Abstract
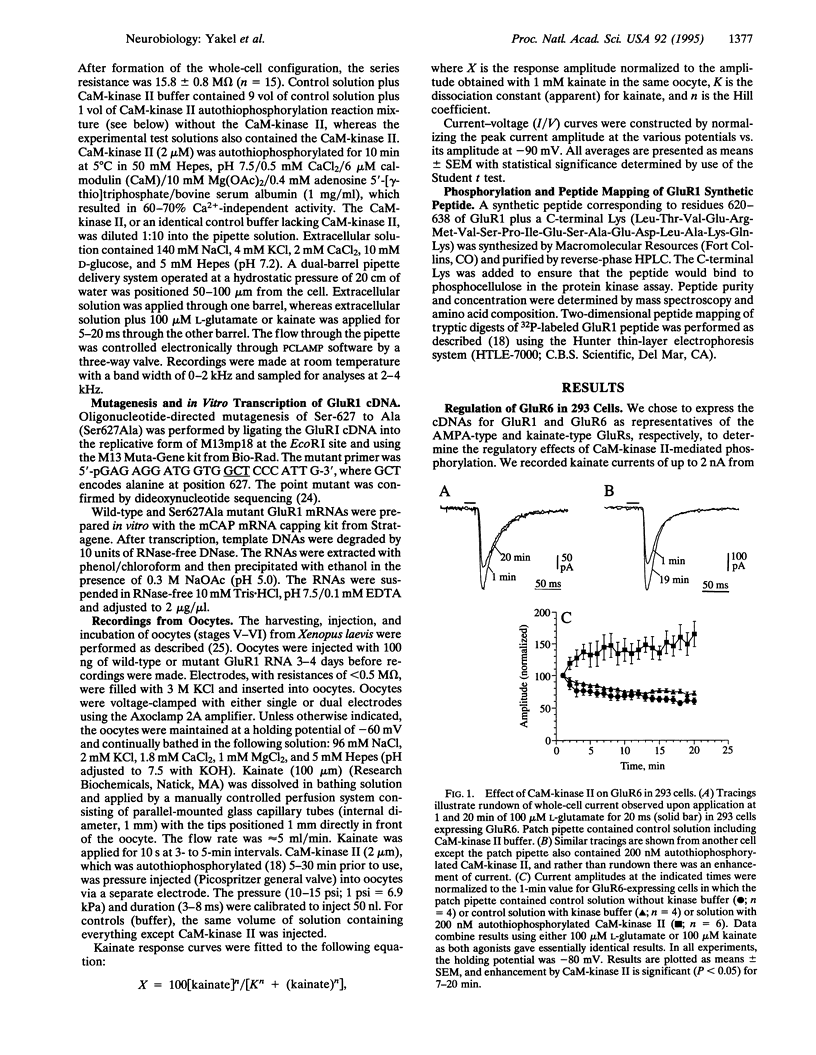
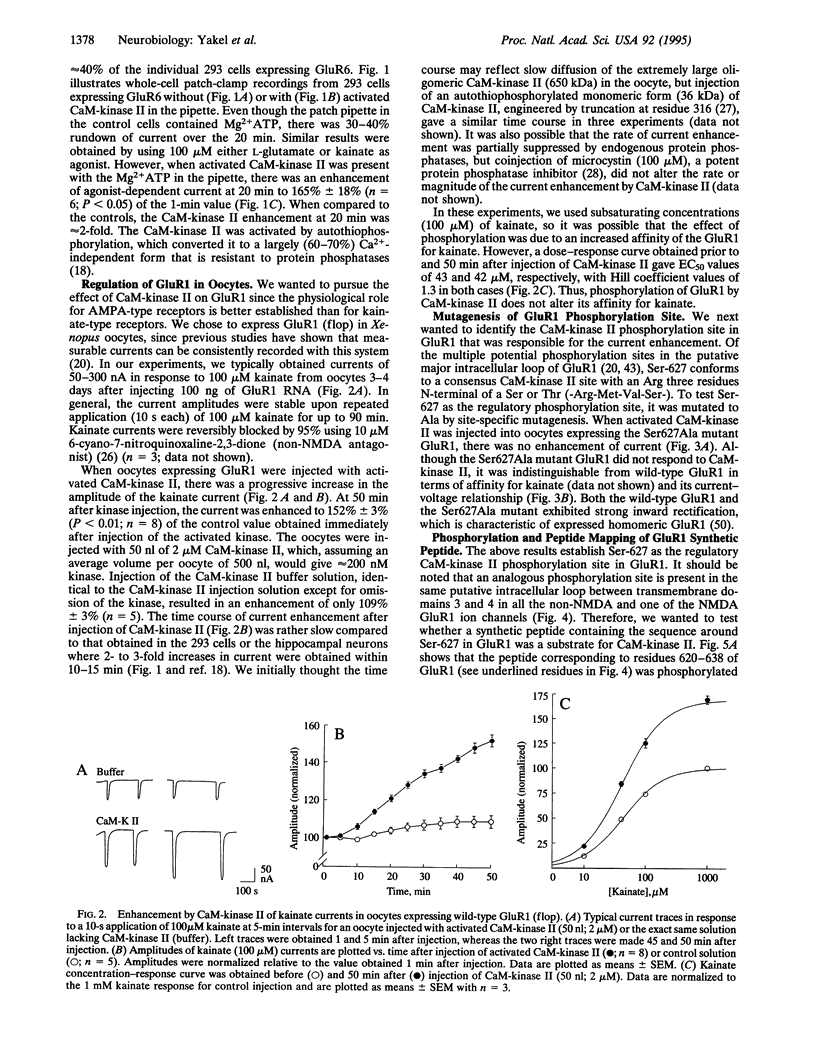
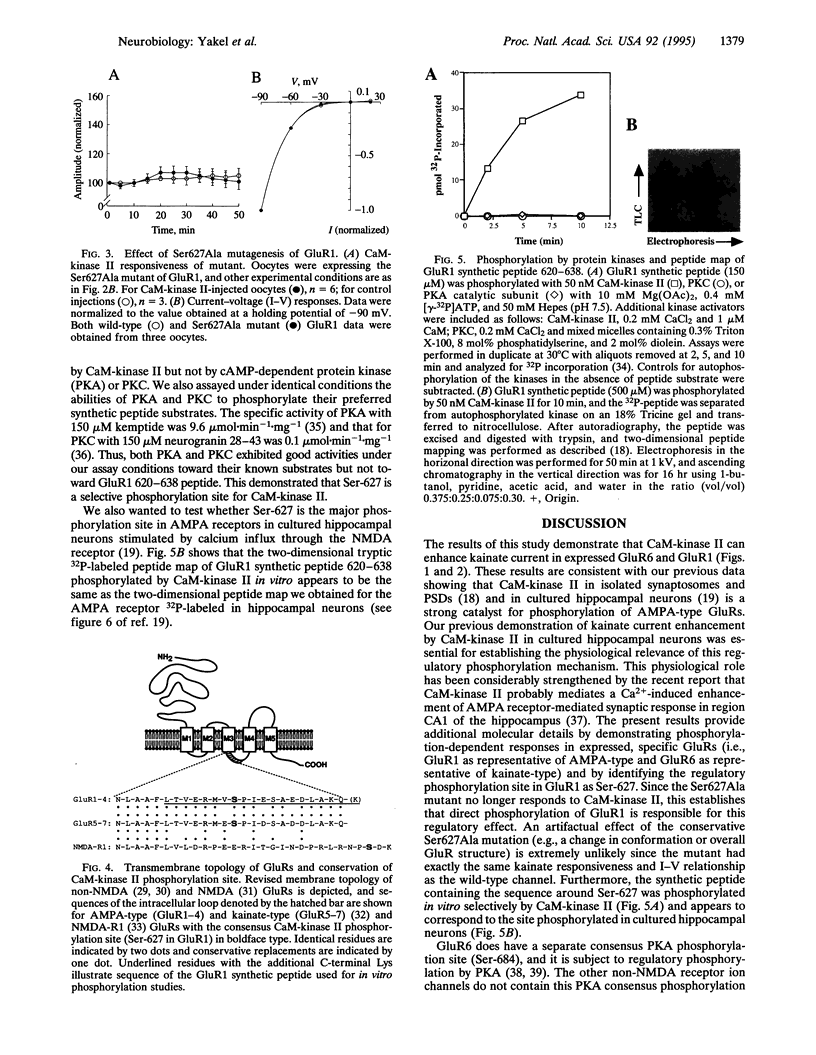
Glutamate receptor ion channels are colocalized in postsynaptic densities with Ca2+/calmodulin-dependent protein kinase II (CaM-kinase II), which can phosphorylate and strongly enhance non-N-methyl-D-aspartate (NMDA) glutamate receptor current. In this study, CaM-kinase II enhanced kainate currents of expressed glutamate receptor 6 in 293 cells and of wild-type glutamate receptor 1, but not the Ser-627 to Ala mutant, in Xenopus oocytes. A synthetic peptide corresponding to residues 620-638 in GluR1 was phosphorylated in vitro by CaM-kinase II but not by cAMP-dependent protein kinase or protein kinase C. The 32P-labeled peptide map of this synthetic peptide appears to be the same as the two-dimensional peptide map of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) glutamate receptors phosphorylated in cultured hippocampal neurons by CaM-kinase II described elsewhere. This CaM-kinase II regulatory phosphorylation site is conserved in all AMPA/kainate-type glutamate receptors, and its phosphorylation may be important in enhancing postsynaptic responsiveness as occurs during synaptic plasticity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekkers J. M., Stevens C. F. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990 Aug 23;346(6286):724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- Bettler B., Egebjerg J., Sharma G., Pecht G., Hermans-Borgmeyer I., Moll C., Stevens C. F., Heinemann S. Cloning of a putative glutamate receptor: a low affinity kainate-binding subunit. Neuron. 1992 Feb;8(2):257–265. doi: 10.1016/0896-6273(92)90292-l. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Collingridge G. L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993 Jan 7;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Boulter J., Hollmann M., O'Shea-Greenfield A., Hartley M., Deneris E., Maron C., Heinemann S. Molecular cloning and functional expression of glutamate receptor subunit genes. Science. 1990 Aug 31;249(4972):1033–1037. doi: 10.1126/science.2168579. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Huang L. Y. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992 Apr 9;356(6369):521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Chen S. J., Klann E., Gower M. C., Powell C. M., Sessoms J. S., Sweatt J. D. Studies with synthetic peptide substrates derived from the neuronal protein neurogranin reveal structural determinants of potency and selectivity for protein kinase C. Biochemistry. 1993 Feb 2;32(4):1032–1039. doi: 10.1021/bi00055a006. [DOI] [PubMed] [Google Scholar]
- Christie M. J., Adelman J. P., Douglass J., North R. A. Expression of a cloned rat brain potassium channel in Xenopus oocytes. Science. 1989 Apr 14;244(4901):221–224. doi: 10.1126/science.2539643. [DOI] [PubMed] [Google Scholar]
- Davies S. N., Lester R. A., Reymann K. G., Collingridge G. L. Temporally distinct pre- and post-synaptic mechanisms maintain long-term potentiation. Nature. 1989 Apr 6;338(6215):500–503. doi: 10.1038/338500a0. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Fine S. M., Unanue E. R., Chaplin D. D. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1 alpha cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5649–5653. doi: 10.1073/pnas.85.15.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K., Soderling T. R., Miyamoto E. Activation of Ca2+/calmodulin-dependent protein kinase II and protein kinase C by glutamate in cultured rat hippocampal neurons. J Biol Chem. 1992 Nov 5;267(31):22527–22533. [PubMed] [Google Scholar]
- Fukunaga K., Stoppini L., Miyamoto E., Muller D. Long-term potentiation is associated with an increased activity of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1993 Apr 15;268(11):7863–7867. [PubMed] [Google Scholar]
- Gasic G. P., Hollmann M. Molecular neurobiology of glutamate receptors. Annu Rev Physiol. 1992;54:507–536. doi: 10.1146/annurev.ph.54.030192.002451. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. doi: 10.1038/342643a0. [DOI] [PubMed] [Google Scholar]
- Honoré T., Davies S. N., Drejer J., Fletcher E. J., Jacobsen P., Lodge D., Nielsen F. E. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. doi: 10.1126/science.2899909. [DOI] [PubMed] [Google Scholar]
- Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. doi: 10.1126/science.2166337. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Kennedy M. B., Bennett M. K., Erondu N. E. Biochemical and immunochemical evidence that the "major postsynaptic density protein" is a subunit of a calmodulin-dependent protein kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7357–7361. doi: 10.1073/pnas.80.23.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E., Chen S. J., Sweatt J. D. Persistent protein kinase activation in the maintenance phase of long-term potentiation. J Biol Chem. 1991 Dec 25;266(36):24253–24256. [PubMed] [Google Scholar]
- Lisman J. The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci. 1994 Oct;17(10):406–412. doi: 10.1016/0166-2236(94)90014-0. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Beattie K. A., Klumpp S., Cohen P., Codd G. A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990 May 21;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Malenka R. C., Nicoll R. A. Mechanisms underlying long-term potentiation of synaptic transmission. Annu Rev Neurosci. 1991;14:379–397. doi: 10.1146/annurev.ne.14.030191.002115. [DOI] [PubMed] [Google Scholar]
- Malinow R., Tsien R. W. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990 Jul 12;346(6280):177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- Manabe T., Renner P., Nicoll R. A. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature. 1992 Jan 2;355(6355):50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E., Yamamoto H., Tan S. E., Brickey D. A., Soderling T. R. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature. 1993 Apr 15;362(6421):640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L. The mechanism of expression of long-term enhancement of hippocampal synapses: current issues and theoretical implications. Annu Rev Physiol. 1993;55:375–396. doi: 10.1146/annurev.ph.55.030193.002111. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Mukherji S., Soderling T. R. Regulation of Ca2+/calmodulin-dependent protein kinase II by inter- and intrasubunit-catalyzed autophosphorylations. J Biol Chem. 1994 May 13;269(19):13744–13747. [PubMed] [Google Scholar]
- Pettit D. L., Perlman S., Malinow R. Potentiated transmission and prevention of further LTP by increased CaMKII activity in postsynaptic hippocampal slice neurons. Science. 1994 Dec 16;266(5192):1881–1885. doi: 10.1126/science.7997883. [DOI] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature. 1993 Feb 18;361(6413):637–641. doi: 10.1038/361637a0. [DOI] [PubMed] [Google Scholar]
- Raymond L. A., Blackstone C. D., Huganir R. L. Phosphorylation of amino acid neurotransmitter receptors in synaptic plasticity. Trends Neurosci. 1993 Apr;16(4):147–153. doi: 10.1016/0166-2236(93)90123-4. [DOI] [PubMed] [Google Scholar]
- Roche K. W., Raymond L. A., Blackstone C., Huganir R. L. Transmembrane topology of the glutamate receptor subunit GluR6. J Biol Chem. 1994 Apr 22;269(16):11679–11682. [PubMed] [Google Scholar]
- Roskoski R., Jr Assays of protein kinase. Methods Enzymol. 1983;99:3–6. doi: 10.1016/0076-6879(83)99034-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A. J., Paylor R., Wehner J. M., Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Silva A. J., Stevens C. F., Tonegawa S., Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992 Jul 10;257(5067):201–206. doi: 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Soderling T. R. Calcium-dependent protein kinases in learning and memory. Adv Second Messenger Phosphoprotein Res. 1995;30:175–189. doi: 10.1016/s1040-7952(05)80007-2. [DOI] [PubMed] [Google Scholar]
- Soderling T. R. Calcium/calmodulin-dependent protein kinase II: role in learning and memory. Mol Cell Biochem. 1993 Nov;127-128:93–101. doi: 10.1007/BF01076760. [DOI] [PubMed] [Google Scholar]
- Stevens C. F., Tonegawa S., Wang Y. The role of calcium-calmodulin kinase II in three forms of synaptic plasticity. Curr Biol. 1994 Aug 1;4(8):687–693. doi: 10.1016/s0960-9822(00)00153-6. [DOI] [PubMed] [Google Scholar]
- Tan S. E., Wenthold R. J., Soderling T. R. Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hippocampal neurons. J Neurosci. 1994 Mar;14(3 Pt 1):1123–1129. doi: 10.1523/JNEUROSCI.14-03-01123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna F. A., Wang L. Y., MacDonald J. F., Hampson D. R. A transmembrane model for an ionotropic glutamate receptor predicted on the basis of the location of asparagine-linked oligosaccharides. J Biol Chem. 1994 May 13;269(19):14159–14164. [PubMed] [Google Scholar]
- Tingley W. G., Roche K. W., Thompson A. K., Huganir R. L. Regulation of NMDA receptor phosphorylation by alternative splicing of the C-terminal domain. Nature. 1993 Jul 1;364(6432):70–73. doi: 10.1038/364070a0. [DOI] [PubMed] [Google Scholar]
- Urushihara H., Tohda M., Nomura Y. Selective potentiation of N-methyl-D-aspartate-induced current by protein kinase C in Xenopus oocytes injected with rat brain RNA. J Biol Chem. 1992 Jun 15;267(17):11697–11700. [PubMed] [Google Scholar]
- Van Essen D. C., Gallant J. L. Neural mechanisms of form and motion processing in the primate visual system. Neuron. 1994 Jul;13(1):1–10. doi: 10.1016/0896-6273(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., Dudek E. M., Browning M. D., MacDonald J. F. Modulation of AMPA/kainate receptors in cultured murine hippocampal neurones by protein kinase C. J Physiol. 1994 Mar 15;475(3):431–437. doi: 10.1113/jphysiol.1994.sp020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Y., Taverna F. A., Huang X. P., MacDonald J. F., Hampson D. R. Phosphorylation and modulation of a kainate receptor (GluR6) by cAMP-dependent protein kinase. Science. 1993 Feb 19;259(5098):1173–1175. doi: 10.1126/science.8382377. [DOI] [PubMed] [Google Scholar]
- Wisden W., Seeburg P. H. Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol. 1993 Jun;3(3):291–298. doi: 10.1016/0959-4388(93)90120-n. [DOI] [PubMed] [Google Scholar]
- Wu K., Carlin R., Siekevitz P. Binding of L-[3H]glutamate to fresh or frozen synaptic membrane and postsynaptic density fractions isolated from cerebral cortex and cerebellum of fresh or frozen canine brain. J Neurochem. 1986 Mar;46(3):831–841. doi: 10.1111/j.1471-4159.1986.tb13047.x. [DOI] [PubMed] [Google Scholar]




