Abstract
Objective:
To introduce allostatic load (AL) as a framework for measuring stress-related outcomes after spinal cord injury (SCI) by identifying the number and nature of biomarkers investigated in existing studies and by generating preliminary data on AL in 30 persons with traumatic SCI.
Methods:
This systematic review and pilot study were conducted at a medical university in the southeastern United States. A review of literature published between 1993 and 2012 identified studies using 2 or more of 5 classes of AL biomarkers. We then collected data on 11 biomarkers (n = 30) from self-selected participants using physical exams and blood and urine specimen collection. These included waist to hip ratio, systolic and diastolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, dihydroepiandrosterone, glycosylated hemoglobin, C-reactive protein, interleukin-6, and cortisol, norepinephrine, and epinephrine normalized by 12-hour creatinine.
Results:
We were unable to identify any studies investigating AL biomarkers from each of the 5 areas or any studies specifically proposing to investigate AL. AL scores were relatively low, with metabolic indicators being the most elevated and neuroendocrine the least elevated.
Conclusions:
AL is a promising, yet underutilized, construct that may be feasibly assessed after SCI.
Key words: allostasis, allostatic load, spinal cord injury, stress
Traumatic spinal cord injury (SCI) typically results in permanent disability and increased risk of health complications and early mortality. In the United States, the primary causes of SCI are motor vehicle crashes, falls, acts of violence, sports, and other unknown etiology.1 After traumatic SCI, individuals face significant physiological and psychological adjustments.2,3 Their response to these and other stressors is influenced greatly by the condition of their body (ie, severity of injury, physical conditioning, presence of comorbidities or risk factors for disease, etc) and how they perceive and interpret the situation (ie, coping styles).4
The traumatic, sudden nature of SCI and the resulting long-term increased vulnerability to secondary health conditions5 suggest the appropriateness of evaluating both physiological and psychological stress paradigms among persons with SCI. Some research has suggested that SCI is associated with posttraumatic stress disorder (PTSD),6–9 although other research has suggested that PTSD rarely occurs in the absence of a depressive disorder.10 A number of studies have examined psychological adjustment to SCI,2,3,11 but further research regarding physiological responses to injury and stressors is warranted.
Two concepts, allostasis and allostatic load (AL), relate to the physiological adaptation to stress and associated costs on the body and brain.4 Allostasis refers to the dynamic regulatory process by which stability is maintained through changes in physiologic systems including autonomic, central nervous, neuroendocrine, cardiovascular, metabolic, and immune systems.12 AL is a measure of the “wear and tear” experienced after chronic allostatic responses to stressful situations; it is the price of adaptation.13 AL may result from recurrent stress and subsequent activation of allostatic systems, failure to shut down the allostatic activity following a stressor, or an inadequate response of an allostatic system ultimately leading to elevated activity of another system.4 The AL model,14 a biologic theory of stress, proposes that the stress response is influenced by a number of factors, including life experiences, genetics, and behavior. Over time, the accumulation of AL can have systemwide adverse effects, contributing to morbidity and mortality.14–17
Measurement of AL was initially operation-alized to reflect levels of physiologic activity across the hypothalamic-pituitary-adrenal axis, the sympathetic nervous system, the cardiovascular system, and metabolic processes through a set of 10 biomarkers, each having been previously linked to increased risk for pathology.4,15 A more diverse and expanded set of biomarkers have since been grouped by (1) anthropometric measures and (2) cardiovascular and respiratory, (3) metabolic, (4) neuroendocrine, and (5) immune system biomarkers.18 In the original index, immune biomarkers were not assessed. A review by Juster et al18 lists 25 biomarkers commonly assessed in AL studies. The number of markers measured in any particular study ranges from 4 to 17.18 Numerous algorithms, formulas, and statistical techniques have been implemented to quantify, or score, AL based on the biomarkers collected. Each biomarker contributes to an overall risk score defined by a critical threshold or cutoff point, whereas the biomarker only counts for the overall score if it is outside the critical threshold.
It is possible that specific stressors associated with long-term injury, in combination with daily life stressors, may make persons with SCI more susceptible to high levels of AL. In studies assessing biomarkers in persons with SCI, there has not been consistency in which biomarkers were assessed or in the cutpoints for some biomarkers. As persons with SCI are at increased risk of secondary conditions and early mortality due to their injury, increased AL is an important concept that could result in even further negative health consequences due to the cumulative wear and tear on body systems and secondary health conditions.
Summary and Purpose
We were unable to identify any studies that explicitly utilized the construct of AL to organize outcome measures in studies of SCI. Therefore, we performed a systematic review to identify studies measuring 2 or more of the 5 categories of biomarkers used to measure AL, so as to identify studies that implicitly measure components of AL. By identifying the biomarkers most widely used in SCI research, we have highlighted gaps in the literature related to the most widely used AL parameters. (It is beyond the scope of this article to review specific findings.)
Our secondary purpose was to generate preliminary data from 30 participants using 11 AL parameters, providing preliminary data on relative frequency of each indicator. This may help to guide future research establishing quartile scores that may be used as potential cutpoints, parameters for power analyses, and selection of specific measures.
Stage I: Systematic Review
A systematic review of literature was conducted using the following databases: PubMed and Cumulative Index to Nursing and Allied Health Literature Plus (CINAHL Plus) through EBSCOhost. The search strategy paired MeSH terms (“spinal cord injuries”) and text words (“cross-sectional studies”) with each of the biomarkers previously indicated in AL literature. The original 10 biomarkers of AL included waist to hip ratio (WHR), systolic and diastolic blood pressure (SBP, DBP), dihydroepiandrosterone sulfate (DHEA-s), cortisol, norepinephrine (NE), epinephrine (Epi), high-density lipoprotein (HDL) cholesterol, total cholesterol to HDL ratio, and glycosylated hemoglobin (HbA1c).15 Six additional biomarkers, including fibrinogen, interleukin-6 (IL-6), C-reactive protein (CRP), albumin, creatinine clearance, and peak respiratory flow, were added to form an expanded set, which provided a more inclusive evaluation of biological dysregulation.19 Additional biomarkers have since been assessed. Our literature review focused on the 25 biomarkers repeatedly used in AL studies, as presented by Juster et al.18
Cross-sectional studies published between 1993 (the year that the term allostatic load was coined) and 2012 were included if they met the following characteristics: participants were adults (>18 years) with chronic (>1 year) traumatic SCI and the study assessed at least 1 biomarker of AL from at least 2 groups (anthropometric, cardiovascular and respiratory,metabolic,immune, or neuroendocrine). Case reports, case series, and studies other than cross-sectional analyses and studies published in languages other than English were excluded. Animal studies were also excluded.
The initial selection excluded obviously unrelated articles retrieved by the searches based on the title alone. The excluded studies were reviewed to ensure no potentially appropriate studies were inadvertently removed. The titles and abstracts of selected studies were then further examined for pertinent information. The references of selected articles and previously published systematic reviews were scanned for applicable articles. Thereafter the articles were evaluated for inclusion and exclusion criteria.
Data were extracted from the selected articles into an electronic data collection form developed for this review that includes information on the study, participants, and outcomes.
Search Results
The PubMed search returned 92 articles, and CINAHL returned 52. Following the selection procedure, we identified 35 studies measuring biomarkers of AL from at least 2 of the 5 groups in individuals with chronic traumatic SCI (Table 1). On average, the studies measured 8 biomarkers (range, 2-13) and 3 groups (range, 2-4).
Table 1. Studies meeting inclusion criteria for assessing individual biomarkers of allostatic load (AL) in SCI populations.
| Author, year | N | Anthropometric | CV and respiratory | Immune | Metabolic | Neuroendocrine | Total AL markers |
|---|---|---|---|---|---|---|---|
| Zhong, 1995 | 197 | 1 | 3 | ||||
| Janssen, 1997 | 37 | 1 | 2 | 8 | |||
| Huang, 2000 | 47 | 1 | 1 | 2 | |||
| Kemp, 2000 | 188 | 1 | 6 | ||||
| Manns, 2005 | 22 | 1 | 1 | 2 | 6 | 10 | |
| Lee, 2006 | 93 | 1 | 1 | 7 | 9 | ||
| Lee, 2006 | 168 | 2 | 2 | 4 | |||
| Bauman, 2007 | 224 | 1 | 2 | 7 | 10 | ||
| Liang, 2007 | 185 | 1 | 2 | 5 | 8 | ||
| Nash, 2007 | 41 | 1 | 2 | 6 | 9 | ||
| Wang, 2007 | 62 | 1 | 2 | 9 | 12 | ||
| Edwards, 2008 | 31 | 1 | 1 | 7 | 9 | ||
| Gibson, 2008 | 69 | 2 | 2 | 1 | 7 | 12 | |
| Huang, 2008 | 42 | 1 | 2 | 3 | |||
| Finnie, 2008 | 75 | 2 | 2 | 1 | 7 | 12 | |
| Liang, 2008 | 131 | 1 | 2 | 1 | 3 | 7 | |
| Liang, 2008 | 129 | 1 | 1 | 2 | |||
| Morse, 2008 | 63 | 1 | 2 | 1 | 4 | ||
| Buchholz, 2009 | 76 | 2 | 2 | 1 | 7 | 12 | |
| Hetz, 2009 | 75 | 1 | 5 | 6 | |||
| Laughton, 2009 | 77 | 1 | 1 | 2 | |||
| Wang, 2009 | 110 | 2 | 2 | 1 | 8 | 13 | |
| Matos, 2010 | 65 | 1 | 2 | 3 | 5 | 11 | |
| Gorgey, 2010 | 10 | 2 | 7 | 9 | |||
| Garshick, 2011 | 59 | 1 | 1 | 2 | 4 | ||
| Gorgey, 2011 | 39 | 1 | 7 | 8 | |||
| Gorgey, 2011 | 13 | 1 | 7 | 8 | |||
| Gorgey, 2011 | 13 | 1 | 7 | 8 | |||
| Groah, 2011 | 121 | 1 | 2 | 8 | 11 | ||
| Groah, 2011 | 125 | 1 | 2 | 2 | 7 | 12 | |
| La Favor, 2011 | 14 | 2 | 2 | 1 | 5 | 10 | |
| Lieberman, 2011 | 38 | 2 | 5 | 7 | |||
| Lieberman, 2011 | 38 | 2 | 5 | 7 | |||
| Wahman, 2011 | 1 | 2 | 3 | 6 | |||
| Matos, 2011 | 34 | 1 | 3 | 3 | 6 | 13 | |
| Krause, 2008 | 30 | 1 | 2 | 2 | 3 | 3 | 11 |
Note: See the supplementary digital content for a more comprehensive table (Table A1) (doi: 10.1310/sci2002-137).
The majority of the studies examined metabolic biomarkers, most often in relation to cardiovascular disease20–36 or metabolic syndrome risk,37–39 but also in association to health outcomes or other biomarker levels.40–42 Immune biomarkers, most commonly CRP, were included in the studies that assessed cardiovascular disease risk, or they were measured as markers of inflammation and associations with other AL biomarkers that were examined.43–46 Studies gathering anthropometric measures of adiposity in association with other AL biomarkers were also common, although waist circumference was often the measure reported rather than the original AL biomarker WHR.47–54 Only one study measured a neuroendocrine biomarker of AL in individuals with chronic, traumatic SCI.47 Investigators have historically focused on examining indicators of health after SCI using either a single biomarker or multiple measures attributed to a specific health outcome (such as bone density, body composition, or cardiovascular risk).
Stage II: Pilot study
Participants
Institutional review board approval was obtained prior to data collection. Participants were 30 self-selected volunteers identified through the South Carolina SCI Association. Inclusion criteria were (1) participant age of at least 18 years old, (2) traumatic SCI with residual impairment, (3) minimum of 2 years post SCI, and (4) ability to travel to the data collection site and participate in the data collection activities.
Procedures
Data were collected over 2 days at a medical university in the southeastern United States. Participants received $250 in remuneration. This included the expense of traveling for the data collection. Eight of the participants required overnight stays, and accommodations were made locally at no cost to the participants. Demographic and injury-specific data were collected on day 1, and participants were provided with the equipment and instructions needed to complete an overnight 12-hour urine collection. They were instructed to begin the urine collection on the evening before their second visit and to bring the urine specimen with them on day 2. The following tests were conducted on day 2 (Table 2): (1) SBP and DBP,55 WHR,56 (3) blood specimen collection via standard phlebotomy techniques, and (4) urine specimen. We processed the blood for fasting total serum cholesterol, HDL, DHEA, and blood HbA1c, CRP, and IL-6. We processed urinary excretion of cortisol, NE, and Epi, normalized by 12-hour creatinine excretion to adjust for body size and renal function.
Table 2. Biomarkers, cutpoint reference values, and the sources of data.
| Biomarkers | Reference value | Data source | ||
|---|---|---|---|---|
| Traditional AL | Anthropometric | High WHR | ≥0.94 Hg | Physical exam |
| CV and respiratory | High SBP | ≥148 mm | Physical exam | |
| High DBP | ≥83 mm Hg | Physical exam | ||
| Neuroendocrine | High Epi | ≥4.99 μg/g creatinine | Urine | |
| High NE | ≥48 μg/g creatinine | Urine | ||
| High cortisol | ≥25.69 μg/g creatinine | Urine | ||
| Low DHEAa | NA | Serum | ||
| Low DHEA-sa | ≤350 ng/mL | Serum | ||
| Metabolic | High HbA1c | ≥7.10 | Blood | |
| Low HDL | ≤37 mg/dL | Serum | ||
| High total cholesterol to HDL ratio | ≥5.92 | Serum | ||
| Expanded | Immune | High CRP | ≥3.19 μg/mL | Blood |
| High IL-6 | ≥4.64 pg/mL | Blood |
Note: CRP = C-reactive protein; CV = cardiovascular; DBP = diastolic blood pressure; DHEA-s = dihydroepiandrosterone sulfate; Epi = epinephrine; HbA1c = glycosylated hemoglobin; HDL = high-density lipoprotein; IL-6 = interleukin-6; NE = norepinephrine; SBP = systolic blood pressure; WHR = waist to hip ratio.
DHEA-s is the sulfated metabolite of DHEA. Stress affects both DHEA and DHEA-s levels, but DHEA-s is typically collected rather than DHEA. We collected DHEA, not DHEA-s, and thus cannot compare to previous allostatic load (AL) reference values.
Waist to hip circumference was measured by procedures outlined in the 1988 Anthropometric Standardization Reference Manual.57 Waist circumference was measured at the narrowest point between the ribs and the iliac crest, and the hip circumference was measured at the maximal site around the buttocks. Measurements were taken while the participant was lying down. Blood pressure was measured using the Hypertension Detection and Follow-up Program protocol.58 Three seated blood pressure readings were completed, and average systolic and diastolic blood pressures were computed from the second and third readings.
Analysis
We used a total of 11 biomarkers, including 9 of the 10 original markers (all except DHEA-s) and 2 relevant immune biomarkers (CRP, IL-6). The addition of these 2 markers follows the later development of the McArthur studies of successful aging and the conclusion that “operationalization of the concept of AL was designed to summarize levels of physiological activity across a range of regulatory systems pertinent to disease risks” and the addition of other markers provides “more comprehensive assessment of cumulative biological dysregulation.”59(1987) As this study was to generate preliminary data of each of the 5 classes of AL markers, all analyses were descriptive in nature with no attempt to statistically compare AL scores as a function of participant characteristics. Analyses were completed using IBM SPSS software (IBM, Armonk, NY). We calculated overall AL scores, based on traditional cutoff scores reported in the literature, as the criterion cutpoints were taken directly from those listed by Seeman et al.59 AL was calculated as the sum of indicators where the participant scored above the reference cutpoint outlined in Table 2. We also identified the portion of individuals meeting the criteria for each marker (ie, outside the reference value). Quartile scores are reported so that alternative cutoff scores may be considered in future research.
Results
All 30 participants were successfully enrolled, and all appropriate measures were obtained (eg, no cases lacked blood draw). Eighty percent of the participants were male. Sixty percent were non-Hispanic White, 36.7% were non-Hispanic Black, and 3.3% Hispanic. Participants were an average of 45.7 ± 12.5 years of age at time of data collection and 13.8 ± 8.8 years post injury. Because participants were self-selected volunteers from the community, with no hospital records or neurologic assessment to establish the ASIA Impairment Scale (AIS),60 we characterized SCI severity using self-report methods. Characterizing SCI severity, 43.3% had cervical injuries with no voluntary, functional movement below the level of injury; 30.0% had noncervical, nonfunctional injuries; and 26.7% had functional movement below the level of injury (self-report proxy for the AIS developed and reported in previous studies).61,62
Overall AL scores were relatively low, with a mean score of 2.03 (range, 0-5). Figure 1 summarizes the distribution of scores. Table 3 summarizes AL scores as a function of demographic and injury characteristics for descriptive purposes. The biomarkers with the greatest number of individuals outside the reference values were low HDL (66.7%), WHR (56.7%), and IL-6 (26.7%) (Table 4). WHR was the only anthropometric measure collected. Each of the 3 metabolic biomarkers had more than 10% of the participants above the cutoff. Conversely, less than 10% of the participants scored above the criterion value on the remaining 6 parameters, with no participant exceeding the cutoff for high diastolic blood pressure. This included both biomarkers from the cardiovascular and respiratory group and all 3 neuroendocrine measures.
Figure 1. Preliminary data with 30 participants and 5 classes of biomarkers.
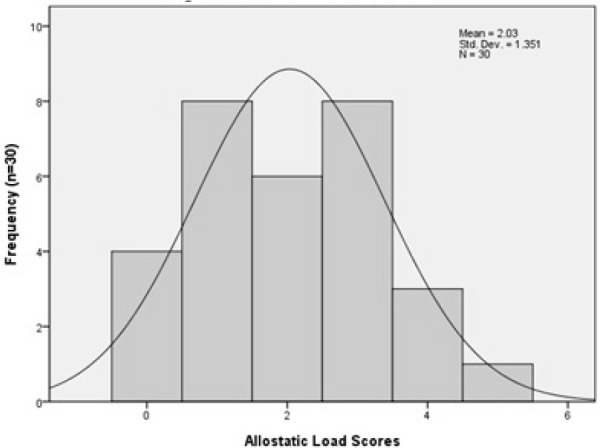
Table 3. Allostatic load by participant characteristics.
| Characteristic | Median | Mean | SD | % AL >0 |
|---|---|---|---|---|
| SCI severity | ||||
| Cervical, Non-F (n=13)* | 3.0 | 2.54 | 1.51 | 84.6 |
| Non-C, Non-F (n=9) | 2.0 | 1.89 | 1.27 | 88.9 |
| Functional movement (n=8) | 1.0 | 1.38 | 0.92 | 75.0 |
| Age, years | ||||
| 18-29 (n=8) | 2.5 | 1.88 | 1.36 | 75.0 |
| 30-44 (n=11) | 1.0 | 1.64 | 1.03 | 81.8 |
| 45+ (n=11) | 2.0 | 2.55 | 1.57 | 90.9 |
| Years post injury | ||||
| 1-10 (n=12) | 2.5 | 1.92 | 1.44 | 75.0 |
| 11-15 (n=9) | 1.0 | 1.44 | 0.73 | 88.9 |
| 16+ (n=9) | 3.0 | 2.78 | 1.48 | 88.9 |
Note: AL = allostatic load; Non-C = noncervical; Non-F = nonfunctional.
Table 4. Descriptive statistics of individual biomarkers.
| Biomarker | Reference value | Mean (SD) | 25th quartile | 50th quartile | 75th quartile | % outside reference | |
|---|---|---|---|---|---|---|---|
| Anthropometric | WHR | ≥ 0.94 | 0.96 (0.13) | 0.86 | 0.96 | 1.03 | 56.7 |
| CV and respiratory | SBP | ≥148 mm Hg | 112.06 (22.29) | 98.67 | 108.00 | 125.25 | 6.7 |
| DBP | ≥ 83 mm Hg | 46.64 (6.48) | 42.00 | 47.17 | 50.75 | 0 | |
| Neuroendocrine | Epi | ≥4.99 μg/g creatinine | 3.13 (0.73) | 3.00 | 3.00 | 3.00 | 3.3 |
| NE | ≥48 μg/g creatinine | 15.23 (14.71) | 10.00 | 10.00 | 14.25 | 3.3 | |
| Cortisol | ≥25.69 μg/g creatinine | 10.18 (8.73) | 5.33 | 7.06 | 11.90 | 3.3 | |
| Metabolic | HbA1C | ≥7.10 % | 5.47 (1.08) | 4.95 | 5.20 | 5.43 | 13.3 |
| HDL | ≤37 mg/dL | 33.73 (8.40) | 28.75 | 31.00 | 40.00 | 66.7 | |
| Total to HDL | ≥5.92 | 4.87 (1.14) | 4.17 | 4.71 | 5.42 | 16.7 | |
| cholesterol ratio | |||||||
| Immuene | CRP | ≥3.19 μg/mL | 1.36 (3.06) | 0.25 | 0.49 | 1.07 | 6.7 |
| IL-6 | ≥4.64 pg/mL | 5.18 (6.71) | 1.66 | 2.70 | 6.47 | 26.7 |
Note: CRP = C-reactive protein; CV = cardiovascular; DBP = diastolic blood pressure; DHEA-s = dihydroepiandrosterone sulfate; Epi = epinephrine; HbA1c = glycosylated hemoglobin; HDL = high-density lipoprotein; IL-6 = interleukin-6; NE = norepinephrine; SBP = systolic blood pressure; WHR = waist to hip ratio.
Discussion
We were unable to find any studies that explicitly applied the AL framework with chronic traumatic SCI, and no studies measured biomarkers within each of the 5 general groups. There were, however, 10 studies that utilized at least 4 of the 5 types of biomarkers, most of which used relatively small sample sizes. Neuroendrocrine measures were the least represented followed by immune measures. Therefore, although the AL paradigm has not been explicitly used after SCI, there is potential for applying the framework in multiple studies. The absence of research with AL (physiologic stress framework) is in contrast with a relatively significant number of studies of PTSD,6,10 which measure psychological response to stress using criteria from the Diagnostic and Statistical Manual of Mental Disorders.63
This is the first study to utilize the AL framework and report at least one measure of each of the 5 outcome indicators. Perhaps not surprising given the self-selected nature of the participant sample, the results indicated relatively low levels of AL, with substantial variation between differing indicators. WHR, the single anthropometric measure, appeared to be most elevated among the participants, although the use of WHR has been questioned in studies of persons with SCI and is not likely the best indicator of body composition with SCI.64 Metabolic indicators were the most consistently elevated, showing promise for future AL studies, whereas neuroendocrine measures were the least highly elevated.
Limitations
This study is limited to preliminary data that may help to identify alternative cutpoints for future research. The small sample size precluded statistical comparisons of AL function of demographic and injury characteristics, even though there were trends in the descriptive data. Therefore, no conclusions may be derived regarding AL levels compared with the general population from the current data, although the basis has been established for doing larger scale follow-up studies. There may be inherent aspects of SCI that may invalidate particular AL indicators with SCI. Well-established issues with the autonomic nervous system65,66 may lead to low prevalence of a particular indicator, such as high diastolic blood pressure (no cases were identified in the current study). Also, SCI is associated with a pattern of secondary conditions that could affect some of the measures. However, this may affect the mechanism by which SCI is associated with a pattern of elevated biomarkers.
Future research
Future research will require larger samples, preferably using population-based cohorts that minimize selection bias based on health or access to treatment. Utilization of a broader number of biomarkers and evaluation of the sensitivity of measures to SCI would help to better quantify AL. Integration of self-report, diagnostic-based indicators (ie, PTSD) and AL biomarkers would provide significant triangulation of methods addressing different types of stress-related conditions. The ultimate utility of stress-based measures would be in their ability to predict future occurrences of secondary health conditions and global health, so that measurement of stress indicators may be used to develop early interventions to improve health and reduce morbidity and excess mortality after SCI.
Conclusion
AL is a promising construct that may feasibly be assessed after SCI with collection of biomarkers. Metabolic indicators appear to be the most significantly elevated among the 5 groups of AL indicators among those with SCI.
Acknowledgments
The contents of this publication were developed under a grant from the US Department of Education, NIDRR grant number H133B090005. However, those contents do not necessarily represent the policy of the Department of Education, and endorsement by the Federal Government should not be assumed.
The authors certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research. The authors declare no conflicts of interest.
References
- 1.Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2012;35(4):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig AR, Hancock KM, Dickson H, Martin J, Chang E. Psychological consequences of spinal injury: A review of the literature. Aust N Z J Psychiatry. 1990;24(3):418–425. [DOI] [PubMed] [Google Scholar]
- 3.Galvin LR GH. The impact of coping on emotional adjustment to spinal cord injury (SCI): Review of the literature and application of a stress appraisal and coping formulation. Spinal Cord. 2001;39(12):615–627. [DOI] [PubMed] [Google Scholar]
- 4.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MP, Molton IR, Groah SL, et al. Secondary health conditions in individuals aging with SCI: Terminology, concepts and analytic approaches. Spinal Cord. 2012;50(5):373–378. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy P, Duff J. Post traumatic stress disorder and spinal cord injuries. Spinal Cord. 2001;39(1):1–10. [DOI] [PubMed] [Google Scholar]
- 7.Hatcher MB, Whitaker C, Karl A. What predicts posttraumatic stress following spinal cord injury? Br J Health Psychol. 2009;14 (Pt 3):541–561. [DOI] [PubMed] [Google Scholar]
- 8.Schonenberg M, Reimitz M, Jusyte A, Maier D, Badke A, Hautzinger M., Depression posttraumatic stress, and risk factors following spinal cord injury [published online ahead of print November 24, 2012]. Int J Behav Med. [DOI] [PubMed] [Google Scholar]
- 9.Post MW, van Leeuwen CM. Psychosocial issues in spinal cord injury: A review. Spinal Cord. 2012;50(5):382–389. [DOI] [PubMed] [Google Scholar]
- 10.Krause JS, Saunders LL, Newman S. Posttraumatic stress disorder and spinal cord injury. Arch Phys Med Rehabil. 2010;91(8):1182–1187. [DOI] [PubMed] [Google Scholar]
- 11.Chevalier Z, Kennedy P, Sherlock O. Spinal cord injury, coping and psychological adjustment: A literature review. Spinal Cord. 2009;47(11):778–782. [DOI] [PubMed] [Google Scholar]
- 12.Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, eds. Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988:629–649. [Google Scholar]
- 13.McEwen BS, Stellar E. Stress and the individual; Mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- 14.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. [DOI] [PubMed] [Google Scholar]
- 15.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation–allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med. 1997;157(19):2259–2268. [PubMed] [Google Scholar]
- 16.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA. 2001;98(8):4770–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosom Med. 2006;68(3):500–507. [DOI] [PubMed] [Google Scholar]
- 18.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. [DOI] [PubMed] [Google Scholar]
- 19.Seeman TE, Crimmins E, Huang MH, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med. 2004;58(10):1985–1997. [DOI] [PubMed] [Google Scholar]
- 20.Janssen TW, van Oers CA, van Kamp GJ, TenVoorde BJ, van der Woude LH, Hollander AP. Coronary heart disease risk indicators, aerobic power, and physical activity in men with spinal cord injuries. Arch Phys Med Rehabil. 1997;78(7):697–705. [DOI] [PubMed] [Google Scholar]
- 21.Bauman WA, Spungen AM. Risk assessment for coronary heart disease in a veteran population with spinal cord injury. Top Spinal Cord Inj Rehabil. 2007;12(4):35–53. [Google Scholar]
- 22.Nash MS, Mendez AJ. A guideline-driven assessment of need for cardiovascular disease risk intervention in persons with chronic paraplegia. Arch Phys Med Rehabil. 2007;88(6):751–757. [DOI] [PubMed] [Google Scholar]
- 23.Wang TD, Wang YH, Huang TS, Su TC, Pan SL, Chen SY. Circulating levels of markers of inflammation and endothelial activation are increased in men with chronic spinal cord injury. J Formos Med Assoc. 2007;106(11):919–928. [DOI] [PubMed] [Google Scholar]
- 24.Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr. 2008;87(3):600–607. [DOI] [PubMed] [Google Scholar]
- 25.Gibson AE, Buchholz AC, Martin Ginis KA. C-Reactive protein in adults with chronic spinal cord injury: Increased chronic inflammation in tetraplegia vs paraplegia. Spinal Cord. 2008;46(9):616–621. [DOI] [PubMed] [Google Scholar]
- 26.Finnie AK, Buchholz AC, Ginis KAM. Current coronary heart disease risk assessment tools may underestimate risk in community-dwelling persons with chronic spinal cord injury. Spinal Cord. 2008;46(9):608–615. [DOI] [PubMed] [Google Scholar]
- 27.Buchholz AC, Martin Ginis KA, Bray SR, et al. Greater daily leisure time physical activity is associated with lower chronic disease risk in adults with spinal cord injury. Appl Physiol Nutr Metab. 2009;34(4):640–647. [DOI] [PubMed] [Google Scholar]
- 28.Wang YH, Chen SY, Wang TD, Hwang BS, Huang TS, Su TC. The relationships among serum glucose, albumin concentrations and carotid atherosclerosis in men with spinal cord injury. Atherosclerosis. 2009;206(2):528–534. [DOI] [PubMed] [Google Scholar]
- 29.Matos-Souza JR, Pithon KR, Ozahata TM, et al. Subclinical atherosclerosis is related to injury level but not to inflammatory parameters in spinal cord injury subjects. Spinal Cord. 2010;48(10):740–744. [DOI] [PubMed] [Google Scholar]
- 30.Groah SL, Hosier H, Ward EA, Nash M, Libin A, Taylor AJ. Cardiometabolic risk clustering and atherosclerosis: Is there a link in spinal cord injury? Top Spinal Cord Inj Rehabil. 2011;16(3):1–13. [Google Scholar]
- 31.Groah SL, Nash MS, Ward EA, et al. Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J Cardiopulm Rehabil Prev. 2011;31(2):73–80. [DOI] [PubMed] [Google Scholar]
- 32.La Favor JD, Hollis BC, Mokshagundam SL, Olive JL. Serum hsCRP and visfatin are elevated and correlate to carotid arterial stiffness in spinal cord-injured subjects. Spinal Cord. 2011;49(9):961–966. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman JA, Hammond FM, Barringer TA, et al. Adherence with the National Cholesterol Education Program guidelines in men with chronic spinal cord injury. J Spinal Cord Med. 2011;34(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieberman JA, Hammond FM, Barringer TA, et al. Comparison of coronary artery calcification scores and National Cholesterol Education Program guidelines for coronary heart disease risk assessment and treatment paradigms in individuals with chronic traumatic spinal cord injury. J Spinal Cord Med. 2011;34(2):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matos-Souza JR, Pithon KR, Oliveira RTD, et al. Altered left ventricular diastolic function in subjects with spinal cord injury. Spinal Cord. 2011;49(1):65–69. [DOI] [PubMed] [Google Scholar]
- 36.Wahman K, Nash MS, Lewis JE, Seiger A, Levi R. Cardiovascular disease risk and the need for prevention after paraplegia determined by conventional multifactorial risk models: The Stockholm Spinal Cord Injury Study. J Rehabil Med. 2011;43(3):237–242. [DOI] [PubMed] [Google Scholar]
- 37.Manns PJ, McCubbin JA, Williams DP. Fitness, inflammation, and the metabolic syndrome in men with paraplegia. Arch Phys Med Rehabil. 2005;86(6):1176–1181. [DOI] [PubMed] [Google Scholar]
- 38.Lee MY, Myers J, Hayes A, et al. C-reactive protein, metabolic syndrome, and insulin resistance in individuals with spinal cord injury. J Spinal Cord Med. 2005;28(1):20–25. [DOI] [PubMed] [Google Scholar]
- 39.Liang H, Chen D, Wang Y, Rimmer JH, Braunschweig CL. Different risk factor patterns for metabolic syndrome in men with spinal cord injury compared with able-bodied men despite similar prevalence rates. Arch Phys Med Rehabil. 2007;88(9):1198–1204. [DOI] [PubMed] [Google Scholar]
- 40.Zhong YG, Levy E, Bauman WA. The relationships among serum uric acid, plasma insulin, and serum lipoprotein levels in subjects with spinal cord injury. Horm Metab Res. 1995;27(6):283–286. [DOI] [PubMed] [Google Scholar]
- 41.Lee MY, Myers J, Abella J, Froelicher VF, Perkash I, Kiratli BJ. Homocysteine and hypertension in persons with spinal cord injury. Spinal Cord. 2006;44(8):474–479. [DOI] [PubMed] [Google Scholar]
- 42.Hetz SP, Latimer AE, Buchholz AC, Martin Ginis KA. Increased participation in activities of daily living is associated with lower cholesterol levels in people with spinal cord injury. Arch Phys Med Rehabil. 2009;90(10):1755–1759. [DOI] [PubMed] [Google Scholar]
- 43.Huang CC, Liu CW, Weng MC, Chen TW, Huang MH. Association of C-reactive protein and insulin resistance in patients with chronic spinal cord injury. J Rehabil Med. 2008;40(10):819–822. [DOI] [PubMed] [Google Scholar]
- 44.Liang H, Mojtahedi MC, Chen D, Braunschweig CL. Elevated C-reactive protein associated with decreased high-density lipoprotein cholesterol in men with spinal cord injury. Arch Phys Med Rehabil. 2008;89(1):36–41. [DOI] [PubMed] [Google Scholar]
- 45.Morse LR, Stolzmann K, Nguyen HP, et al. Association between mobility mode and C-reactive protein levels in men with chronic spinal cord injury. Arch Phys Med Rehabil. 2008;89(4):726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garshick E, Stolzmann KL, Gagnon DR, Morse LR, Brown R. Systemic inflammation and reduced pulmonary function in chronic spinal cord injury. PM R. 2011;3(5):433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang TS, Wang YH, Chen SY. The relation of serum leptin to body mass index and to serum cortisol in men with spinal cord injury. Arch Phys Med Rehabil. 2000;81(12):1582–1586. [DOI] [PubMed] [Google Scholar]
- 48.Kemp BJ, Spungen AM, Adkins RH, Krause JS, Bauman WA. The relationships among serum lipid levels, adiposity, and depressive symptomatology in persons aging with spinal cord injury. J Spinal Cord Med. 2000;23(4):216–220. [DOI] [PubMed] [Google Scholar]
- 49.Liang H, Tomey K, Chen D, Savar NL, Rimmer JH, Braunschweig CL. Objective measures of neighborhood environment and self-reported physical activity in spinal cord injured men. Arch Phys Med Rehabil. 2008;89(8):1468–1473. [DOI] [PubMed] [Google Scholar]
- 50.Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE. Lowering Body Mass Index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47(10):757–762. [DOI] [PubMed] [Google Scholar]
- 51.Gorgey AS, Chiodo AE, Zemper ED, Hornyak JE, Rodriguez GM, Gater DR. Relationship of spasticity to soft tissue body composition and the metabolic profile in persons with chronic motor complete spinal cord injury. J Spinal Cord Med. 2010;33(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gorgey AS, Gater DR. A preliminary report on the effects of the level of spinal cord injury on the association between central adiposity and metabolic profile. PM R. 2011;3(5):440–446. [DOI] [PubMed] [Google Scholar]
- 53.Gorgey AS, Gater DR. Regional and relative adiposity patterns in relation to carbohydrate and lipid metabolism in men with spinal cord injury. Appl Physiol Nutr Metab. 2011;36(1):107–114. [DOI] [PubMed] [Google Scholar]
- 54.Eriks-Hoogland I, Hilfiker R, Baumberger M, Balk S, Stucki G, Perret C. Clinical assessment of obesity in persons with spinal cord injury: Validity of waist circumference, body mass index, and anthropometric index. J Spinal Cord Med. 2011;34(4):416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor J. The hypertension detection and follow-up program: a progress report. Circ Res. 1977;40(5 Suppl 1):I106–109. [PubMed] [Google Scholar]
- 56.Lohman T, Roche A, Martorell R. Anthropometric Standardization Reference Manual Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 57.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 58.Hypertension Detection and Follow-up Program Cooperative Group. Variability of blood pressure and results of screening in the hypertension detection and follow-up program. J Chronic Dis. 1978;31:651–667. [DOI] [PubMed] [Google Scholar]
- 59.Seeman TE, Crimmins E, Huang MH, et al. Cumulative biological risk and socio-economic differences in mortality: MacArthur Studies of successful aging. Soc Sci Med. 2004;58(10):1985–1997. [DOI] [PubMed] [Google Scholar]
- 60.Committee Membership; Burns S, Biering-Sørensen F, Donovan W, et al. International standards for neurological classification of spinal cord injury, revised 2011. Top Spinal Cord Inj Rehabil. 2012; 18(1):85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krause JS, Carter RE, Pickelsimer E, Wilson D. A prospective study of health and risk of mortality after spinal cord injury. Arch Phys Med Rehabil. 2008;89(8):1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krause JS, Carter RE, Pickelsimer E. Behavioral risk factors of mortality after spinal cord injury. Arch Phys Med Rehabil. 2009;90(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Psychiatric Association American. Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.). Washington, DC: Author; 2000. [Google Scholar]
- 64.Rajan S, McNeely MJ, Warms C, Goldstein B. Clinical assessment and management of obesity in individuals with spinal cord injury: A review. J Spinal Cord Med. 2008;31(4):361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garstang SV, Miller-Smith SA. Autonomic nervous system dysfunction after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18(2):275–296, vi-vii. [DOI] [PubMed] [Google Scholar]
- 66.Krassioukov A. Autonomic dysreflexia: current evidence related to unstable arterial blood pressure control among athletes with spinal cord injury. Clin J Sport Med. 2012;22(1):39–45. [DOI] [PubMed] [Google Scholar]


