Abstract
Pelvic insufficiency fractures may occur in the absence of trauma or as a result of low-energy trauma in osteoporotic bone. With a growing geriatric population, the incidence of pelvic insufficiency fracture has increased over the last 3 decades and will continue to do so. These fractures can cause considerable pain, loss of independence, and economic burden to both the patient and the health care system. While many of these injuries are identified and treated based on plain radiographs, some remain difficult to diagnose. The role of advanced imaging in these cases is discussed. In addition to treating the fracture, medical comorbidities contributing to osteoporosis should be identified and corrected. Specific attention has been given to 25-OH serum vitamin D screening and repletion. Treatment generally consists of providing pain control and assisting patients with mobilization while allowing weight bearing as tolerated. In those unable to do so, invasive techniques such as sacroplasty as well as internal fixation may be beneficial. The role of operative fixation in insufficiency fractures is also discussed.
Keywords: pelvic insufficiency fractures, geriatric trauma, osteoporosis, fragility fracture
Introduction
Pelvic insufficiency fractures were first described by Lourie in 1982, when he reported 3 cases of spontaneous sacral fractures in patients with severe osteoporosis.1 These fractures may occur in osteoporotic bone in the absence of trauma or due to low-energy mechanisms, such as a fall, that would not typically be expected to cause a fracture of the pelvic ring.2 As many as two-thirds have been reported in the absence of trauma,3 although more frequently they occur in the setting of a ground level fall.4,5 Since the original description, recognition of this injury has improved and we now recognize that these injuries can cause significant pain and disability despite often having unimpressive if not minimal radiographic findings. While nonoperative management remains the mainstay of treatment inclusive of pharmacologic intervention, pelvic internal fixation and sacroplasty have been proposed for refractory cases.
Epidemiology
As would be expected, the majority of pelvic insufficiency fractures occur in elderly patients. In a retrospective review, the average age of patients sustaining low-energy pelvic fractures was 69 years.6 The burden of insufficiency fractures is expected to grow as the population ages. It is projected that the population older than 65 years will nearly double to over 80 million by 2050 to make up 21% of the population.7 Melton et al described the contribution of age and gender on the incidence of pelvic insufficiency fractures in a retrospective review of a Mayo Medical database. A total of 198 patients with 204 fractures were identified between 1968 and 1977 with a total incidence of 37/100 000. The incidence in women (47.5/100 000) was nearly twice that in men (24.4/100 000) and increased with age. The incidence rose from 7.6 and 56.9/100 000 to 220.3 and 446.3/100 000 in men and women from age 55 and 74 years to over 85 years, respectively.6 Figure 1 demonstrates that the sharp increase in incidence in women occurs around the age of 60, whereas the incidence in men does not increase rapidly until after the age of 75.6
Figure 1.
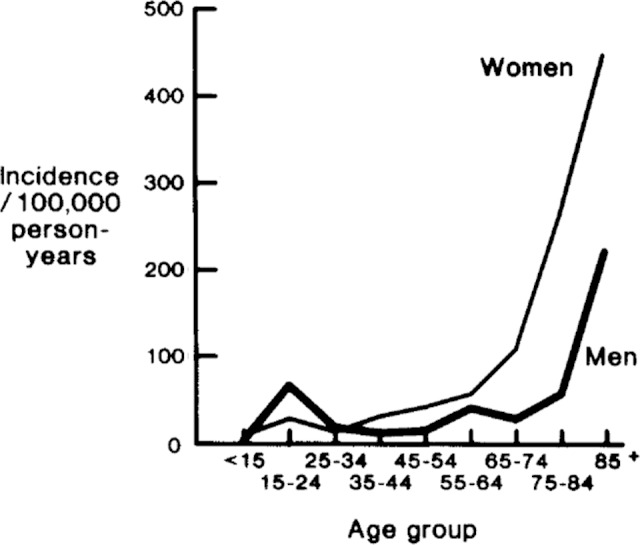
Sex- and age-specific incidence of all pelvic fractures among residents of Rochester, Minnesota (1968-1977). Melton et al with permission.6
Aside from population aging, Kannus et al demonstrated an age-independent increase of 23% per year in incidence of osteoporotic pelvic fractures over a 27-year period from 1970 to 1997.8 It is predicted that the incidence of osteoporotic pelvic fractures will continue to increase by 60% to 100% by 2030.9,10 These fractures occur at a high economic cost due to delays in diagnosis, need for advanced imaging, prolonged inpatient stays, and ongoing assistance requirements of these patients. Over 2 million osteoporosis-related fractures occurred in 2005 at a cost of US$17 billion.10 Pelvic fractures accounted for 7% of the incidence and 5% of the cost of these fractures.10
Risk Factors
The definition of pelvic insufficiency fractures implies that they occur when bone fails under normal physiologic loads. Therefore, any condition that lowers bone density may be a risk factor. Osteoporosis is certainly the most prevalent underlying condition. In one study, 93% (107 of 115) of patients with low-energy pelvic fractures had a Singh index of 4 or less indicating osteoporosis.4 Breuil et al found that 53.8% of patients had a prior diagnosis of osteoporosis and 57.6% had sustained a prior fracture; however, only 30.9% had received treatment.11 In addition, they found a high rate of vitamin D deficiency (25 of 31), secondary hyperparathyroidism (16 of 31), and hypocalcemia (14 of 49). The DEXA scans showed osteoporosis (T score <-2.5) in 12 of the 19 patients who were able to tolerate Dual Energy X-ray Absorptiometry (DEXA) scan while in the hospital.11
Pelvic radiation in the treatment of malignancy is also a risk factor for the later development of insufficiency fractures. The proposed mechanism is that irradiation damages local circulation impeding bone turnover and remodeling.12 Data from the Surveillance, Epidemiology, and End Results (SEER) cancer registry have demonstrated this increased risk. Baxter et al conducted a retrospective cohort study of SEER data in 2855 women who underwent pelvic irradiation versus 3573 women who did not undergo pelvic irradiation for their malignancies. There was a significantly higher 5-year rate of fracture in the radiation group versus the nonradiation group.13 Housman et al conducted a similar review of SEER data in men with prostate cancer and found a 30% lower incidence of pelvic fracture in men treated with brachytherapy versus external beam 3-dimensional conformal radiation therapy.14
Total hip arthroplasty has also been cited as a risk factor for the development of insufficiency fractures of the pubic rami and acetabulum.15–19 One possible etiology is that patients are relatively immobile in the period leading up to their arthroplasty causing disuse osteopenia.18 As pain relief is achieved and mobility improved following arthroplasty, the increased activity may lead to stress fractures postoperatively. Additionally, stress risers are created in the adjacent pelvis due to the mismatch of the relatively stiff implants and osteoporotic bone making the pelvis vulnerable to this condition.
Although there are case reports of pelvic insufficiency fractures among patients with rheumatoid arthritis (RA),20–22 no good series exist characterizing the true incidence against a control group. It stands to reason, however, that patients with RA would be more likely to sustain insufficiency fractures due to their osteopenia and higher rates of corticosteroid use as would other patients on chronic steroid use.
Diagnosis
Diagnosing pelvic insufficiency fractures requires suspicion of the injury and a knowledge of clinical context due to a varied and often insidious onset in presentation. Often, patients present with intractable low back or pelvic pain and the loss of mobility and independence. Symptoms are exacerbated by weight bearing and relieved by rest.23 The diagnosis is often delayed, sometimes by as much as up to 2 months,3 due to the presentation in the absence of trauma or following minor trauma in which pelvic ring injury would not be expected. Complaints of low back pain or pelvic pain in this setting may be misinterpreted as exacerbations secondary to osteoarthritis, RA, trochanteric bursitis, spinal stenosis, or lumbar disc herniation.24
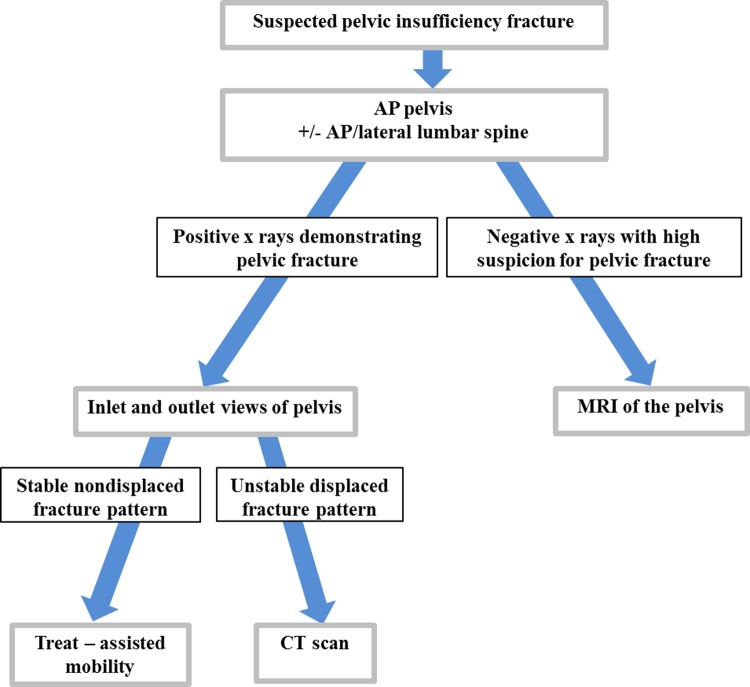
Also contributing to delayed diagnosis is the difficulty in interpreting plain radiographs. Once injury is suspected, an anteroposterior pelvis and possibly lumbar spine should be obtained. If x-rays demonstrate rami and/or sacral fractures, inlet and outlet views of the pelvis should be ordered. It is possible to treat the injury on the basis of the radiographs alone, but further imaging may be desired depending on injury characteristics. If initial x-rays are negative, while under high suspicion, a bone scan, magnetic resonance imaging (MRI), or computed tomography (CT) scan should follow. Overlying bowel gas makes it difficult or impossible to identify minimally displaced sacral fractures on plain films. Anterior fractures of the pubic rami, while not obscured by bowel gas, are often minimally displaced or simply appear as atypical bony changes and can be confused with malignancy.25 The limitations of x-ray and need for advanced imaging in diagnosing insufficiency fractures are illustrated in a study by Grasland. In 16 patients with sacral insufficiency fractures, only 8 could be identified on x-ray. Bone scans showed sacral uptake in all 16 patients. Of the patients, 9 underwent additional CT scan and sacral fracture was visualized in all 9 patients. Likewise, sacral fracture was identified in 1 patient who underwent MRI.26
Bone scans were the advanced imaging technique of choice for several years. The characteristic Honda or “H” sign is frequently referred to as being diagnostic of sacral insufficiency fracture. This was confirmed in a study by Fujii et al, who found a positive predictive value of the Honda sign of 94% as being diagnostic for a sacral fracture.27 Conversely, the absence of the Honda sign did not rule out sacral fracture, as only 63% of patients with sacral insufficiency fractures demonstrated this sign on bone scan.27 In addition, the sacroiliac (SI) joints will show increased uptake even in normal individuals making interpretation of bone scans difficult. The MRI and CT have supplanted the bone scan as the second line of diagnostic imaging after radiographs due to these limitations and perhaps greater familiarity with these modalities.
Computed tomography is certainly the test of choice in characterizing the detail of pelvic ring injuries in most circumstances due to its ability to highlight bony detail. Typically, we will obtain a CT scan in a patient in whom pelvic x-rays demonstrate a fracture if we wish to learn more about the fracture pattern to guide treatment. Magnetic resonance imaging, however, is more sensitive at detecting occult pelvic insufficiency fractures because of the signal detection of edema and bleeding in the bone (Figure 2). A retrospective comparison of MRI versus CT in detecting pelvic insufficiency fractures showed that 128 (99%) of 129 of fractures were identified on MRI versus only 89 (69%) of 129 were identified on CT.28 A prospective study by Henes et al confirmed this finding. In all, 96.3% of 122 fractures could be identified on MRI versus only 77% on CT.29 In addition, the interobserver reliability was higher for MRI (κ = .947) versus that for CT (κ = .730).29 Figure 3 summarizes our diagnostic imaging ordering process and rationale.
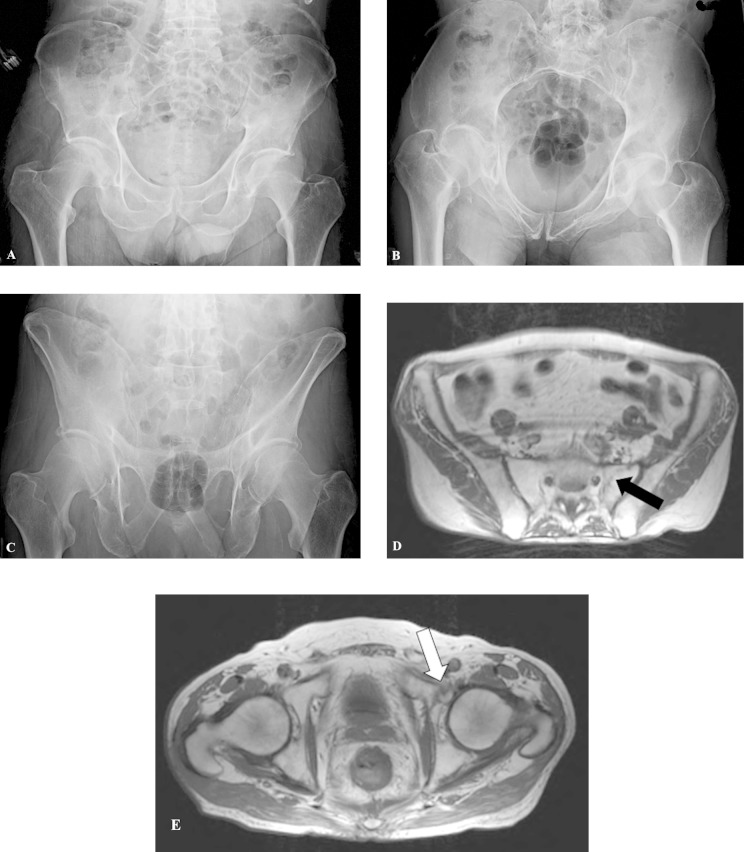
Figure 2.
This patient is a 71–year-old man who sustained a ground level fall with inability to bear weight on his left side. Initial x-rays of the pelvis were negative (A-C). Magnetic resonance imaging (MRI) scan demonstrated a nondisplaced left sacral fracture (D) and left superior pubic ramus fracture (E).
Figure 3.
Diagnostic imaging algorithm.
Treatment
Acute Phase
Low-energy pelvic ring injuries in geriatric patients should undergo similar assessment and demand the attention that is given to other pelvic ring injuries. A thorough assessment should be done to evaluate associated injuries. Severe hemorrhage has been documented in several case reports.30–36 A retrospective review35 showed that 8 (2.4%) of 328 patients with low-energy pelvic ring and 1 (2%) of 51 patients with low-energy acetabular fractures developed severe hemorrhage requiring embolization. An additional 2 patients developed significant hemorrhage with pelvic contusions without fracture. The mean hemoglobin drop in these patients was 4.0 g/dL. Hemoglobin stabilized in all patients following embolization. Several reasons are thought to contribute to hemorrhage in the elderly patients with a low-energy pelvic fracture. Arteriosclerosis may contribute by decreasing compliance and making vessels more fragile and likely to rupture. Often the elderly patients may be on blood thinning agents or medications contributing to coagulopathy. Vasospasm may also be impaired in sclerotic arteries. Additionally, the lack of gluteal muscle tone may impair tamponading effects. It is important that patients, in general, should undergo admission and serial hemoglobin evaluation every 6 hours for 24 hours.30–36
Nonoperative
Following the initial assessment and resuscitation phase, a decision must be made on how to best manage these patients. The majority of patients can be treated nonoperatively. The goals are defined by pain control and mobilization of the patient in an attempt to prevent complications of immobility. Patients are typically allowed to weight bear as tolerated; however, many are unable to do so due to pain and focus should be on bed to chair transfers. A walker helps to offload the weight-bearing axis through the pelvis. A discussion with the patient, family, and caregivers outlining expectations and goals is important. Similar to hip fractures on the femoral side, loss of independence and functional status is common.
Retrospective reviews by Taillandier, Breuil, and Koval demonstrated an average length of hospital stay of 14 to 45 days.2,5,11 In Koval series, 95% (36 of 38) of patients were able to return home; however, they did not comment on the need for further assistance. The studies by Taillander, Breuil, and Morris would suggest a much greater loss of independence. In Taillandier series, only 24 (59%) of 41 patients who had been previously self-sufficient recovered self-sufficiency at 1 year.2 All patients in Breuil series lived at home prior to fracture, and only 31% (19 of 60) were able to return home from the hospital.11 At a mean of 29-month follow-up, 74.5% (38 of 51) had returned home but the majority (60%) required assistance for activities of daily living, while only 18% (9 of 51) had required assistance prior to fracture.11 Morris found that of 87 patients living at home prior to fracture, only 11 (13%) were able to return home independently, 55 (63%) returned home with assistance, and 16 required a residential nursing home.4
Adverse events were common in these series of nonoperatively treated patients occurring in approximately 50% of patients in the Taillandier and Breuil series. Urinary tract infections comprised 50% of adverse events, bedsores 33%, depression or altered cognitive functions 18%, followed by thromboembolic events in 3% of patients.11 Morris found a 1-year mortality rate of 27%, with a subsequent annual mortality rate of 10% after the first year.4 Taillandier et al found a 1-year mortality rate of 14.3% that was higher than the expected 1-year mortality rate of 5.08% in patients of the same age without fractures; however, this was not statistically significant (P = .08).2 Given the frequency and characteristics of adverse events, the focus should be on mobility and nursing care with frequent turns, pressure reducing mattresses, and reducing the length of time that indwelling Foley catheters are in place. Mechanical deep vein thrombosis prophylaxis should be used in all patients. The risks of pharmacologic prophylaxis must be weighed on an individual basis.
Patients are best managed in a team approach by members of the orthopedic, medicine, and perhaps endocrinology services. Once through the initial phase of injury, a thorough laboratory work-up should be undertaken to evaluate reversible secondary causes of osteoporosis such as hyperthyroidism, hypothyroidism, vitamin D deficiency, or hypogonadism.23 Common laboratories should include thyroid stimulating hormone, parathyroid hormone (PTH), calcium, phosphorus, 25-hydroxyvitamin D, urinary calcium, creatinine, albumin, and liver function tests. Serum and urine protein electrophoresis should be obtained if multiple myeloma is suspected.23
Obviously medical treatment should be directed at correcting identified abnormalities. Specific attention has been given to vitamin D supplementation as many geriatric patients are vitamin D deficient. Our practice is to check serum 25-OH vitamin D levels in all patients over the age of 45 with insufficiency fractures. If low, the goal is to replete the serum 25-OH vitamin D level to the normal range of 30 to 80 ng/dL as quickly as possible with a repletion dose of 50 000 IU ergocalciferol weekly. Once in the normal range, patients are maintained on 2000 IU vitamin D daily.37
Antiresorptive agents, such as bisphosphonates, are mainstays in the treatment of osteoporosis. Randomized trials have demonstrated a reduction in the risk of hip and spine fractures in osteoporotic women.38,39 Calcitonin, another antiresorptive agent, is easily administered as a subcutaneous injection or nasal spray and is approved for the treatment of postmenopausal osteoporosis and may have the added benefit of pain reduction.23 While the long-term benefit in fracture reduction has been well established, their use in the acute fracture setting is less clear. Because osteoblast and osteoclast activity are coupled, osteoblast activity and therefore bone formation may also be reduced when antiresorptive agents are used.23
Teraperatide, or recombinant PTH, has seen more recent attention in the treatment of osteoporosis and related fractures. Teraperatide increases the number of osteoblasts by both stimulating formation and preventing apoptosis.40 It has been shown to increase bone density, cortical thickness, and bone strength as well as reduce fracture risk.40,41 As a catabolic agent, some benefits of administration have been shown during the fracture healing phase. A randomized trial comparing PTH with placebo in the treatment of osteoporotic pelvic fractures demonstrated a faster healing time, reduction in pain, and faster functional improvements in the group receiving PTH.42 Sixty-five women were randomized to receive 100 μg of PTH 1 to 84 administered once daily as a subcutaneous injection or placebo. All patients were given 1000 mg of calcium and 800 international units of vitamin D daily through fracture healing and for 24 months after fracture healing as treatment of their osteoporosis. There were 21 patients in the treatment group and 44 in the control group. The median time to fracture healing was 7.8 weeks in the treatment group compared with 12.6 weeks in the control group (P < .001). By week 8, all fractures in the treatment group had healed versus 9.1% in the control group. The mean time for healing in the control group was 14.9 weeks. All fractures in both groups had healed by 18 weeks. At week 8, patients in the treatment group also demonstrated significant improvement in the mean timed get up and go test (22.9 seconds) compared with the control group (54.3 seconds; P < .001). The mean visual analog score from week 0 to week 8 improved from 7.6 to 3.2 in the treatment group while only improving from 7.7 to 6.5 in the control group.
Operative
In patients who fail medical and nonoperative therapy, operative intervention may be considered with sacroplasty, internal fixation, or a combination of the 2. Sacroplasty was first reported in 2000, in the treatment of metastatic lesions of S1 extending the principles of vertebroplasty to the sacrum.43,44 The proposed concept was that filling the sacral defect with polymethylmethacrylate cement would reduce micromotion and therefore pain. The first case report of sacroplasty as a treatment for a pelvic insufficiency fracture was by Garant in 2002.45 In this report, a bedridden patient with compression fractures at T8, L3, L5, and the sacrum underwent vertebroplaty and sacroplasty. She was able to sit up and bear weight on postoperative day 1 and was pain free at 9 months following the procedure.
The success of early case reports led Frey to conduct a prospective observational cohort study to evaluate the incidence of complications and mid-term outcomes of percutaneous sacroplasty in the treatment of osteoporotic sacral insufficiency fractures.46 This study included 52 patients (40 females) with an average age of 75.9 who had no improvement with conservative care for a mean of 34.5 days. Mean visual analogue score (VAS) at baseline was 8.1 and improved to 3.6 at 30 minutes following the procedure. Mean VAS at weeks 2, 24, and 52 was 2.7, 1.4, and 0.8, respectively. All but 2 patients reported 75% to 100% satisfaction. In all, 13% of patients were pain free within 30 minutes of the procedure, and 25% were pain free at 2 weeks. One patient developed S1 radicular pain during the procedure, and injection was terminated. The radiculopathy was treated with a steroid injection and resolved after 7 days. All sacral fractures treated in this study occurred in zone 1, and the authors comment that use of sacroplasty in zone 2 or 3 fracture could risk cement leakage into the presacral space, spinal canal, and sacral foramen. Cement leakage could cause neurologic injury via direct compression or thermal necrosis. They concluded that sacroplasty was safe and effective in treatment of zone 1 insufficiency fractures but should not be used in zone 2 or 3.
Bayley et al conducted a literature review of the sacroplasty in the treatment of pelvic insufficiency fractures in 2008.47 A total of 15 articles including 108 patients were identified; however, there were no level 1, 2, or 3 study. Combined overall VAS improvement in these studies was from 8.9 presacroplasty to 2.6 postsacroplasty. Only 1 case was refractory to treatment. Complications included insignificant cement leakage into the S1 foramen in 1 case, posteriorly in 1 case, into the SI joint in 1 case, anteriorly in 4 cases, and IV in 1 case. Gupta et al performed a similar literature review in 2012 that included 109 patients.48 They reported a longevity of pain reduction of up to 1.5 years. The only complication identified in this review was the S1 radiculopathy that occurred in the Frey study.
While studies indicate that sacroplasty is safe and effective, some authors have expressed concerns that cement injection into the sacrum cannot restore biomechanical strength to the sacrum. Polymethylmethacrylate is excellent at resisting compressive loads such as those seen in the lumbar spine and would be seen in vertebroplasty for treatment of lumbar compression fractures. Polymethylmethacrylate is poor however at resisting shear loads such as those born by the sacrum. A biomechanical study of stiffness in osteoporotic pelvises showed that stiffness was not restored to baseline by sacroplasty.49 The authors point out, however, that stiffness of the entire pelvis was measured, not just that at the fracture site. In contrast, a finite element analysis found that sacroplasty restored the maximal principle stress of a sacral fracture model to 83% of the baseline value.50 It is unclear how these biomechanical studies translate into actual patients.
Concerns over the biomechanics of sacroplasty and limitations of the technique to the treatment of zone 1 injuries have lead to the investigation of operative techniques. In a biomechanical analysis comparing sacroplasty, unilateral iliosacral screw fixation, and trans-sacral screw fixation, Mears et al found that all techniques were effective at decreasing motion of the sacral fracture. Following cyclical loading, there was a greater increase in motion in the sacroplasty group, followed by the unilateral iliosacral screw fixation group, with the least increase in motion in the trans-sacral screw group, although the differences were not statistically significant (Figure 4).51
Figure 4.
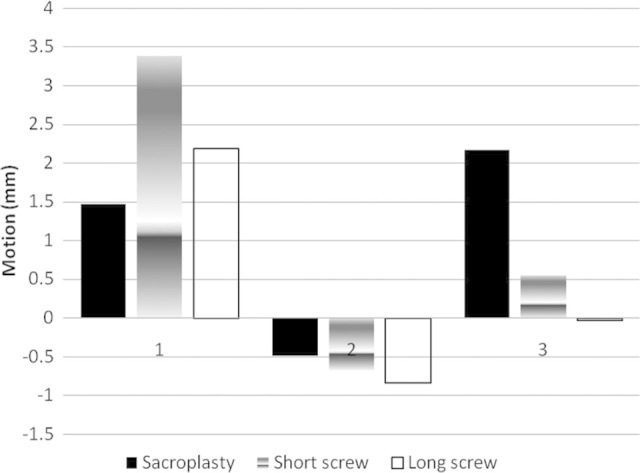
1, Demonstrates increase in motion after creation of a sacral fracture. 2, Decrease in motion (Mm) following fixation of a sacral fracture model after fixation with sacroplasty, unilateral sacroiliac (SI) screw, or trans-sacral screw. 3, Nonsignificant increases in motion following cyclical loading. Mears et al with permission.51
Again, higher level of evidence on surgical technique is lacking as the majority of articles on the subject are limited to descriptions of surgical techniques, case reports, and small case series. Opinion is varied regarding the optimal technique. Some authors recommend iliosacral screw fixation alone due to concern that cement augmentation would obscure the ability to evaluate fracture healing on follow-up imaging.23 Additionally, a space occupying mass inside the bone will never allow for incorporation with native bone and restoration of physiologic load transmission in patients under medical treatment to improve bonestock. Others comment that it is difficult to generate compression with iliosacral or trans-sacral screws and recommend augmentation.51–54 A case series of 19 patients (15 women) with an average age of 71.5 treated with a “trans-iliac-sacral-iliac-bar” (TISIB) without augmentation demonstrated excellent results. They reported near immediate pain relief in all patients and improved mobility, no neurologic complications, and an average length of stay of 5 days. Patients were allowed to weight bear as tolerated following the procedure with 8 being able to walk unaided and 11 requiring walking aids or assistance.55 The authors did comment that the technique may not be possible in all patients however. In severely osteoporotic bone, it may be difficult to visualize sacral landmarks for safe screw insertion and intraoperative CT may be required. In addition, the pelvic anatomy may not allow for TISIB placement. An anatomic evaluation of 20 patients demonstrated that TISIB placement would be possible in 10 of the 10 men at S1 and S2, but only 5 of the 10 women at S1 and 8 of the 10 women at S2.56
At our institution, we have not used cement augmentation when treating these injuries operatively as we have had success in treating these injuries without augmentation. Additionally, our goal is ultimately to get these fractures to heal, which may be difficult with the space occupying cement in the fracture zone. If screw purchase is poor posteriorly, we will place multiple screws and or trans-iliac-trans-sacral screws engaging the iliac wing on the far side.
Although attention in the literature has focused on the posterior sacral lesions common to stress fractures, anterior lesions of the rami must be diagnosed and possibly addressed as well. These lesions should be considered in the treatment scheme of internal fixation to augment posterior fixation in order to decrease motion of the injured hemipelvis. Posterior internal fixation should be prioritized since the majority of weight bearing is through the posterior lumbopelvic region, however once stabilized, anterior rami fractures can be fixed with retrograde or antegrade ramus screws or via open reduction internal fixation directly. It is our preference to begin with posterior fixation, then perform a stress examination under anesthesia. If there is instability of the anterior fracture, then anterior fixation is added. We tend to add anterior fixation if there is any doubt as this can be placed with a minimally invasive technique with little time and morbidity added to the procedure.
Figure 5 demonstrates a fracture that was treated with posterior only fixation, while Figures 6 and 7 demonstrate posterior and anterior fixation using a minimally invasive technique. We have previously described this technique using plates and screws or an occipitocervical plate-rod construct.57–59 A 3-cm incision is made over the iliac crest from the anterior superior iliac spine toward the gluteus tubercle and a separate 6 to 8 cm pfanensteil incision is made centered over the symphisis. A subcutaneous tunnel is created between the 2 incisions, superficial to the external oblique fascia, inguinal ligament, and rectus sheath. Polyaxial pedicle screws are inserted into the pubic body, and the contoured rod is then slid in the tunnel between the 2 incisions. Two or three screws are then placed into the ilac wing through the plate portion of the rod to secure it proximally.
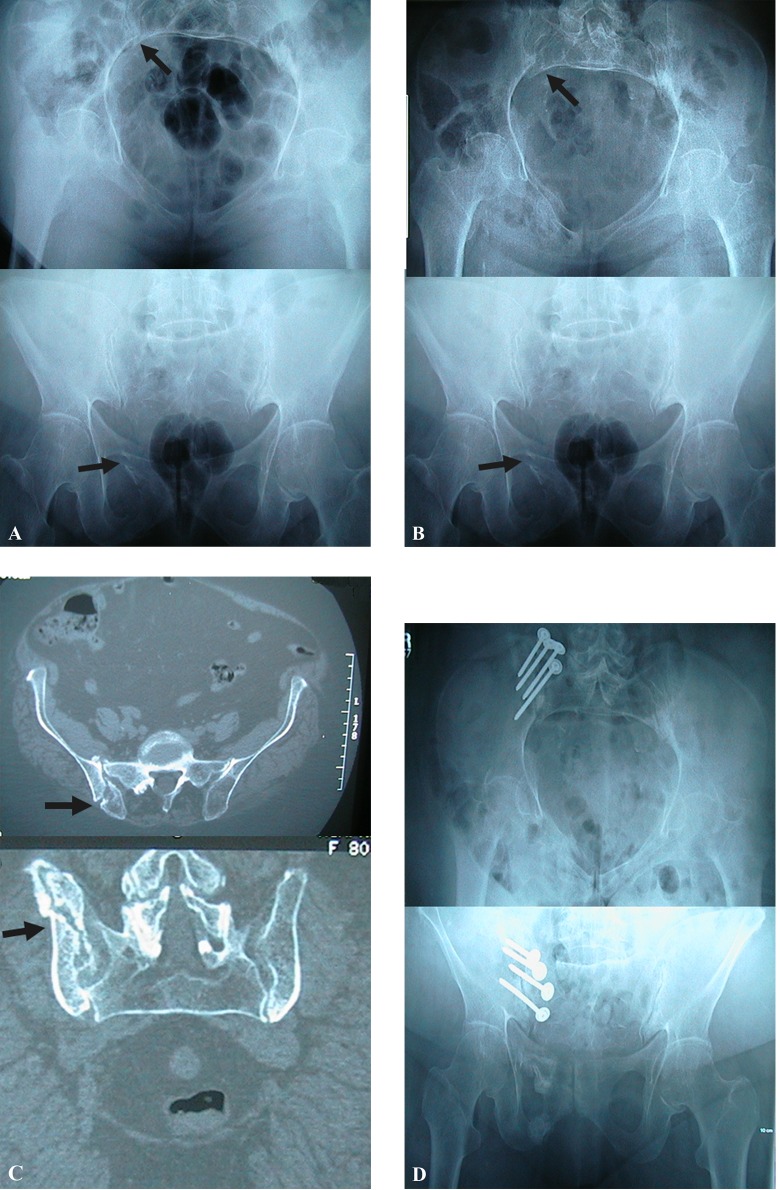
Figure 5.
Subtle signs of a fragility fracture from a lateral compression type 1 (LC I) fracture depicted by arrows on inlet and outlet x-ray view (A). Twelve-week postinjury, the patient was developing worsening pain having been advised to be weight bearing as tolerated. Greater displacement is depicted at the site of the arrows (B). Computed tomography (CT) scan of pelvis demonstrating an un-united posterior pelvic ring crescent type fracture (C). Three months after an open reconstruction with bone grafting and fixation of the posterior pelvis, the patient was asymptomatic with weight bearing (D).
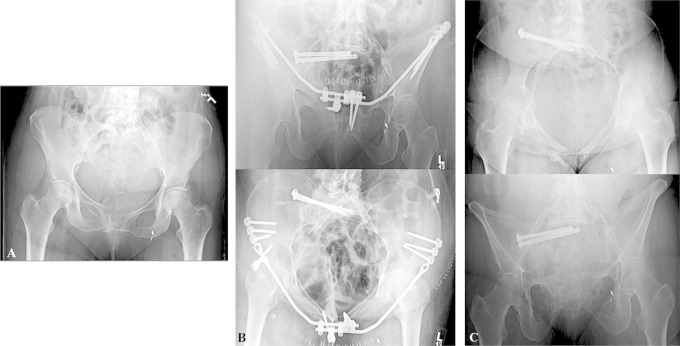
Figure 6.
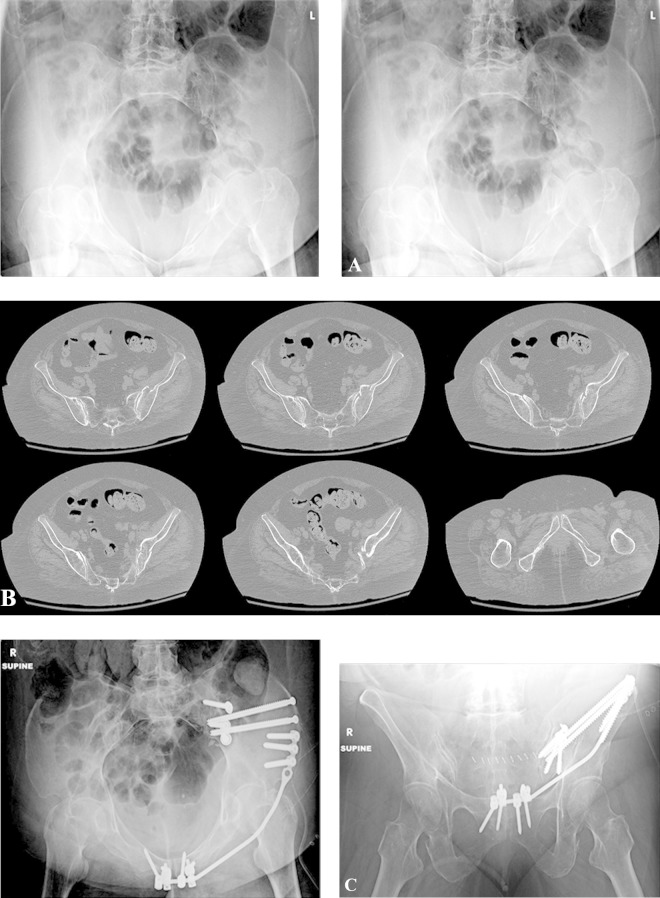
Patient with right pubic rami fractures and sacral fracture that displaced after 2 weeks of nonoperative management (A). Postoperative inlet and outlet x-rays of pelvis showing minimally invasive osteosynthesis with posterior iliosacral screws and an anterior pelvic bridge technique57 (B). Six-month post-healing after anterior hardware was removed electively (C).
Figure 7.
A 52-year-old female who fell from her wheelchair at home. Preoperative anteroposterior inlet and outlet x-rays (A) and computed tomography (CT) scan (B) demonstrating a large posterior crescent fracture and superior and inferior rami fractures anteriorly. C, Postoperative inlet and outlet views demonstrating fixation of the posterior crescent fracture and anterior fixation using the pelvic bridge. 6.5 and 7.3 screws were used due to poor bone quality.
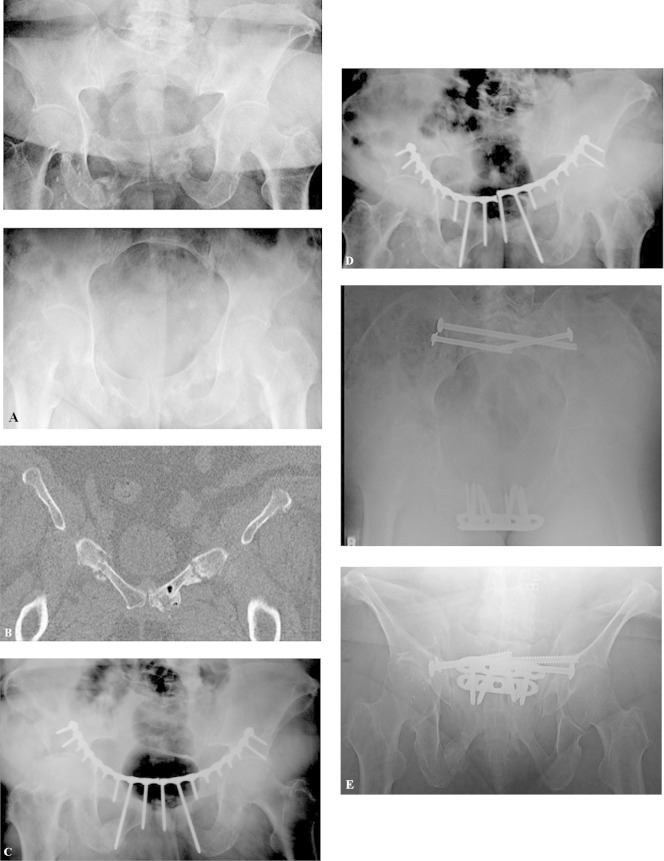
Pelvic insufficiency fractures may go on to nonunion after both operative and nonoperative treatment. Mears and Velyvis60 reported on a case series of 44 nonunions treated with in situ fixation. Of these, 36 (82%) healed after in situ fixation and 7 of the 8 healed after additional surgery. Despite union, 13 (30%) patients had persistent pain at 1 year. Of the 8 patients, 6 who failed to heal after their initial operation had undergone prior radiation therapy. After 1 year, 20 (55%) of the 44 patients were highly satisfied, 12 (27%) satisfied, and 8 (18%) were unsatisfied. In all, 17 (39%) patients experienced improvement in their walking ability (ie, from walker to cane or from cane to no cane), while 27 (61%) patients had no change in their walking ability. The authors state, and we agree, that anterior and posterior fixation should be used in the treatment of pelvic insufficiency nonunions. Figure 8 demonstrates the importance of posterior fixation. This case is a nonunion that occurred in a patient with anterior fixation only of bilateral rami fractures. Likely, this patient failed due to residual instability and cycling of the plate as a result of a stiff anterior construct with motion through the posterior pelvis, which was not fixed. The patient went on to heal after stabilizing the posterior pelvis and symphysis plating.
Figure 8.
A 64- year old female began having pelvic pain pushing a wheel chair up a hill. Six-month follow-up x-rays and computed tomography (CT) showed nonhealing rami fractures (A and B). She underwent anterior pelvic fixation and 7-month postop films showed failure of fixation with single leg stance views on the right (C) and left (D). (E) Revision surgery stabilizing anterior and posterior pelvis after healing at last 2-year follow-up. Patient symptoms resolved and she became full weight bearing with no pain or assistive devices for ambulation.
Summary and Recommendations
Pelvic insufficiency fractures are increasing in incidence and can cause considerable disability in the elderly patients. Many studies indicate prolonged periods of immobility, the potential for complications, and loss of independence. Limitations of these studies, however, are that most were carried out on inpatients and there are likely a number of these fractures that are treated on an outpatient basis, perhaps with less disability. The severity of pelvic insufficiency fractures and implications on patients’ lives however should be taken seriously.
Diagnosis of These Injuries Can Be Difficult and May Require Advanced Imaging Bone scans, while helpful, have largely been supplanted by MRI and CT. Magnetic resonance imaging has been shown to be more sensitive than CT in the diagnosis of suspected sacral insufficiency fractures.
Medical treatment of these patients is paramount in terms of both prevention, through osteoporosis screening and treatment, and in the treatment of fractures once they occur. The vast majority of patients are successfully treated nonoperatively with assisted mobilization, analgesia, and reversal of secondary causes of osteoporosis when indicated. Consideration should be given to treatment with PTH as it has been shown in a randomized controlled trial to significantly improve fracture healing time and lessen pain.
While seemingly benign injuries, not all pelvic insufficiency fractures do well with nonoperative treatment. We typically follow patients weekly with serial radiographs for the first 2 to 3 weeks, then monthly until healing. Many patients will have persistent pain at 4 to 6 weeks; however, those who are still unable to mobilize due to pain are offered surgical intervention. Zone 1 fractures may be treated with sacroplasty. Alternatively, iliosacral screws or trans-iliac-trans-sacral screws may be used in zone 1, 2, or 3 sacral fractures. This may be augmented with anterior fixation depending on an intraoperative stress examination or fracture pattern. Surgical techniques continue to evolve, and more studies are needed to identify the optimal surgical technique.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P. A. Cole receives consulting fees/honoraria from AONA and AO International, is a consultant to J&J (Synthes-Depuy), and has stock/stock options with BoneFoams Inc, LLC.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Lourie H. Spontaneous osteoporotic fracture of the sacrum. an unrecognized syndrome of the elderly. JAMA. 1982;248 (6):715–717. [PubMed] [Google Scholar]
- 2. Taillandier J, Langue F, Alemanni M, Taillandier-Heriche E. Mortality and functional outcomes of pelvic insufficiency fractures in older patients. Joint Bone Spine. 2003;70 (4):287–289. [DOI] [PubMed] [Google Scholar]
- 3. Finiels H, Finiels PJ, Jacquot JM, Strubel D. Fractures of the sacrum caused by bone insufficiency. Meta-analysis of 508 cases. Presse Med. 1997;26 (33):1568–1573. [PubMed] [Google Scholar]
- 4. Morris R, Sonibare A, Green D, Masud T. Closed pelvic fractures: characteristics and outcomes in older patients admitted to medical and geriatric wards. Postgrad Med J. 2000;76 (900):646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koval KJ, Aharonoff GB, Schwartz MC, et al. Pubic rami fracture: a benign pelvic injury? J Orthop Trauma. 1997;11 (1):7–9. [DOI] [PubMed] [Google Scholar]
- 6. Melton LJ III, Sampson JM, Morrey BF, Ilstrup DM. Epidemiologic features of pelvic fractures. Clin Orthop Relat Res. 1981;(155):43–47. [PubMed] [Google Scholar]
- 7. US census data. 2012 national population projections: summary tables. Web site http://www.census.gov/population/projections/data/national/2012/summarytables.html. Published May 15, 2013. Accessed March 1, 2014.
- 8. Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M. Epidemiology of osteoporotic pelvic fractures in elderly people in Finland: sharp increase in 1970-1997 and alarming projections for the new millennium. Osteoporos Int. 2000;11 (5):443–448. [DOI] [PubMed] [Google Scholar]
- 9. Kannus P, Palvanen M, Parkkari J, Niemi S, Jarvinen M. Osteoporotic pelvic fractures in elderly women. Osteoporos Int. 2005;16 (10):1304–1305. doi:10.1007/s00198-005-1941-1. [DOI] [PubMed] [Google Scholar]
- 10. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the united states, 2005-2025. J Bone Miner Res. 2007;22 (3):465–475. doi:10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 11. Breuil V, Roux CH, Testa J, et al. Outcome of osteoporotic pelvic fractures: an underestimated severity. survey of 60 cases. Joint Bone Spine. 2008;75(5):585–588. doi:10.1016/j.jbspin.2008.01.024; 10.1016/j.jbspin.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 12. Moreno A, Clemente J, Crespo C, et al. Pelvic insufficiency fractures in patients with pelvic irradiation. Int J Radiat Oncol Biol Phys. 1999;44 (1):61–66. [DOI] [PubMed] [Google Scholar]
- 13. Baxter NN, Habermann EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294 (20):2587–2593. doi:10.1001/jama.294.20.2587. [DOI] [PubMed] [Google Scholar]
- 14. Housman D, Savage C, Zelefsky M, Elkin E. Pelvic fracture after radiation therapy for localized prostate cancer: a population based study. Int J Rad Onc Biol Phys. 2010;78 (3):S64. [Google Scholar]
- 15. Oh I, Hardacre JA. Fatigue fracture of the inferior pubic ramus following total hip replacement for congenital hip dislocation. Clin Orthop Relat Res. 1980;(147):154–156. [PubMed] [Google Scholar]
- 16. Launder WJ, Hungerford DS. Stress fracture of the pubis after total hip arthroplasty. Clin Orthop Relat Res. 1981;(159):183–185. [PubMed] [Google Scholar]
- 17. Marmor L. Stress fracture of the pubic ramus stimulating a loose total hip replacement. Clin Orthop Relat Res. 1976;(121):103–104. [PubMed] [Google Scholar]
- 18. Smith D, Zuckerman JD. Bilateral stress fractures of the pubic rami following THA—an unusual case of groin pain. Bull NYU Hosp Jt Dis. 2010;68 (1):43–45. [PubMed] [Google Scholar]
- 19. Kanaji A, Ando K, Nakagawa M, Fukaya E, Date H, Yamada H. Insufficiency fracture in the medial wall of the acetabulum after total hip arthroplasty. J Arthroplasty. 2007;22 (5):763–767. doi:10.1016/j.arth.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 20. Peh WC, Gough AK, Sheeran T, Evans NS, Emery P. Pelvic insufficiency fractures in rheumatoid arthritis. Br J Rheumatol. 1993;32 (4):319–324. [DOI] [PubMed] [Google Scholar]
- 21. Hoshino Y, Doita M, Yoshikawa M, Hirayama K, Sha N, Kurosaka M. Unstable pelvic insufficiency fracture in a patient with rheumatoid arthritis. Rheumatol Int. 2004;24 (1):46–49. doi:10.1007/s00296-003-0331-2. [DOI] [PubMed] [Google Scholar]
- 22. Thakral R, Kiely Patrick J, Mahalingham K. Unstable pelvic insufficiency fracture of the pelvis in rheumatoid arthritis patient: evaluation and treatment. Eur J Orthop Surg Trauma. 2010;20 (3):237–240. [Google Scholar]
- 23. Tsiridis E, Upadhyay N, Giannoudis P. Sacral insufficiency fractures: current concepts of management. Osteoporosis Int. 2006;17 (12):1716–1725. [DOI] [PubMed] [Google Scholar]
- 24. Babayev M, Lachmann E, Nagler W. The controversy surrounding sacral insufficiency fractures: to ambulate or not to ambulate? Am J Phys Med Rehabil. 2000;79 (4):404–409. [DOI] [PubMed] [Google Scholar]
- 25. Peh WC, Khong PL, Yin Y, et al. Imaging of pelvic insufficiency fractures. Radiographics. 1996;16 (2):335–348. doi:10.1148/radiographics.16.2.8966291. [DOI] [PubMed] [Google Scholar]
- 26. Grasland A, Pouchot J, Mathieu A, Paycha F, Vinceneux P. Sacral insufficiency fractures: an easily overlooked cause of back pain in elderly women. Arch Intern Med. 1996;156 (6):668–674. [DOI] [PubMed] [Google Scholar]
- 27. Fujii M, Abe K, Hayashi K, et al. Honda sign and variants in patients suspected of having a sacral insufficiency fracture. Clin Nucl Med. 2005;30 (3):165–169. [DOI] [PubMed] [Google Scholar]
- 28. Cabarrus MC, Ambekar A, Lu Y, Link TM. MRI and CT of insufficiency fractures of the pelvis and the proximal femur. AJR Am J Roentgenol. 2008;191(4):995–1001. doi:10.2214/AJR.07.3714; 10.2214/AJR.07.3714. [DOI] [PubMed] [Google Scholar]
- 29. Henes FO, Nuchtern JV, Groth M, et al. Comparison of diagnostic accuracy of magnetic resonance imaging and multidetector computed tomography in the detection of pelvic fractures. Eur J Radiol. 2012;81 (9):2337–2342. doi:10.1016/j.ejrad.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 30. Coupe NJ, Patel SN, McVerry S, Wynn-Jones CH. Fatal haemorrhage following a low-energy fracture of the pubic ramus. J Bone Joint Surg Br. 2005;87 (9):1275–1276. doi:10.1302/0301-620X.87B9.16696. [DOI] [PubMed] [Google Scholar]
- 31. Henning P, Brenner B, Brunner K, Zimmermann H. Hemodynamic instability following an avulsion of the corona mortis artery secondary to a benign pubic ramus fracture. J Trauma. 2007;62 (6):E14–E17. doi:10.1097/01.ta.0000210355.44804.24. [DOI] [PubMed] [Google Scholar]
- 32. Loffroy R, Yeguiayan JM, Guiu B, Cercueil JP, Krause D. Stable fracture of the pubic rami: a rare cause of life-threatening bleeding from the inferior epigastric artery managed with transcatheter embolization. CJEM. 2008;10 (4):392–395. [DOI] [PubMed] [Google Scholar]
- 33. Macdonald D, Tollan C, Robertson I, Rana B. Massive haemorrhage after a low-energy pubic ramus fracture in a 71-year-old woman. Postgrad Med J. 2006;82 (972):e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meyers TJ, Smith WR, Ferrari JD, Morgan SJ, Franciose RJ, Echeverri JA. Avulsion of the pubic branch of the inferior epigastric artery: a cause of hemodynamic instability in minimally displaced fractures of the pubic rami. J Trauma. 2000;49 (4):750–753. [DOI] [PubMed] [Google Scholar]
- 35. Krappinger D, Zegg M, Jeske C, El Attal R, Blauth M, Rieger M. Hemorrhage after low-energy pelvic trauma [published online August 11, 2011]. J Trauma. 2011. doi:10.1097/TA.0b013e31822ad41f. [DOI] [PubMed] [Google Scholar]
- 36. Theodorides AA, Morgan BW, Simmons D. Haemodynamic instability resulting from a low energy pubic ramus fracture in a 78-year-old woman. A case report and review of the literature. Injury. 2011;42 (7):722–724. doi:10.1016/j.injury.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 37. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357 (3):266–281. [DOI] [PubMed] [Google Scholar]
- 38. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. fracture intervention trial research group. Lancet. 1996;348 (9041):1535–1541. [DOI] [PubMed] [Google Scholar]
- 39. McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. hip intervention program study group. N Engl J Med. 2001;344 (5):333–340. doi:10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 40. Body JJ, Gaich GA, Scheele WH, et al. A randomized doubleblind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2002;87 (10):4528–4535. doi:10.1210/jc.2002-020334. [DOI] [PubMed] [Google Scholar]
- 41. Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16 (10):1846–1853. doi:10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 42. Peichl P, Holzer LA, Maier R, Holzer G. Parathyroid hormone 1-84 accelerates fracture-healing in pubic bones of elderly osteoporotic women. J Bone Joint Surg Am. 2011;93 (17):1583–1587. doi:10.2106/JBJS.J.01379. [DOI] [PubMed] [Google Scholar]
- 43. Dehdashti AR, Martin JB, Jean B, Rufenacht DA. PMMA cementoplasty in symptomatic metastatic lesions of the S1 vertebral body. Cardiovasc Intervent Radiol. 2000;23 (3):235–237. [DOI] [PubMed] [Google Scholar]
- 44. Marcy PY, Palussiere J, Descamps B, et al. Percutaneous cementoplasty for pelvic bone metastasis. Support Care Cancer. 2000;8 (6):500–503. [DOI] [PubMed] [Google Scholar]
- 45. Garant M. Sacroplasty: a new treatment for sacral insufficiency fracture. J Vasc Interv Radiol. 2002;13 (12):1265–1267. [DOI] [PubMed] [Google Scholar]
- 46. Frey ME, Depalma MJ, Cifu DX, Bhagia SM, Carne W, Daitch JS. Percutaneous sacroplasty for osteoporotic sacral insufficiency fractures: a prospective, multicenter, observational pilot study. Spine J. 2008;8 (2):367–373. doi:10.1016/j.spinee.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 47. Bayley E, Srinivas S, Boszczyk BM. Clinical outcomes of sacroplasty in sacral insufficiency fractures: a review of the literature. Eur Spine J. 2009;18 (9):1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gupta AC, Yoo AJ, Stone J, et al. Percutaneous sacroplasty. J Neurointerv Surg. 2012;4 (5):385–389. doi:10.1136/neurintsurg-2011-010136. [DOI] [PubMed] [Google Scholar]
- 49. Waites MD, Mears SC, Richards AM, Mathis JM, Belkoff SM. A biomechanical comparison of lateral and posterior approaches to sacroplasty. Spine (Phila Pa 1976). 2008;33 (20):E735–E738. doi:10.1097/BRS.0b013e31817ecc22. [DOI] [PubMed] [Google Scholar]
- 50. Whitlow CT, Yazdani SK, Reedy ML, Kaminsky SE, Berry JL, Morris PP. Investigating sacroplasty: technical considerations and finite element analysis of polymethylmethacrylate infusion into cadaveric sacrum. AJNR Am J Neuroradiol. 2007;28 (6):1036–1041. doi:10.3174/ajnr.A0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mears SC, Sutter EG, Wall SJ, Rose DM, Belkoff SM. Biomechanical comparison of three methods of sacral fracture fixation in osteoporotic bone. Spine (Phila Pa 1976). 2010;35 (10):E392–E395. doi:10.1097/BRS.0b013e3181cb4fcd. [DOI] [PubMed] [Google Scholar]
- 52. Soles GL, Ferguson TA. Fragility fractures of the pelvis. Curr Rev Musculoskelet Med. 2012;5 (3):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tjardes T, Paffrath T, Baethis H, et al. Computer assisted percutaneous placement of augmented iliosacral screws: a reasonable alternative to sacroplasty. Spine (Phila Pa 1976). 2008;33 (13):1497–1500. doi:10.1097/BRS.0b013e318175c25c. [DOI] [PubMed] [Google Scholar]
- 54. Sciubba DM, Wolinsky JP, Than KD, Gokaslan ZL, Witham TF, Murphy KP. CT fluoroscopically guided percutaneous placement of transiliosacral rod for sacral insufficiency fracture: case report and technique. AJNR Am J Neuroradiol. 2007;28 (8):1451–1454. doi:10.3174/ajnr.A0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vanderschot P, Kuppers M, Sermon A, Lateur L. Trans-iliac-sacral-iliac-bar procedure to treat insufficiency fractures of the sacrum. Indian J Orthop. 2009;43 (3):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vanderschot P, Meuleman C, Lefevre A, Broos P. Trans iliac-sacral-iliac bar stabilisation to treat bilateral lesions of the sacro-iliac joint or sacrum: anatomical considerations and clinical experience. Injury. 2001;32 (7):587–592. [DOI] [PubMed] [Google Scholar]
- 57. Cole PA, Gauger EM, Anavian J, Ly TV, Morgan RA, Heddings AA. Anterior pelvic external fixator versus subcutaneous internal fixator in the treatment of anterior ring pelvic fractures. J Orthop Trauma. 2012;26 (5):269–277. doi:10.1097/BOT.0b013e3182410577. [DOI] [PubMed] [Google Scholar]
- 58. Moazzam C, Heddings AA, Moodie P, Cole PA. Anterior pelvic subcutaneous internal fixator application: an anatomic study. J Orthop Trauma. 2012;26 (5):263–268. doi:10.1097/BOT.0b013e31823e6b82. [DOI] [PubMed] [Google Scholar]
- 59. Hiesterman TG, Hill BW, Cole PA. Surgical technique: a percutaneous method of subcutaneous fixation for the anterior pelvic ring: the pelvic bridge. Clin Orthop Relat Res. 2012;470 (8):2116–2123. doi:10.1007/s11999-012-2341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mears DC, Velyvis JH. In situ fixation of pelvic nonunions following pathologic and insufficiency fractures. J Bone Joint Surg Am. 2002;84-A(5):721–728. [DOI] [PubMed] [Google Scholar]