least 60 patients per group. Randomization was through a random number producing algorithm. Separate but parallel, permuted randomization schemes were used to assign patients to a treatment group at both sites. A permuted block of size N = 4 was used. Prior to the initiation of the study at each site, a random number sequence generated by the Statistical Package for the Social Sciences (SPSS) version 21 created a randomization schedule from a series of permuted blocks. Patients were randomly assigned to the treatment if the permuted number in the block was even and to the sham intervention if the permuted number within the block was odd.
Blinding
Although the treating clinicians could not be blinded to the intervention, the patients, the screening clinicians, and the statistician evaluating the outcome data were all blinded to the treatment allocation.
Results
Recruitment
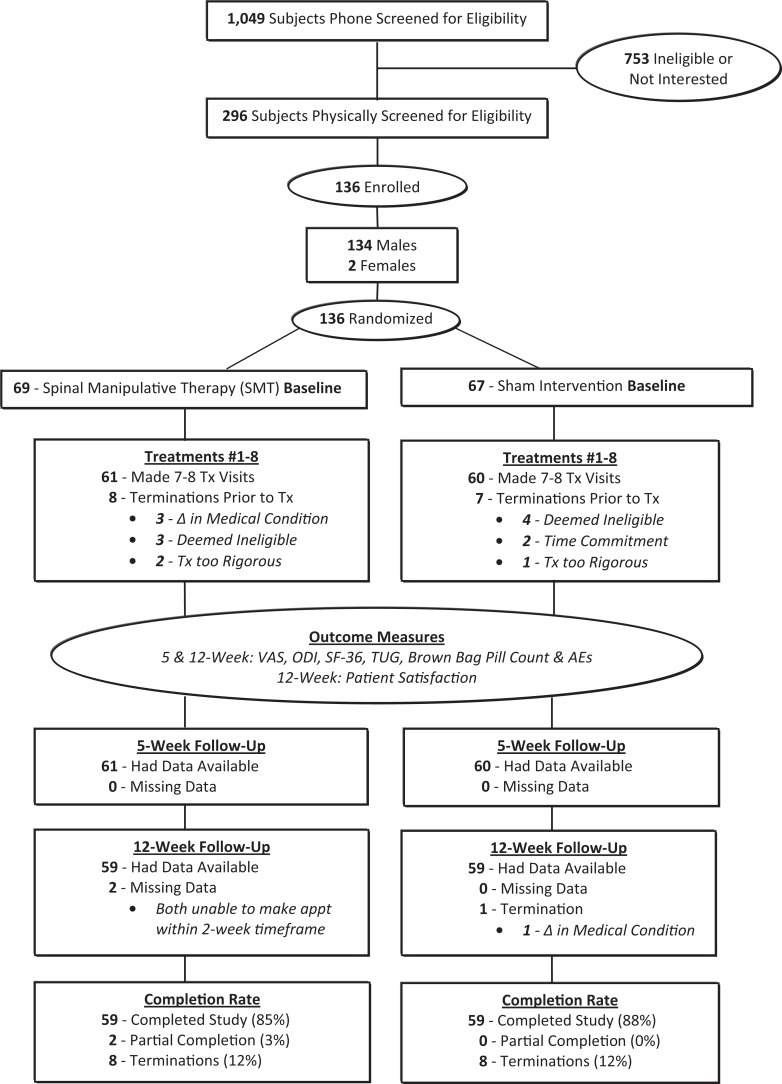
Patients were recruited through direct mailing, posters, and physician recruitment. A total of 1049 patients were phone screened, and of these 296 were physically screened and of those 136 were included in the study with 69 randomized to SMT and 67 randomized to the sham intervention group (see Figure 1). There were 84 (62%) patients enrolled at the VA ROPC site with a 42 (50%) patients in each group, SMT and sham intervention, respectively. There were 52 (38%) patients enrolled at the VA WNYHS site with 27 (52%) patients in the SMT group and 25 (48%) patients in the sham intervention group. Patient recruitment began in 2008, and the last follow-up visit was completed in 2011.
Figure 1.
Study flow sheet.
Baseline Data
We present the demographic and health status characteristics of the included patients in Table 1. As seen in Table 1, the sample was overwhelmingly male, white, and not employed. The mean age of the sample was 77 years, and the mean education level, as defined by the last completed grade, was grade 13. Between one-quarter to one-third of the patients reported that they never exercised, and a majority of the patients reported exercising at least twice a week or more. A majority of the patients reported arthritis on their clinical history and a small number of patients sampled reported a depression diagnosis (12% in the SMT group and 16% of the sham intervention group). In addition, since this was a multi-site study, we compared the patient characteristics between the 2 sites and found no significant differences in any of the patients’ characteristics between the sites (Table 2).
Table 1.
Comparison of Patient Demographics Between Treatment Groups.
| Variable | Treatment group | |
|---|---|---|
| SMT, n = 69 (St dev) | Sham intervention, n = 67 (St dev) | |
| Mean age | 76.99 (6.77) | 77.04 (6.81) |
| Mean height, inches | 67.91 (2.07) | 67.66 (3.27) |
| Mean weight, pounds | 202.93 (36.14) | 196.26 (38.90) |
| Mean BMI | 30.89 (5.06) | 30.08 (5.18) |
| Mean last grade completed | 13.08 (2.61) | 13.64 (2.49) |
| Arthritis | 68.10% | 67.20% |
| Osteoporosis | 7.20% | 3.00% |
| Depression | 11.60% | 16.40% |
| Male | 98.60% | 98.50% |
| White | 85.50% | 94.00% |
| African American | 13.00% | 4.50% |
| Hispanic | 1.50% | 0% |
| Currently employed | 10.10% | 16.40% |
| Drink alcohol | 39.10% | 46.30% |
| Current smoker | 2.90% | 10.40% |
| Exercise | ||
| Never | 23.50% | 32.80% |
| Once a week | 13.20% | 11.90% |
| 2-3 times/week | 33.80% | 20.90% |
| 4-5 times/week | 11.80% | 9.00% |
| More than 5 times/week | 17.60% | 25.40% |
Abbreviations: BMI, body mass index; SMT, spinal manipulative therapy; St dev, standard deviation.
Table 2.
Mean Intention-to-Treat Outcome Scores as a Function of Treatment Group (SMT, Sham Intervention) and Time of Measurement (Baseline, 5-Week, and 12-Week Follow-up).a
| Outcome | Treatment group | Time of measurement | ||
|---|---|---|---|---|
| Baseline, mean (± 95% CI) | 5-week follow-up, mean (± 95% CI) | 12-week follow-up, mean (± 95% CI) | ||
| VASb | SMT | 61.45 (57.56-65.33) | 35.13 (29.85-40.41) | 39.27 (33.74-44.81) |
| Sham Intervention | 58.06 (54.12-62.00) | 37.61 (32.25-42.97) | 41.49 (35.87-47.11) | |
| SF-36 pain subscalec | SMT | 5.75 (5.42-6.13) | 6.78 (6.42-7.16) | 6.73 (6.31-7.16) |
| Sham Intervention | 5.97 (5.61-6.33) | 6.50 (6.13-6.88) | 6.62 (6.19-7.06) | |
| ODId | SMT | 36.70e (3.80-39.70) | 31.30 (28.10-34.50) | 27.90 (24.50-31.30) |
| Sham Intervention | 35.60 (32.60-38.60) | 32.20 (28.90-35.40) | 32.00 (28.60-35.40) | |
| SF-36 physical function subscalef | SMT | 1.87 (1.76-1.98) | 1.92 (1.81-2.04) | 1.92 (1.80-2.04) |
| Sham Intervention | 1.81 (1.70-1.92) | 1.91 (1.79-2.02) | 1.93 (1.80-2.05) | |
| TUGe | SMT | 15.44 (14.13-16.76) | 14.28 (13.20-15.35) | 13.91 (12.76-15.06) |
| Sham Intervention | 14.53 (13.23-15.83) | 13.89 (12.82-14.95) | 13.85 (12.71-14.99) | |
Abbreviations: CI, confidence interval; ODI, Oswestry Disability Index; SMT, spinal manipulative therapy; TUG, Timed Up and Go; VAS, Visual Analog Scale.
a n = 69 SMT, n = 67 sham intervention.
b The higher the number the higher the reported pain on a 100-point scale.
c The higher the number the less self-reported pain. The computed SF-36 pain subscale scores range from 2 to 12.
d The higher the number the more disability reported due to pain on a 100-point scale.
e In seconds.
f The higher the number the less self-reported limitations in physical function. The computed SF-36 physical function subscale scores range from 2 to 12.
Of the 69 patients randomized to the SMT group, 19 (28%) underwent HVLA spinal manipulation, 57 (83%) underwent flexion distraction, and 29 (42%) underwent mobilization. These numbers reflect the clinician’s ability to perform more than 1 type of SMT throughout the course of treatment including performing more than 1 during a treatment session. Although the authors recognize that this limits the ability to identify which specific component or type of SMT may have been clinically effective, we feel that this increases the generalizability of the study, as this is typical in a normal chiropractic treatment encounter. There were no significant differences in the number of patients undergoing any of these therapies between sites.
Effectiveness of the sham intervention
Since we designed the study as a randomized placebo trial utilizing a sham intervention, the logic of our design depended on the patients accepting the sham intervention as a realistic treatment. We tested this by examining the patients’ initial expectations regarding their treatment in both groups. If we realistically designed the sham intervention as a placebo, we would expect no significant differences in patients’ expectations at the start of the study.
We compared the patient’s treatment confidence self-report at the end of the first treatment session (after randomization) using a 1-way analysis of variance with treatment group as the between-patient factor. We found no significant difference (F 1,124 = 1.23, P = .27) between patients in the SMT group (mean = 7.89, standard error [SE] = .24) and the sham intervention group (mean = 7.51, SE = .25). The patients in the sham intervention group were as confident in their treatment as patients in the SMT group.
Next, we compared the patients’ mBNS treatment expectation scores at the end of the first treatment session using a 1-way analysis of variance with the treatment group as the between-patient factor. We found no significant difference (F 1,124 = 0.42, P = .51) in the treatment expectation score between patients who underwent the first sham intervention (mean = 3.88, SE = .08) and patients who underwent the first chiropractic treatment (mean = 3.08, SE = .08). The sham intervention patients’ initial expectations about the treatment were as positive as the expectations of the patients in the SMT group. This pattern of results suggests that we effectively designed our sham intervention as a realistic placebo.
Statistical Analysis
To test our hypotheses, we chose a difference score strategy. The advantage is that difference scores adjust for differences in patients’ baseline scores by subtracting the baseline score from the follow-up measures. We are aware of the controversies surrounding different approaches (eg, analyses of covariance and difference scores) in analyzing treatment effects.31–33 We feel that the use of difference scores is in keeping with the nature of the study hypotheses and the proven reliability of the measures. As a double check, we reran the analyses following an analysis of covariance approach and obtained the same pattern of significance.
A difference score (baseline − follow-up) was computed for each of the outcome measures at the 5-week follow-up and again at the 12-week follow-up. We then compared the difference scores of the SMT group to the sham intervention group at the 5-week and 12-week follow-up time points, using a general linear model procedure following a univariate analysis of variance model approach with the treatment group as the between-patient factor.
We used an intention-to-treat approach that included all enrolled patients who met inclusion criteria regardless of whether they completed the study or dropped out. Fifteen patients dropped out. There was no significant difference in the dropout rate across the 2 groups. For missing data, we substituted the mean score of the patient’s treatment group at that point in time. With treatment effects expected to dissipate over time,33 we felt a mean substitution would be a more conservative strategy than to populate missing values by the last previous valid data point for the patient, as often is done in clinical trials. We also reran the analyses where we deleted list-wise patients with missing data. The pattern of significance in these analyses was parallel to the intention-to-treat analyses.
Given that this was a multi-site study, we tested for the presence of site differences before combining the data. We ran preliminary analyses that included study location as a between-patient factor. We found no significant site of treatment main effects or site of treatment × treatment group interaction effects on the outcome measures. With the absence of site of treatment effects, we pooled data from the 2 treatment sites for our analyses.
We tested our primary hypothesis, which predicted a significant reduction in pain in the SMT group compared to the sham intervention, by comparing the pain difference scores (ie, VAS, SF-36 Pain Subscale) in the SMT treatment group and the sham intervention group at the 5- and 12-week follow-up. We would expect a significantly larger difference score in the SMT group compared to the sham intervention group, which would reflect a greater improvement in the SMT group from baseline compared to the sham intervention group. We found no statistically significant differences in the pain difference scores in the SMT group compared to the sham intervention group at the 5- or the 12-week follow-up as seen in Table 3. Our primary hypothesis was not supported.
Table 3.
Mean Intention-to-Treat Outcome Measures Difference Scores as a Function of Treatment Group (SMT, Sham Intervention) at 5-Week and 12-Week Follow-Ups.a
| Outcome | Treatment group SMT | Difference score | |
|---|---|---|---|
| Baseline to 5-week follow-up, mean ( ± 95% CI) | Baseline to 12-week follow-up, mean ( ± 95% CI) | ||
| VASb | SMT | 26.32 (20.48-32.16)c | 22.18 (16.43-27.93)d |
| Sham Intervention | 20.45 (14.18-26.71) | 16.57 (10.10-23.03) | |
| SF-36 pain subscalee | SMT | −1.01 (−1.42 to −.60)f | −0.96 (−1.40 to −.51)g |
| Sham Intervention | −0.53 (−.95 to −.11) | −0.65 (−1.11 to −.19) | |
| ODIb | SMT | 5.45 (2.82-8.07)h | 8.80 (5.93-11.75)i |
| Sham Intervention | 3.42 (.08-6.04) | 3.55 (.81-6.29) | |
| SF-36 physical function subscalee | SMT | −0.06 (−.14 to .03)j | −0.05 (−.15 to .04)k |
| Sham Intervention | −0.10 (−.19 to −.01) | −0.12 (−.21 to −.02) | |
| TUGe | SMT | −1.17 (−2.11 to −.23)l | −1.53 (−2.50 to −.57)m |
| Sham Intervention | −0.65 (−1.39 to .09) | −0.68 (−1.41 to .05) | |
Abbreviations: CI, confidence interval; ODI, Oswestry Disability Index; SMT, spinal manipulative therapy; TUG, Timed Up and Go; VAS, Visual Analog Scale.
a n = 69 SMT, n = 67 sham intervention.
b The larger the difference score, the greater improvement from baseline.
c VAS difference between groups F 1,134 = 1.87, P = .17.
d VAS difference between groups F 1,134 = 1.68, P = .19.
e The greater the negative difference score, the greater improvement from baseline.
f SF36 Pain Scale difference between groups F 1,134 = 2.67, P = .10.
g SF36 Pain Scale difference between groups F 1,134 = .91, P = .34.
h ODI difference between groups F 1,134 = 1.19, P = .27.
i ODI difference between groups F 1,134 = 6.95, P < .001.
j SF-36 Physical Function Scale difference between groups F 1,134 = .42, P = .52.
k SF-36 Physical Function Scale difference between groups F 1,134 = 1.01, P = .31.
l TUG difference between groups F 1,134 = .75, P = .38.
m TUG difference between groups F 1,134 = 2.02, P = .16.
We tested our secondary hypotheses of significant improvement in disability and function in the SMT group compared to the sham intervention group, by comparing the function difference scores (ie, ODI, SF-36 physical function subscale, TUG) in the SMT treatment group and the sham intervention group at the 5- and 12-week follow-up. Again, we would expect a significantly larger difference score in the SMT group compared to the sham intervention group. As seen in Table 3, contrary to our hypothesis, we did not find significant differences in the changes in SMT group’s functional outcomes compared to the sham intervention group’s outcomes, as measured by difference scores, at the 5-week follow-up. But, at the 12-week follow-up. we found a statistically significantly improvement in the ODI outcome in the SMT group compared to the sham intervention group, thus partially supporting our secondary hypothesis. There were no other significant differences between the SMT group and the sham intervention group’s difference scores on the other functional outcomes.
Secondary Analyses
When we inspected Table 2, we noticed a potentially interesting pattern. As described earlier, there were no differences between the outcome difference scores of the 2 groups at the 5-week follow-up, but the outcome scores appeared to improve from baseline to the 12-week follow-up in both the groups. We explored this by performing a 2 × 2 repeated-measures analysis of variance with time as the repeated measure and treatment group as the between-patient factor on the study outcomes. We found a significant time of measurement main effect on all the outcomes (VAS F 1,134 = 118.97, P < .001; SF-36 Pain Scale F 1,119 = 27.23, P < .001; ODI F 1,134 = 22.76, P < .001; SF-36 physical function subscale F 1,134 = 6.38, P < .01; TUG F 1,134 = 9.27, P < .01), which indicated that there was an overall improvement in patient outcome from baseline in both the groups. We did not find a significant treatment group by time interaction on any of the outcome measures, which indicated that there was no difference in the rate of improvement between the treatment and the sham control groups. One group did not improve more than the other.
Harms
Adverse event (AE) and serious AE (SAE) data were tracked for each group (SMT and sham intervention). For purpose of this protocol, an AE was defined as any undesirable medical event with new onset or significant exacerbation during the course of the study, regardless of whether or not it was considered to be related to study treatment. Each clinician rated each AE as to severity (a clinical judgment): mild, moderate, or severe. An SAE was defined as any AE occurring during the study or within 30 days of conclusion of study participation, resulting in any one of the following outcomes: death, life-threatening persistent or significant disability/incapacity, hospitalization (when the result of an AE occurring during the study; hospitalization for an elective procedure or for treatment of a preexisting condition not worsened during the study was not considered an SAE; admission to the emergency department for 23 hours or less was not considered a hospitalization), congenital anomaly, important medical event (ie, an event that in the opinion of the investigator may jeopardize the participant and may require medical or surgical intervention to prevent one of the outcomes listed earlier). The Data Safety and Management Board (DSMB) met 4 times during the study (at 25%, 50%, 75%, and final enrollment); the DSMB evaluated the reported AEs and SAEs and found no issues with the reporting of these events and no trends that would require alteration in the study methods. A total of 250 AEs were reported over the course of the study with 56% in the SMT group and 44% in the sham intervention group. Of the 141 AEs reported in the SMT group, the DSMB judged 35 as definitely or probably associated with the intervention. Of the 109 AEs reported in the sham intervention group, the DSMB judged 10 as definitely or probably associated with the intervention.
Most AEs were mild to moderate and were related to musculoskeletal soreness. There were no differences in the frequency of the AEs between the SMT and sham intervention groups of the study, F 1,246 = .05, P = not significant. There were no differences in the severity between the SMT and the sham intervention groups of the study, F 1,246 = .02, P = not significant. Only 10% of the AEs were judged to be definitely related to the study. Preexisting conditions accounted for 42% of the AEs, and new events accounted for 58% of the events. The proportion of new events was the same in both groups during the study period; there were 6 SAEs reported after the start of the treatments (5 in the SMT group and 1 in the sham intervention group). Of the SAEs, there were 2 episodes of syncope, 1 chest pain, 1 paresthesia in the group which was thought to be a myocardial infarction, 1 myocardial infarction, and 1 fall and injury to neck. The DSMB judged that none of the SAEs were associated with the study interventions.
Discussion
We describe the first placebo-controlled trial utilizing a sham intervention evaluating the effect of SMT on pain, disability, and physical function in older adult veterans who are naive to chiropractic care. The strength of this study design is that it utilized a sham intervention that was seen as credible, as assessed by the patients’ mBNS treatment confidence self-reports at the start of the treatment.
We found no support for our primary hypothesis. The SMT did not produce a significantly greater reduction in pain compared to the sham intervention group at either the 5-week or 12-week follow-up. We found weak support for our secondary hypothesis. There was a statistically significant but modest improvement in disability, as measured by the ODI, in the SMT group compared to the sham intervention group at the 12-week follow-up.
Although statistically significant, the question still remains whether the change in ODI score is clinically important. In our study, the patients’ ODI scores improved an average of 8.8 points compared to 3.6 points in the sham intervention group. Comparing our results against some recent literature that attempts to define clinically significant improvement in ODI,34 we fall below their benchmark of 10 points of improvement. At the very least, this tempers our conclusions regarding the effectiveness of SMT. It is unclear whether the magnitude of change in ODI represents an upper limit of SMT’s efficacy, reflects attenuation of SMT’s effectiveness resulting from less than perfect treatment fidelity, or is due to important individual differences in treatment responsiveness.
The hypothesized mechanisms by which SMT may affect disabilities are varied.35 Reduced disability associated with SMT is thought to be associated with improvement in neuromuscular function,35 pain,36–38 and range of motion.40 Although throughout the study (baseline, 5-week, and 12-week follow-up) there was a consistent significant correlation between the pain score and the functional score (ie, if there was more pain, there was less function, the change in pain [VAS], SF-36 pain subscale), change function (ODI, SF-36 function subscale) between baseline and 12-week follow-up was not significant (r < .11). These findings suggest that changes in pain may not necessarily lead to improvement in pain-related disability.30 Another possible explanation for the modest improvement in function in the SMT group at the 12-week follow-up may be related to an increase in range of motion that may decrease the fear associated with movement, thus allowing the patient to be more active. Future studies are needed to further quantify the extent of improvement in disability in patients who receive SMT and systematically examine what factors may contribute to the effectiveness of SMT.
We found in our secondary analysis of the results in Table 2 that there was a significant improvement in the pain and function scores from baseline to follow-up in both the SMT and the sham intervention group. This may point to a general “nonspecific therapeutic effect” that was present in both the groups. We found no differences between sites suggesting that these “nonspecific” effects were not unique to one provider. The relationship established between the clinician and the patient may by itself produce therapeutic effects and serve as a critical ingredient in treatment of CLBP. Future studies are needed to better define the specific versus nonspecific effects of management strategies for CLBP. The current study also provides data, which demonstrate that the use of SMT in older adults is safe compared to an inert sham intervention with relatively minor AEs.
Generalizability
This sham intervention controlled trial was performed at 2 different sites utilizing 2 different VA facilities, 1 large VAMC and 1 VA Community Based Outpatient Clinic with almost all male patients. The use of only veterans in studies has been criticized in the past41; however, the data are directly relevant to the treatment of veterans within VA. Future replication studies in non-VA settings and with female patients are called for. The study evaluated a broadly defined treatment method that was designed to be representative of the “normal” chiropractic encounter including the use of both high-velocity, low-amplitude SMT and allowing for more gentle treatment such as flexion distraction.
Limitations
One issue that has plagued previous spinal manipulation research is the lack of high-quality, placebo-controlled trials. A recent systematic review reported on only 8 placebo-controlled clinical trials assessing spinal manipulation.15 The current study demonstrates that a sham intervention can be a “believable” intervention to the patients as reflected in their confidence and mBNS ratings. However, one limitation of the sham intervention was that it did involve some tactile stimulation, albeit minimal, over the area of pain. Although the pressure of the ultrasound probe was kept low, there was a remote possibility that it may have had some therapeutic value.
An additional limitation to this study is that it was only performed in VA facilities, with a predominantly male population. The use of only veterans in studies has been criticized in the past41; however, the data are directly relevant to the treatment of veterans within VA and useful for future replication studies. Although there were no significant differences in baseline comorbidities, the authors recognize that clinical research bears the risk of confounders that are not identified in the study. Additionally, the average age of the patients was 77 and as would be expected in this group, they were not employed. One factor that may have played a role in the nonspecific effects of treatment was that the appointments got them out of their home, therefore increasing activity. Additionally, they interacted with the provider and the staff at the clinic. These “nonspecific” factors may have played a role in the overall improvement in both the groups.
Another limitation is that all patients were given a standardized brochure from the Arthritis Foundation. Educational booklets have demonstrated effectiveness in treating back pain in the past; however, since both groups received the booklet this did not threaten the internal validity of the study. It does, however, raise some questions regarding the extent to which the nonspecific therapeutic effects found in both groups were due in part to the booklet.19
Another limitation in the current study was that the clinicians were not blinded as to which intervention they were delivering. The authors attempted to mitigate study bias by blinding the patients, the screening clinician, and the statistician evaluating the outcome measures to the treatment allocation.
The study design included a validated measure of physical function (TUG).26 The TUG was originally designed to assess risk of falls in older adults.42 We found no support for our hypothesized improvement in the TUG scores in the SMT group compared to sham intervention. Holding aside measurement confounds resulting from the change in measurement procedures half way through the study, as described earlier, one explanation for this finding is that the disability of these older adults with CLBP was not related to the type of physical function measured by TUG.
Interpretation
The data from this trial demonstrate that the use of a broadly defined SMT in older veterans who were naive to chiropractic is safe. We found SMT did not lead to improvement in pain from baseline compared to a sham intervention. The data provide weak evidence, at best, of a specific therapeutic effect of SMT reducing physical disability compared to a sham intervention at the 12-week follow-up. At the same time, our secondary analyses indicate the presence of nonspecific therapeutic effects in the SMT leading to improvement in outcomes in both the SMT and the sham intervention groups from baseline to the 5-week follow-up. The doctor–patient interaction can have significant therapeutic value, and this study demonstrated that the use of even an inert placebo treatment, such as “detuned ultrasound,” may have resulted in clinically significant improvements in CLBP.
Conclusion
Our placebo-controlled trial utilizing a sham intervention did not demonstrate superiority of SMT over sham intervention in older veterans with CLBP in our primary outcome measures of pain. Although the SMT resulted in statistically greater improvement in CLBP-related disability, as measured by the ODI, than a sham intervention group at 12 week follow-up, it failed to reach the threshold of clinically significant improvement. The fact that both the SMT and the sham groups showed improvement in the outcome measure from baseline to the 5- and 12-week follow-up in our secondary analyses demonstrates a nonspecific therapeutic effect of the intervention. Further studies are needed to assess the specific and nonspecific therapeutic effects of SMT in older adults. In addition, given fact that there are approximately 2.2 million younger Operation Enduring Freedom and Operation Iraqi Freedom Veterans and that many have chronic pain, specifically LBP,40 there is a need to evaluate the role of chiropractic care in this population utilizing a placebo-controlled trial. There are some data, which support that chiropractic care may have efficacy41,42; however, there is a need to study this intervention more rigorously. Future trials may want to consider similar placebo-controlled designs to assess the efficacy of this nonpharmacological intervention to address the important problem of pain in younger veterans.
Acknowledgment
1. This work was supported by a Merit Review grant from the United States (U.S.) Department of Veterans Affairs Clinical Sciences Research and Development.
2. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Authors’ Note: The study was a product of a Department of Veterans Affairs (VA) Clinic Science and Research and Development (CSR&D) grant.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The principal investigator is a full-time employee of the VA. ClinicalTrials.gov Identifier: NCT00475787.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article:Drs Dougherty, Dunn and Katz are VA employees and did not receive financial compensation for participation in the study. Dr. Karuza and Ms. Savino received compensation through salary support, but no direct compensation.
References
- 1. Smith M, Davis MA, Stano M, Whedon JM. Aging baby boomers and the rising cost of chronic back pain: secular trend analysis of longitudinal Medical Expenditures Panel Survey data for years 2000 to 2007. J Manipulative Physiol Ther. 2013;36 (1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bressler HB, Keyes WJ, Rochon PA, Badley E. The prevalence of low back pain in the elderly. A systematic review of the literature. Spine (Phila Pa 1976). 1999;24 (17):1813–1819. [DOI] [PubMed] [Google Scholar]
- 3. Rice DP, Fineman N. Economic implications of increased longevity in the United States. Annu Rev Public Health. 2004;25:457–473. [DOI] [PubMed] [Google Scholar]
- 4. Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the veterans health administration. J Am Geriatr Soc. 2004;52 (8):1271–1276. [DOI] [PubMed] [Google Scholar]
- 5. Morasco BJ, Duckart JP, Carr TP, Deyo RA, Dobscha SK. Clinical characteristics of veterans prescribed high doses of opioid medications for chronic non-cancer pain. Pain. 2010;151 (3):625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289 (9):1107–1116. [DOI] [PubMed] [Google Scholar]
- 7. Scudds RJ, McD RJ. Empirical evidence of the association between the presence of musculoskeletal pain and physical disability in community-dwelling senior citizens. Pain. 1998;75 (2-3):229–235. [DOI] [PubMed] [Google Scholar]
- 8. The management of chronic pain in older persons: AGS panel on chronic pain in older persons. American geriatrics society. J Am Geriatr Soc. 1998;46 (5):635–651. [DOI] [PubMed] [Google Scholar]
- 9. Hertzman-Miller RP, Morgenstern H, Hurwitz EL, et al. Comparing the satisfaction of low back pain patients randomized to receive medical or chiropractic care: results from the UCLA low-back pain study. Am J Public Health. 2002;92 (10):1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nyiendo J, Haas M, Goldberg B, Sexton G. Pain, disability, and satisfaction outcomes and predictors of outcomes: a practice-based study of chronic low back pain patients attending primary care and chiropractic physicians. J Manipulative Physiol Ther. 2001;24 (7):433–439. [PubMed] [Google Scholar]
- 11. Gemmell HA, Hayes BM. Patient satisfaction with chiropractic physicians in an independent physicians' association. J Manipulative Physiol Ther. 2001;24 (9):556–559. [DOI] [PubMed] [Google Scholar]
- 12. Dougherty PE, Hawk C, Weiner DK, Gleberzon B, Andrew K, Killinger L. The role of chiropractic care in older adults. Chiropr Man Therap. 2012;20 (1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rubinstein SM, van MM, Assendelft WJ, de Boer MR, van Tulder MW. Spinal manipulative therapy for chronic low-back pain: an update of a Cochrane review. Spine (Phila Pa 1976). 2011;36 (13):E825–E846. [DOI] [PubMed] [Google Scholar]
- 14. Moore RA, Derry S, McQuay HJ, et al. Clinical effectiveness: an approach to clinical trial design more relevant to clinical practice, acknowledging the importance of individual differences. Pain. 2010;149 (2):173–176. [DOI] [PubMed] [Google Scholar]
- 15. Ernst E, Harkness E. Spinal manipulation: a systematic review of sham-controlled, double-blind, randomized clinical trials. J Pain Symptom Manage. 2001;22 (4):879–889. [DOI] [PubMed] [Google Scholar]
- 16. Hawk C, Long CR, Rowell RM, Gudavalli MR, Jedlicka J. A randomized trial investigating a chiropractic manual placebo: a novel design using standardized forces in the delivery of active and control treatments. J Altern Complement Med. 2005;11 (1):109–117. [DOI] [PubMed] [Google Scholar]
- 17. Gleberzon BJ. A narrative review of the published chiropractic literature regarding older patients from 2001-2010. J Can Chiropr Assoc. 2011;55 (2):76–95. [PMC free article] [PubMed] [Google Scholar]
- 18. Bronfort G, Goldsmith CH, Nelson CF, Boline PD, Anderson AV. Trunk exercise combined with spinal manipulative or NSAID therapy for chronic low back pain: a randomized, observer-blinded clinical trial. J Manipulative Physiol Ther. 1996;19 (9):570–582. [PubMed] [Google Scholar]
- 19. Cherkin DC, Deyo RA, Battie M, Street J, Barlow W. A comparison of physical therapy, chiropractic manipulation, and provision of an educational booklet for the treatment of patients with low back pain. N Engl J Med. 1998;339 (15):1021–1029. [DOI] [PubMed] [Google Scholar]
- 20. Evans DW, Lucas N. What is ‘manipulation’? A reappraisal. Man Ther. 2010;15 (3):286–291. [DOI] [PubMed] [Google Scholar]
- 21. Maher CG, Latimer J, Hodges PW, et al. The effect of motor control exercise versus placebo in patients with chronic low back pain [ACTRN012605000262606]. BMC Musculoskelet Disord. 2005;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Balthazard P, de GP, Rivier G, Demeulenaere P, Ballabeni P, Deriaz O. Manual therapy followed by specific active exercises versus a placebo followed by specific active exercises on the improvement of functional disability in patients with chronic non specific low back pain: a randomized controlled trial. BMC Musculoskelet Disord. 2012;13:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haas M, Groupp E, Kraemer DF. Dose-response for chiropractic care of chronic low back pain. Spine J. 2004;4 (5):574–583. [DOI] [PubMed] [Google Scholar]
- 24. Soer R, Koke AJ, Vroomen PC, et al. Extensive validation of the pain disability index in three groups of patients with musculoskeletal pain. Spine (Phila Pa 1976). 2013;38 (9):E562–E568. [DOI] [PubMed] [Google Scholar]
- 25. Chapman JR, Norvell DC, Hermsmeyer JT, et al. Evaluating common outcomes for measuring treatment success for chronic low back pain. Spine (Phila Pa 1976). 2011;36 (21 suppl):S54–S68. [DOI] [PubMed] [Google Scholar]
- 26. Zhu K, Devine A, Dick IM, Prince RL. Association of back pain frequency with mortality, coronary heart events, mobility, and quality of life in elderly women. Spine (Phila Pa 1976). 2007;32:2012–2018. [DOI] [PubMed] [Google Scholar]
- 27. Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry. 1972;3 (4):257–260. [Google Scholar]
- 28. Boonstra AM, Schiphorst Preuper HR, Reneman MF, Posthumus JB, Stewart RE. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int J Rehabil Res. 2008;31 (2):165–169. [DOI] [PubMed] [Google Scholar]
- 29. Myers JL, Well AD. Research Design and Statistical Analysis. New York, NY: Harper Collins; 1991. [Google Scholar]
- 30. Norman GR. Issues in the use of change scores in randomized trials. J Clin Epidemiol. 1989;42 (11):1097–1105. [DOI] [PubMed] [Google Scholar]
- 31. Miller GA, Chapman JP. Misunderstanding analysis of covariance. J Abnorm Psychol. 2001;110 (1):40–48. [DOI] [PubMed] [Google Scholar]
- 32. Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine. 2008;33 (1):90–94. [DOI] [PubMed] [Google Scholar]
- 33. Maigne JY, Vautravers P. Mechanism of action of spinal manipulative therapy. Joint Bone Spine. 2003;70 (5):336–341. [DOI] [PubMed] [Google Scholar]
- 34. Millan M, Leboeuf-Yde C, Budgell B, Amorim MA. The effect of spinal manipulative therapy on experimentally induced pain: a systematic literature review. Chiropr Man Therap. 2012;20 (1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Coronado RA, Gay CW, Bialosky JE, Carnaby GD, Bishop MD, George SZ. Changes in pain sensitivity following spinal manipulation: a systematic review and meta-analysis. J Electromyogr Kinesiol. 2012;22 (5):752–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clark BC, Goss DA, Jr, Walkowski S, Hoffman RL, Ross A, Thomas JS. Neurophysiologic effects of spinal manipulation in patients with chronic low back pain. BMC Musculoskelet Disord. 2011;12:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Millan M, Leboeuf-Yde C, Budgell B, Descarreaux M, Amorim MA. The effect of spinal manipulative therapy on spinal range of motion: a systematic literature review. Chiropr Man Therap. 2012;20 (1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grover FL, Shroyer AL. Clinical science research. J Thorac Cardiovasc Surg. 2000;119 (4 pt 2):S11–S21. [DOI] [PubMed] [Google Scholar]
- 39. Dobson F, Hinman RS, Hall M, Terwee CB, Roos EM, Bennell KL. Measurement properties of performance-based measures to assess physical function in hip and knee osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2012;20 (12):1548–1562. [DOI] [PubMed] [Google Scholar]
- 40. Haskell SG, Ning Y, Krebs E, et al. Prevalence of painful musculoskeletal conditions in female and male veterans in 7 years after return from deployment in operation enduring freedom/operation Iraqi freedom. Clin J Pain. 2012;28 (2):163–167. [DOI] [PubMed] [Google Scholar]
- 41. Dunn AS, Green BN, Formolo LR, Chicoine D. Retrospective case series of clinical outcomes associated with chiropractic management for veterans with low back pain. J Rehabil Res Dev. 2011;48 (8):927–934. [DOI] [PubMed] [Google Scholar]
- 42. Dunn AS, Green BN, Formolo LR, Chicoine DR. Chiropractic management for veterans with neck pain: a retrospective study of clinical outcomes. J Manipulative Physiol Ther. 2011;34 (8):533–538. [DOI] [PubMed] [Google Scholar]