Introduction
Cardiovascular disease (CVD) is a leading cause of death in persons with spinal cord injury (SCI) who survive at least 1 year post injury.1 Persons with SCI who are younger than 45 years of age are 4 times more likely to die of cardiac causes than their age-matched counterparts without SCI.2 There is evidence that people with SCI are at increased risk for CVD compared to the general population,2–4 however prospective trials documenting true incidence have not confirmed this. These figures are not surprising, due to the effect of paralysis on body composition, challenges to physical activity, and the body’s response to physical activity after SCI. Individuals with SCI may also be less likely, depending on level of injury, to experience angina or other cardiac-related symptoms as a warning sign of cardiac compromise due to sympathetic nervous system disruption.5
Because measures of risk, and subsequent preventive practices, are based on and designed for the general population and because persons with SCI experience CVD earlier and with fewer cardiac symptoms than the general population, there is a need to better characterize the risks in this population.6 One of the significant risks of CVD in persons with SCI is their tendency to develop cardiometabolic syndrome, defined as a cluster of risk factors that includes obesity, insulin resistance, diabetes mellitus, dyslipidemia, and subclinical atherosclerosis.7
Cardiometabolic risk (CMR) has been a principal research focus of the Rehabilitation Research and Training Center (RRTC) on Secondary Conditions in the Rehabilitation of Individuals with Spinal Cord Injury. Research conducted during a previous cycle of SCI-RRTC (2003-2009) funding demonstrated that 76.9% of subjects exhibited risk clustering, with elevated low-density lipoprotein cholesterol (LDL-C) occurring in 64% of subjects and depressed high-density lipoprotein cholesterol (HDL-C) occurring in 53% of subjects (42% and 11%, respectively, for males and females). Further, overweight/obesity was the most prevalent CMR (74%) among 121 community-dwelling persons with chronic SCI.8
Body mass index (BMI) provides a proxy measure of body fat, as the ratio of weight (kg) and height (m2). Although BMI may be adequate for providing a gross estimate of obesity in the general population, it is problematic after SCI due to the significant changes in body composition that are nearly universal and are characterized by a loss of lean and increase in fat mass. As a result, the BMI scale significantly underestimates obesity in individuals after SCI9 and therefore may not signal actual risk. Similarly, although optimization of the lipid profile is integral to general population CVD prevention,6 the way in which lipid patterns may differ after SCI and how those differences impact CVD risk in persons with SCI have not received much attention.10
RRTC investigators reported the state of the science on CMR in SCI as part of a pre-conference11,12 to the 40th anniversary meeting of the American Spinal Injury Association (ASIA). Investigators focused their presentation of the current state of knowledge in the area of CMR after SCI through the lens of their ongoing work on 2 key risks within the CMR constellation: dyslipidemia and obesity. The sections that follow describe the state of current knowledge in the areas of lipid and body composition risk factors as they apply to prevention and treatment of CVD in persons with SCI.
Body Composition and CVD Risk After SCI
Obesity figures prominently among the correlates of CVD,13and the added risk of CVD in people with SCI may be related to excess body fat.14 Given the comparable prevalence of metabolic syndrome, men with SCI experience a higher rate of abdominal obesity than men without SCI, a characteristic hypothesized to contribute to the higher rate of CVD among men with versus without SCI.15 Yet the effect of paralysis on the reliability of anthropometrics commonly used in general population assessments of body fat, for example, abdominal circumference, skin-fold thickness, and sagittal abdominal depth (standing posture assumed), is not clear.16
The 4-compartment model17 that considers body composition as the aggregate of its aqueous, protein, mineral, and fat fractions is the present gold standard.16 The collection of these measures, however, is a time-and resource-intensive process and is not feasible during routine obesity screening procedures; it is even less feasible for people with SCI. For the general population, the Quetelet or body mass index (BMI)18provides a low-cost and reasonably reliable means of estimating percent body fat (%BF) for managing CVD and other risks.19,20 BMI was originally developed in the 1800s to classify a population of sedentary people according to “thickness or thinness.” For the past several decades, especially after being introduced as a screening tool by the World Health Organization in 1998,21the numerical BMI has been used by clinicians to estimate disease risk. Persons with SCI, however, have higher total fat tissue and %BF for any given BMI than persons without SCI due to the loss of muscle mass and physical inactivity that accompany paralysis.21,22
A study by the SHAPE SCI Research Group suggests a BMI of 22 (kg/m2) as the threshold for overweight in persons with SCI (male gender, irrespective of level of injury) and a BMI of 25 as the categorical definition of obese.23 These SCI-specific BMI cutoffs contrast with those published by the National Heart, Lung, and Blood Institute for the general population, which define the thresholds for overweight and obese as 25 and 30, respectively.24 However, after stratifying individuals with SCI according to these more stringent BMI cutoff recommendations, Libin et al did not find that persons within the more conservative definitions demonstrated better metabolic health than did individuals under the more generous, NHLBI general population definitions.25 This finding suggests that if an indirect measure, such as BMI, is to be useful for approximating body fat, its accuracy needs to be improved or another measure needs to be developed specifically for people with SCI.
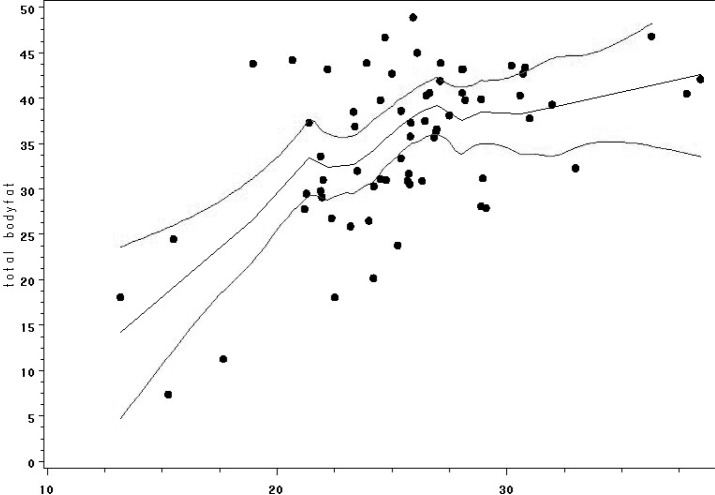
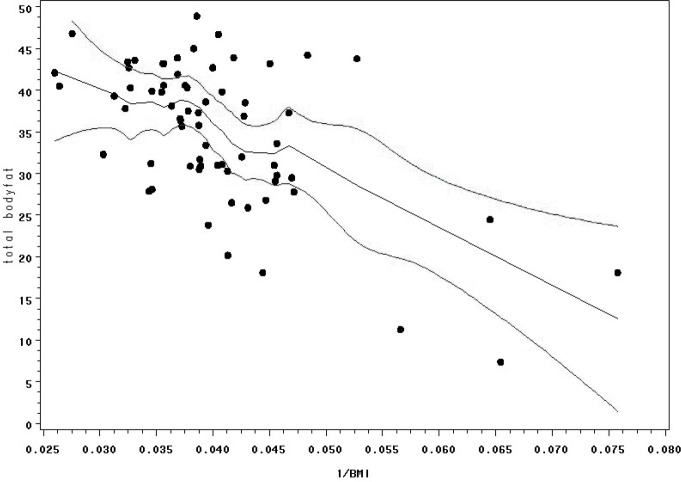
Health risk calculators are more comprehensive, and they commonly incorporate other normative data based on standard measurements such as age, gender, and ethnicity.26 Because risk factors interact, the factors themselves and the interaction among factors need to be considered27 to provide a useful, algorithmic tool for both individuals and health care providers in assessing adiposity for CVD and other risk management. RRTC investigators are currently in the early phases of development of a body fat calculator for people with SCI based on health risk calculator methods and utilizing height, weight, dual-energy x-ray absorptiometry (DXA)–determined body fat, and other key demographic and SCI-specific variables. Participants to date include 101 individuals with chronic SCI who are at least 1 year post injury, with no diagnosed history of CVD or diabetes. Calculated BMI plotted against %BF yielded a nonlinear distribution (Figure 1). Linearity was improved, however, by plotting the reciprocal BMI (1/BMI) against %BF (Figure 2). Then, 1/BMI, age, gender, race, ASIA classification, and duration of SCI were included as potential predictors in a discrete linear regression model to test the violations of the assumptions of a linear regression model were presumed, and a bootstrapping procedure (1,000 bootstrap samples of the same size as the original sample) was utilized to counter this effect. Similarly, bootstrapping methods were used to internally validate the resulting transform equating 1/BMI and other body composition predictors to measured %BF. Refinement of the BMI risk calculator for SCI populations is ongoing, however preliminary evidence suggests that 1/BMI and level of injury are statistically significant (P < .0001) indicators of body fat, with duration of injury being borderline significant (P = .065) after adjusting for age, gender, and race.
Figure 1. Loess plot with 95% confidence interval between body mass index (BMI) and percent body fat (PBF).
Figure 2. Loess plot with 95% confidence interval between reciprocal body mass index (1/BMI) and percent body fat (PBF).
To our knowledge, the current study is the first to use data simulation methods to compensate for the small sample sizes that typify SCI research28 in an effort to develop a health risk calculator tailored to persons with SCI.29 Findings demonstrate that a specific combination of variables has the potential to provide a more accurate, indirect measure of adiposity in persons with SCI than does BMI alone. Our goal is for individuals with SCI and health care providers to use this algorithm, embedded into a simple health risk calculator, to better assess percent body fat and to use that information in preventive decision making.
Lipid Profile as a Predictor of CVD Risk After SCI
To better understand the differences in the lipid profiles of persons with SCI versus those of the general population and to provide evidence to improve lipid screening and treatment practices for individuals with SCI, RRTC investigators from 4 collaborating institutions (MedStar Georgetown University Hospital, MedStar National Rehabilitation Hospital, University of Miami Miller School of Medicine, and the University of Washington) conducted a systematic review of the literature and meta-analysis.6 Lipid data from 50 relevant studies were extracted from 4,512 persons with SCI and 1,252 persons without SCI (controls) who were otherwise demographically similar. Persons with SCI registered significantly lower average levels of HDL-C than did controls: 41.0 mg/dL (SD 5.8) versus 49.6 mg/dL (SD 6.8), P < .001 (Table 1). This finding is consistent with those of prior studies.30–37 The current analysis further found that persons with SCI had significantly higher ratios of average total cholesterol (TC) to average HDL-C than did controls. There was no difference between persons with SCI and able-bodied individuals with respect to total cholesterol, non–HDL-C, and triglyceride levels.
Table 1. Comparison of relevant lipid levels in persons with and without SCI.
| SCI | Non-SCI | ||
|---|---|---|---|
| Serum lipids | Mean (SD), mg/dL | Mean (SD), dL | p |
| TC | 183.4 (15.1) | 194.9 (19.7) | .019 |
| LDL-C | 115.5 (14.7) | 118.0 (25.4) | .64 |
| HDL-C | 41.0 (5.8) | 49.6 (5.8) | <.001 |
| Triglycerides ° | 134.0 (45.6) | 125.0 (74.2) | .57 |
| Non–HDL-C | 142. (14.1) | 145.9 (19.6) | .53 |
| TC:HDL-C | 4.5 (0.6) | 4.0 (0.6) | .002 |
Note: HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; SCI = spinal cord injury; TC = total cholesterol.
Low levels of HDL-C correlate with higher CVD morbidity and mortality independent of non–HDL-C and triglyceride values38,39 and hypertension.40 The National Cholesterol Education Program’s Adult Treatment Panel III (ATPIII) guidelines set the categorical risk threshold for low HDL-C at 40 mg/dL.41 This cutoff represents a 5 mg/dL increase over the previous recommendation of ATPII42 in response to more recent research.43 In the specific context of metabolic syndrome, the International Diabetes Foundation recommends an even more conservative lower bound of 50 mg/dL for HDL-C for females.44
Studies have demonstrated that for every increase of 1 mg/dL in HDL-C, CVD risk decreases by 2% to 3% (for men and women, respectively)45and that for every increase in HDL-C of 1 SD (15 mg/ dL), CVD risk decreases by 22%.38 Further, the total cholesterol (TC) to HDL-C ratio has been shown to be 40% more informative of CVD risk than non–HDL-C values and more than twice as informative as TC alone.40 Low HDL-C, however, typically presents as part of a lipid triad where it occurs in conjunction with elevated triglycerides and small LDL particles.41,46,47 When low HDL-C manifests in isolation from other lipid abnormalities, it may signal an additional risk factor, as suggested in studies of certain ethnic populations.48 Present guidelines, however, would classify the average person with SCI, as represented by data from the RRTC study, as low risk based on the single risk factor of low HDL-C.41 Given the disproportionate CVD burden borne by persons with SCI vis-à-vis the general population, the suitability of these guidelines for the SCI population is the subject of debate.6
A final relevant issue presented by RRTC researchers involved the understanding of CVD in people with SCI being largely dependent on CMR as opposed to hard cardiovascular endpoints such as myocardial infarction, stroke, and cardiovascular death. Risk factors are inherently problematic as they correlate with the disease in question but they do not identify the disease itself.49,50 Technologic advances, notably in imaging, have made it possible to identify and track surrogates of end-organ disease instead of relying on risk factors alone. The American College of Cardiology/ American Heart Association recommends the use of imaging modalities, such as carotid intima media thickness (CIMT) and coronary artery calcium scoring (CACS), for the general population when assignment of CVD risk is not straightforward.51 A study comparing risk stratified according to the ATPIII guidelines with CACS findings in 38 men with SCI revealed considerable disagreement over the determination of eligibility for lipid treatment.52 Currently, the SCI-RRTC is in the final phase of a study that also examines the relationship between lipid profiles in persons with SCI and imaging surrogates of CVD burden: CACS, CIMT, and coronary CT angiography.
Limitations
Although CVD risk is a significant factor in morbidity and mortality after SCI, it is not the sole factor. Better lipid screening protocols and more accurate BMI calculations may reduce CVD risk, but their impact on other risks after SCI, such as pressure ulcer development, is equivocal. Very low (as well as very high) BMI is associated with increased incidence of pressure ulcers.53 CVD risk management after SCI, therefore, must be undertaken within a holistic program of prevention.
Conclusions
Current research on cardiometabolic risk in the SCI population underscores the need for early and routine monitoring for major CMR factors, among which dyslipidemia and overweight/ obesity figure prominently. Although lipid risk stratification and treatment are not clearly defined for the SCI population, there needs to be an increased awareness of lipid abnormalities that may be intrinsic to SCI, such as isolated low HDL-Other populations with risk factors for CVD (eg, diabetes, familial hyperlipidemia, and tobacco use41,54,55; autoimmune chronic inflammatory disease, chronic kidney disease, and status post solid organ transplant47) have received increased acknowledgment with more stringent lipid panel screening at earlier ages and increased frequency. It may be of benefit to implement similar screening for the SCI population as research continues to further define how best to prevent CVD in this group of people.
Currently, clinicians must use the existing measures and guidelines for the general population to screen for overweight/obesity in persons with SCI, although research consistently demonstrates that these metrics (eg, BMI) underestimate true %BF and confound determination of actual CVD risk. More research is needed on adjusting BMI calculations for persons with SCI, taking into consideration age-, gender-, and race/ethnic-specific BMI cutoff values to assist with risk stratification. There is a need for more clinically friendly and accurate surveillance tools to allow clinicians to intervene earlier to reduce obesity-related CVD.
It is not feasible to image and measure actual disease progression (eg, coronary artery calcium scoring and carotid intima thickness as a measure of atherosclerosis; quantification of body composition, fat versus lean body mass, via DXA scan) as part of a general population prevention and treatment program. However, the existence and research use of those imaging technologies provide a means for optimizing risk assessment algorithms based on more easily implemented indirect measures, such as lipid and BMI profiles. For populations who diverge from the physiologic norm, such as persons with SCI, the ability to tailor risk assessment individually to inform prevention and treatment promises even greater impact. In an era where “patient-centeredness” is a well-recognized tenet of quality health care, the goal of 21st century health care is the realization of ever-increasing personalization in both treatment and prevention of disease to maximize the health and well-being of all individuals.
Acknowledgments
Conflicts of interest: The authors have no conflicts of interest to disclose.
Financial support/disclosures: The conference panels described in this article, as well as the research supporting the meta-analysis of lipid panels after SCI and the development of an SCI-specific BMI calculator, were supported by grant H133B090002 from the National Institute on Disability and Rehabilitation Research, US Department of Education.
Statement of ethics: The conference panels described in this article, as well as the research supporting the meta-analysis of lipid panels after SCI and the development of an SCI-specific BMI calculator, were approved by the institutional review boards of MedStar Health and the University of Miami, Miller School of Medicine.
References
- 1.DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. [DOI] [PubMed] [Google Scholar]
- 2.Whiteneck GG, Charlifue SW, Frankel HL, et al. Mortality, morbidity, and psychosocial outcomes of persons spinal cord injured more than 20 years ago. Paraplegia. 1992;30(9):617–630. [DOI] [PubMed] [Google Scholar]
- 3.Warburton D, Krassioukov A, Sproule S. Cardiovascular health and exercise rehabilitation in spinal cord injury. Top Spinal Cord Inj Rehabil. 2007;13:98–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yekutiel M, Brooks ME, Ohry A, Yarom J, Carel R. The prevalence of hypertension, ischaemic heart disease and diabetes in traumatic spinal cord injured patients and amputees. Paraplegia. 1989;27(1):58–62. [DOI] [PubMed] [Google Scholar]
- 5.Groah SL, Weitzenkamp D, Sett P, Soni B, Savic G. The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord. 2001;39(6):310–317. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert ON, Croffoot JR, Taylor AJ, Nash MS, Schomer K, Groah S. Serum lipid concentrations among persons with spinal cord injury: A systematic review and meta-analysis of the literature. Atherosclerosis. 2014;232(2):305–312. [DOI] [PubMed] [Google Scholar]
- 7.Bauman WA, Kahn NN, Grimm DR, Spungen AM. Risk factors for atherogenesis and cardiovascular autonomic function in persons with spinal cord injury. Spinal Cord. 1999;37:601–616. [DOI] [PubMed] [Google Scholar]
- 8.Groah SL, Nash MS, Ward EA, et al. Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prevent. 2011;31(2):73–80. [DOI] [PubMed] [Google Scholar]
- 9.Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47(10):757–762. [DOI] [PubMed] [Google Scholar]
- 10.Wilt TJ, Carlson KF, Goldish GD, et al. Carbohydrate and lipid disorders and relevant considerations in persons with spinal cord injury. Evid Rep Technol Assess. 2008;163:1–95. [PMC free article] [PubMed] [Google Scholar]
- 11.Nash M, Gilbert O, Kressler J, Taylor A. Cardiometabolic Panel. Presented at: The State of the Science of Prevention and Management of Secondary Health Conditions in People After Spinal Cord Injury; 2013; Chicago, Illinois. [Google Scholar]
- 12.Tinsley EA, Ballard P, Libin A. Body composition. Presented at: The State of the Science of Prevention and Management of Secondary Health Conditions in People after Spinal Cord Injury; 2013; Chicago, Illinois. [Google Scholar]
- 13.Bigaard J, Frederiksen K, Tjønneland A, et al. Body fat and fat-free mass and all-cause mortality. Obesity. 2004;12(7):1042–1049. [DOI] [PubMed] [Google Scholar]
- 14.Bauman WA, Spungen AM. Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord. 2008;46(7):466–476. [DOI] [PubMed] [Google Scholar]
- 15.Liang H, Chen D, Wang Y, Rimmer JH, Braunschweig CL. Different risk factor patterns for metabolic syndrome in men with spinal cord injury compared with able-bodied men despite similar prevalence rates. Arch Phys Med Rehabil. 2007;88(9):1198–1204. [DOI] [PubMed] [Google Scholar]
- 16.Gater DR, Clasey JL. Body composition assessment in spinal cord injury clinical trials. Top Spinal Cord Inj Rehabil. 2006;11(3):36–49. [Google Scholar]
- 17.Heymsfield SB, Lichtman S, Baumgartner RN, et al. Body composition of humans: Comparison of two improved four-compartment models that differ in expense, technical complexity, and radiation exposure. Am J Clin Nutr. 1990;52(1):52–58. [DOI] [PubMed] [Google Scholar]
- 18.Eknoyan G. Adolphe Quetelet (1796–1874)—the average man and indices of obesity. Nephrol Dial Transplant. 2008;23(1):47–51. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO consultation. Geneva: Author; 2000. [PubMed] [Google Scholar]
- 20.Taylor AE, Ebrahim S, Ben-Shlomo Y, et al. Comparison of the associations of body mass index and measures of central adiposity and fat mass with coronary heart disease, diabetes, and all-cause mortality: a study using data from 4 UK cohorts. Am J Clin Nutr. 2010;91(3):547–556. [DOI] [PubMed] [Google Scholar]
- 21.Gater DR., Jr.Obesity after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18(2):333–351, vii. [DOI] [PubMed] [Google Scholar]
- 22.Groah SL, Nash MS, Ljungberg IH, et al. Nutrient intake and body habitus after spinal cord injury: An analysis by sex and level of injury. J Spinal Cord Med. 2009;32(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laughton GE, Buchholz AC, Martin Ginis KA, et al. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47:757–762. [DOI] [PubMed] [Google Scholar]
- 24.National Heart, Lung, and Blood Institute in cooperation with The National Institute of Diabetes and Digestive and Kidney Diseases. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda, MD: National Institutes of Health;1998. [PubMed] [Google Scholar]
- 25.Libin A, Tinsley EA, Nash MS, et al. Cardiometabolic risk clustering in spinal cord injury: Results of exploratory factor analysis. Top Spinal Cord Inj Rehabil. 2013;19(3):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Agostino RBS, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation. 2008;117(6):743–753. [DOI] [PubMed] [Google Scholar]
- 27.Wierzbicki AS, Twomey PJ, Reynolds TM. Cardiovascular risk assessment. Lancet. 2005;365(9467):1305–1306. [DOI] [PubMed] [Google Scholar]
- 28.Groah SL, Libin A, Lauderdale M, Kroll T, DeJong G, Hsieh J. Beyond the evidence-based practice paradigm to achieve best practice in rehabilitation medicine: A clinical review. Phys Med Rehabil. 2009;1(10):941–950. [DOI] [PubMed] [Google Scholar]
- 29.Barnett SD, Heinemann AW, Libin A, et al. Small N designs for rehabilitation research. J Rehabil Res Dev. 2012;49(1):175–186. [DOI] [PubMed] [Google Scholar]
- 30.Bauman WA, Adkins RH, Spungen AM, Kemp BJ, Waters RL. The effect of residual neurological deficit on serum lipoproteins in individuals with chronic spinal cord injury. Spinal Cord. 1998;36(1):13–17. [DOI] [PubMed] [Google Scholar]
- 31.Demirel S, Demirel G, Tukek T, Erk O, Yilmaz H. Risk factors for coronary heart disease in patients with spinal cord injury in Turkey. Spinal Cord. 2001;39(3):134–138. [DOI] [PubMed] [Google Scholar]
- 32.Heldenberg D, Rubinstein A, Levtov O, Werbin B, Tamir I. Serum lipids and lipoprotein concentrations in young quadriplegic patients. Atherosclerosis. 1981;39(2):163–167. [DOI] [PubMed] [Google Scholar]
- 33.Liang H, Chen D, Wang Y, Rimmer JH, Braunschweig CL. Different risk factor patterns for metabolic syndrome in men with spinal cord injury compared with able-bodied men despite similar prevalence rates. Arch Phys Med Rehabil. 2007;88(9):1198–1204. [DOI] [PubMed] [Google Scholar]
- 34.Nikkila EA, Kuusi T, Myllynen P. High density lipoprotein and apolipoprotein A-i during physical inactivity. Demonstration at low levels in patients with spine fracture. Atherosclerosis. 1980;37(3):457-462. [DOI] [PubMed] [Google Scholar]
- 35.Schmid A, Halle M, Stutzle C, et al. Lipoproteins and free plasma catecholamines in spinal cord injured men with different injury levels. Clin Physiol. 2000;20(4):304–310. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y-H, Chen S-Y, Wang T-D, Hwang B-S, Huang T-S, Su T-C. The relationships among serum glucose, albumin concentrations and carotid atherosclerosis in men with spinal cord injury. Atherosclerosis. 2009;206(2):528–534. [DOI] [PubMed] [Google Scholar]
- 37.Zlotolow SP, Levy E, Bauman WA. The serum lipoprotein profile in veterans with paraplegia: The relationship to nutritional factors and body mass index. J Am Paraplegia Soc. 1992;15(3):158–162. [DOI] [PubMed] [Google Scholar]
- 38.Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emerging Risk Factors Collaboration; Angelantonio ED, Sarwar N, Perry P. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewington S, Whitlock G, Clarke R, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 12007;370(9602):1829–1839. [DOI] [PubMed] [Google Scholar]
- 41.Adult Treatment Panel III. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Final Report. Bethesda, MD: National Cholesterol Education Program; 2002. [PubMed] [Google Scholar]
- 42.National Cholesterol Education Program. Second report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel II). Circulation. 1994;1333–1445. [DOI] [PubMed] [Google Scholar]
- 43.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998:1837–1847. [DOI] [PubMed] [Google Scholar]
- 44.International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. Brussels, Belgium: Author; 2006. [Google Scholar]
- 45.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15. [DOI] [PubMed] [Google Scholar]
- 46.Austin MA, King MC, Vranizan KM, Krauss RM. Atherogenic lipoprotein phenotype. A proposed genetic marker for coronary heart disease risk. Circulation. 1990;82(2):495–506. [DOI] [PubMed] [Google Scholar]
- 47.Catapano AL, Reiner Z, De Backer G, et al. ESC/EAS guidelines for the management of dyslipidaemias; The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Atherosclerosis. 2011;217(1):3–46. [DOI] [PubMed] [Google Scholar]
- 48.Huxley RR, Barzi F, Lam TH, et al. Isolated low levels of high-density lipoprotein cholesterol are associated with an increased risk of coronary heart disease: An individual participant data meta-analysis of 23 studies in the Asia-Pacific region. Circulation. 2011;124(19):2056–2064. [DOI] [PubMed] [Google Scholar]
- 49.Cohn JN. Introduction to surrogate markers. Circulation. 2004;109(25 Suppl 1): IV20–21. [DOI] [PubMed] [Google Scholar]
- 50.Cohn JN, Quyyumi AA, Hollenberg NK, Jamerson KA. Surrogate markers for cardiovascular disease: Functional markers. Circulation. 2004;109(25 Suppl 1): IV31–46. [DOI] [PubMed] [Google Scholar]
- 51.Greenland P, Alpert JS, Beller GA, et al. 2010ACCF/ AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):50–103. [DOI] [PubMed] [Google Scholar]
- 52.Lieberman JA, Hammond FM, Barringer TA, et al. Comparison of coronary artery calcification scores and National Cholesterol Education program guidelines for coronary heart disease risk assessment and treatment paradigms in individuals with chronic traumatic spinal cord injury. J Spinal Cord Med. 2011;34(2):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.VanGilder C, MacFarlane G, Meyer S, Lachenbruch C. Body mass index, weight, and pressure ulcer prevalence: An analysis of the 2006-2007 International Pressure Ulcer Prevalence Surveys. J Nurs Care Qual. 2009;24(2):127–135. [DOI] [PubMed] [Google Scholar]
- 54.Berg AO, Allan JD. Introducing the third US Preventive Services Task Force. Am J Prev Med. 2001;20(3 Suppl):3–4. [DOI] [PubMed] [Google Scholar]
- 55.Ravnskov U. American College of Physicians guidelines on cholesterol screening. Ann Intern Med. 1996;125(12):1010–1011. [DOI] [PubMed] [Google Scholar]