Abstract
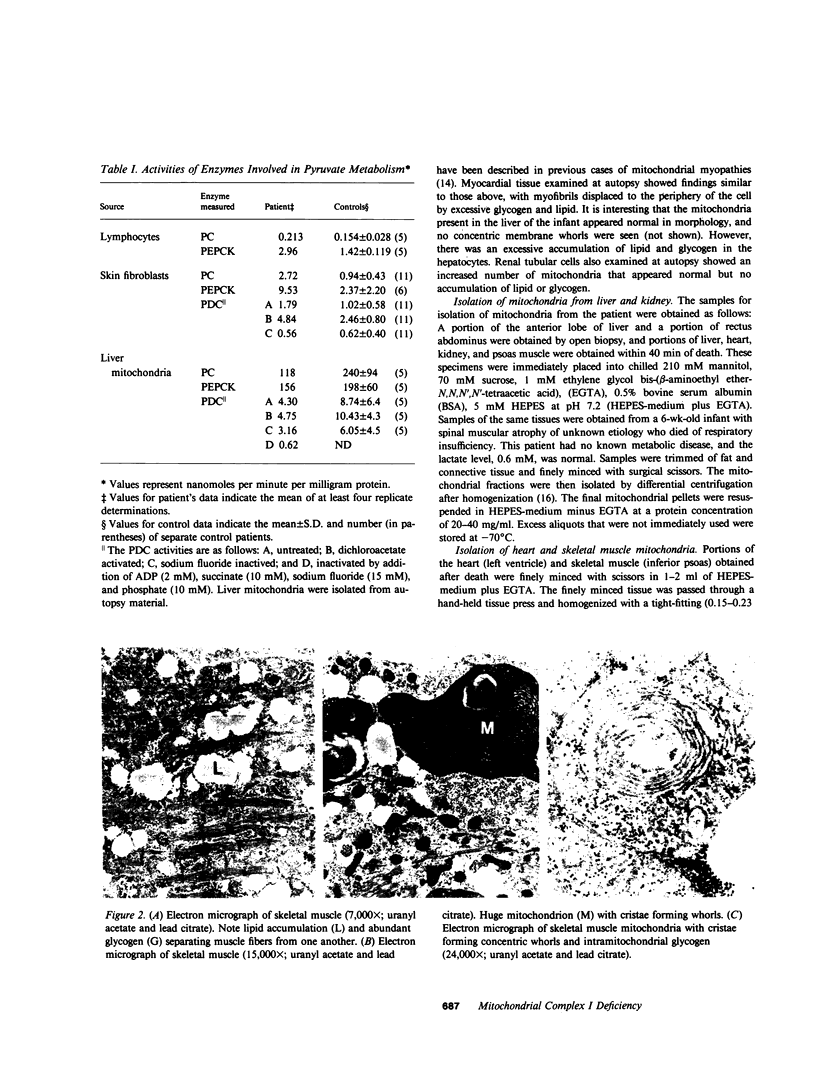
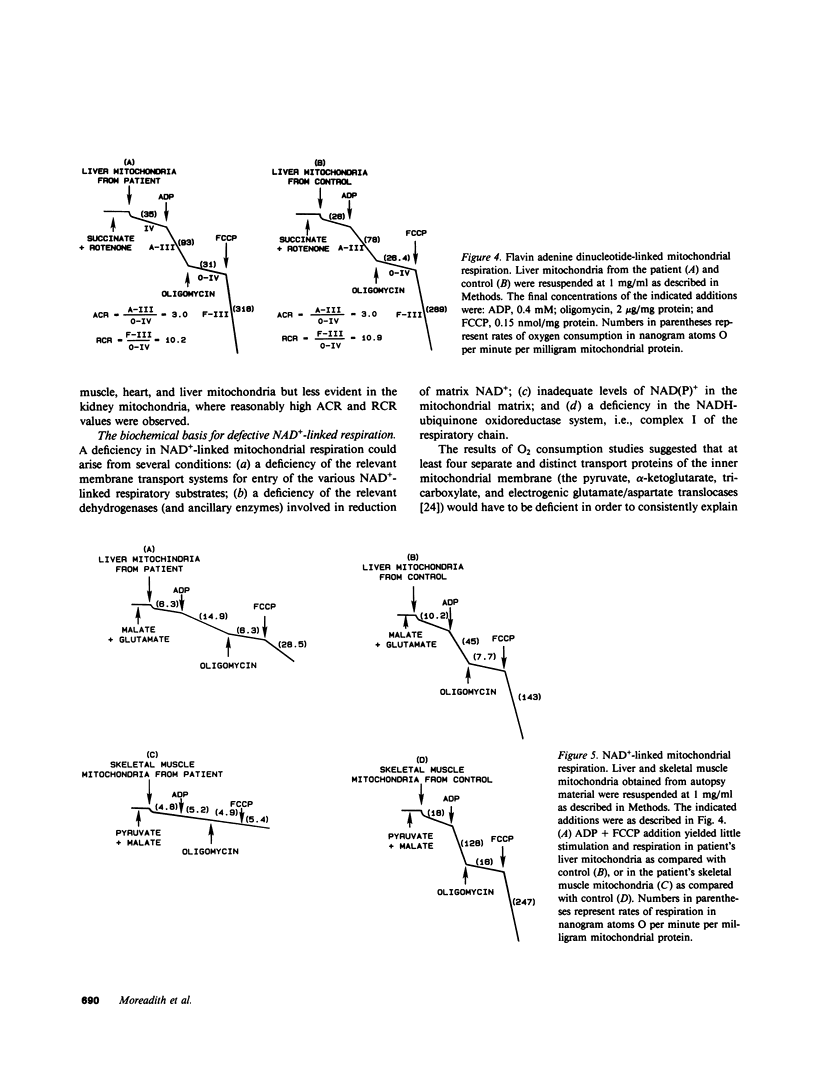
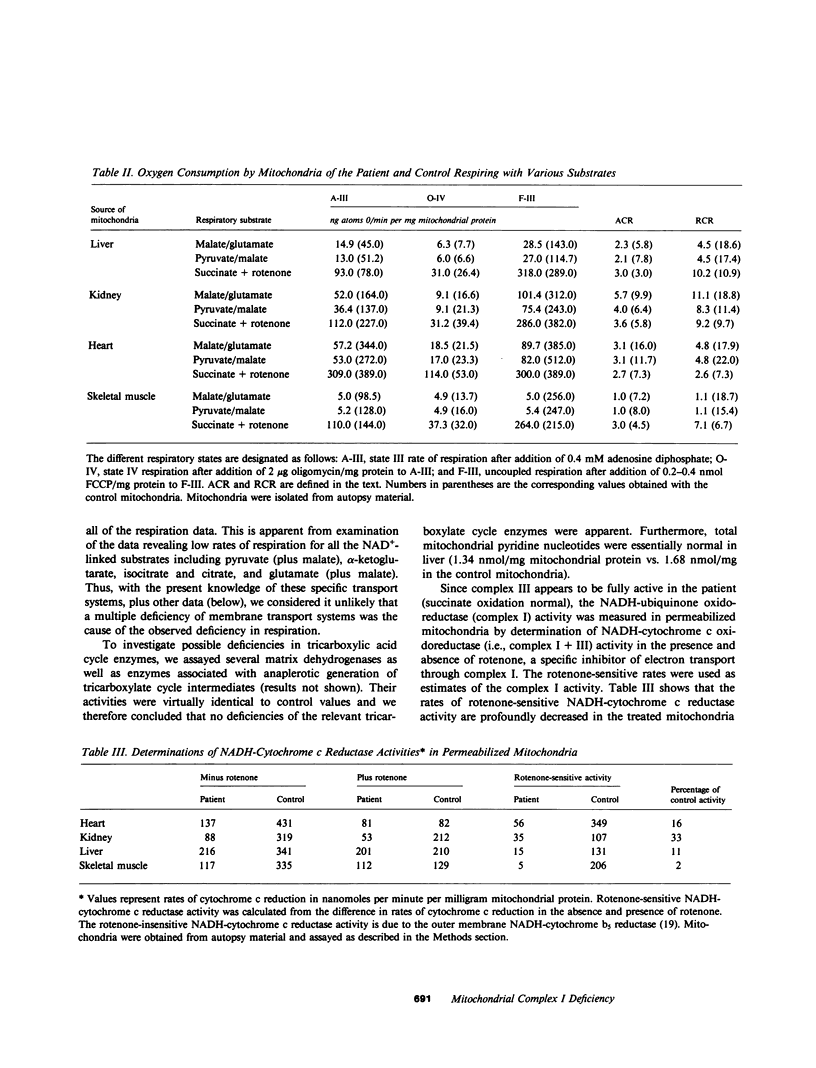
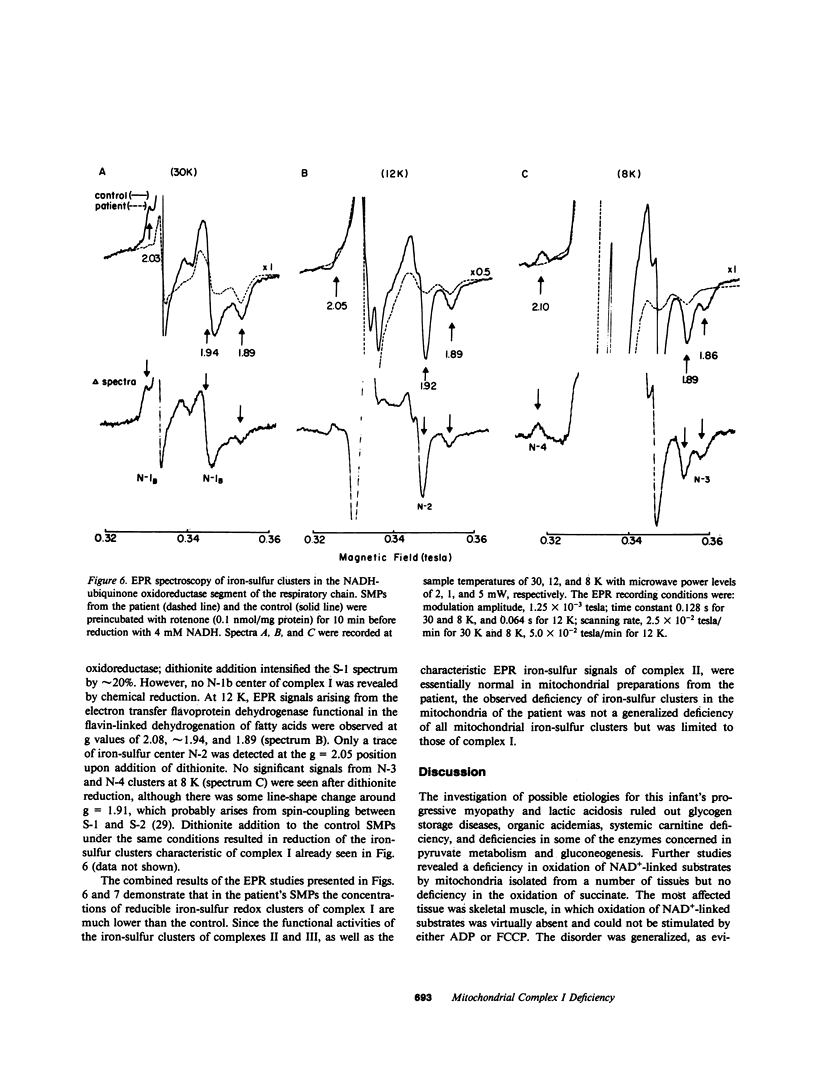
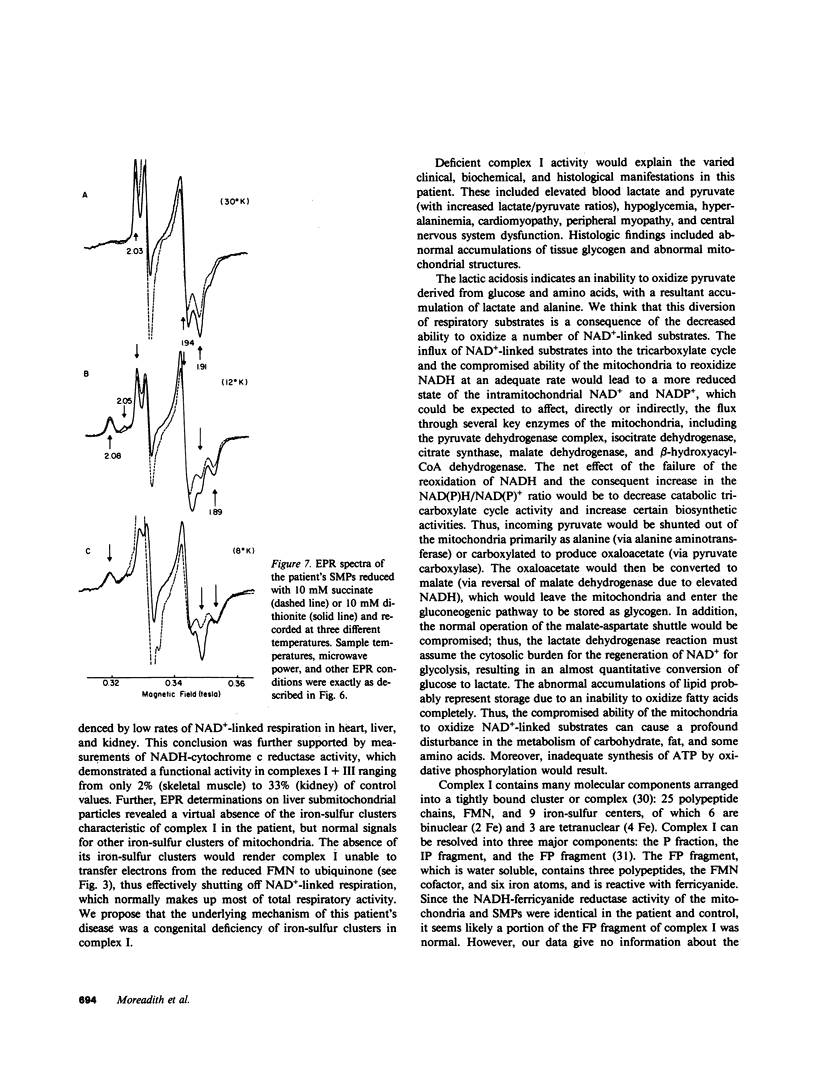
We report the case of an infant with hypoglycemia, progressive lactic acidosis, an increased serum lactate/pyruvate ratio, and elevated plasma alanine, who had a moderate to profound decrease in the ability of mitochondria from four organs to oxidize pyruvate, malate plus glutamate, citrate, and other NAD+-linked respiratory substrates. The capacity to oxidize the flavin adenine dinucleotide-linked substrate, succinate, was normal. The most pronounced deficiency was in skeletal muscle, the least in kidney mitochondria. Enzymatic assays on isolated mitochondria ruled out defects in complexes II, III, and IV of the respiratory chain. Further studies showed that the defect was localized in the inner membrane mitochondrial NADH-ubiquinone oxidoreductase (complex I). When ferricyanide was used as an artificial electron acceptor, complex I activity was normal, indicating that electrons from NADH could reduce the flavin mononucleotide cofactor. However, electron paramagnetic resonance spectroscopy performed on liver submitochondrial particles showed an almost total loss of the iron-sulfur clusters characteristic of complex I, whereas normal signals were noted for other mitochondrial iron-sulfur clusters. This infant is presented as the first reported case of congenital lactic acidosis caused by a deficiency of the iron-sulfur clusters of complex I of the mitochondrial electron transport chain.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albracht S. P., Slater E. C. EPR studies at 20 degrees K on the mitochondrial respiratory chain. Biochim Biophys Acta. 1971 Sep 7;245(2):503–507. doi: 10.1016/0005-2728(71)90168-x. [DOI] [PubMed] [Google Scholar]
- Atkin B. M., Utter M. F., Weinberg M. B. Pyruvate carboxylase and phosphoenolpyruvate carboxykinase activity in leukocytes and fibroblasts from a patient with pyruvate carboxylase deficiency. Pediatr Res. 1979 Jan;13(1):38–43. doi: 10.1203/00006450-197901000-00009. [DOI] [PubMed] [Google Scholar]
- Capeillere-Blandin C., Ohnishi T. Investigation of the iron-sulfur clusters in some mitochondrial mutants of Saccharomyces cerevisiae. A possible correlation between Rieske's iron-sulfur cluster and cytochrome b. Eur J Biochem. 1982 Feb;122(2):403–413. doi: 10.1111/j.1432-1033.1982.tb05895.x. [DOI] [PubMed] [Google Scholar]
- HOWELL R. R., ASHTON D. M., WYNGAARDEN J. B. Glucose-6-phosphatase deficiency glycogen storage disease. Studies on the interrelationships of carbohydrate, lipid, and purine abnormalities. Pediatrics. 1962 Apr;29:553–565. [PubMed] [Google Scholar]
- Hatefi Y. Introduction--preparation and properties of the enzymes and enzymes complexes of the mitochondrial oxidative phosphorylation system. Methods Enzymol. 1978;53:3–4. doi: 10.1016/s0076-6879(78)53004-8. [DOI] [PubMed] [Google Scholar]
- Haworth J. C., Robinson B. H., Perry T. L. Lactic acidosis due to pyruvate carboxylase deficiency. J Inherit Metab Dis. 1981;4(2):57–58. doi: 10.1007/BF02263589. [DOI] [PubMed] [Google Scholar]
- Heffner R. R., Barron S. A. The early effects of ischemia upon skeletal muscle mitochondria. J Neurol Sci. 1978 Oct;38(3):295–315. doi: 10.1016/0022-510x(78)90137-5. [DOI] [PubMed] [Google Scholar]
- Heron C., Smith S., Ragan C. I. An analysis of the polypeptide composition of bovine heart mitochondrial NADH-ubiquinone oxidoreductase by two-dimensional polyacrylamide-gel electrophoresis. Biochem J. 1979 Aug 1;181(2):435–443. doi: 10.1042/bj1810435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommes F. A., Bendien K., Elema J. D., Bremer H. J., Lombeck I. Two cases of phosphoenolpyruvate carboxykinase deficiency. Acta Paediatr Scand. 1976 Mar;65(2):233–240. doi: 10.1111/j.1651-2227.1976.tb16543.x. [DOI] [PubMed] [Google Scholar]
- Igarashi Y., Otomo H., Narisawa K., Tada K. A new variant of glycogen storage disease type 1: probably due to a defect in the glucose-6-phosphate transport system. J Inherit Metab Dis. 1980;2(3):45–49. doi: 10.1007/BF01801717. [DOI] [PubMed] [Google Scholar]
- LaNoue K. F., Schoolwerth A. C. Metabolite transport in mitochondria. Annu Rev Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]
- Lindblad B., Lindblad B. S., Olin P., Svanberg B., Zetterström R. Methylmalonic acidemia. A disorder associated with acidosis, hyperglycinemia, and hyperlactatemia. Acta Paediatr Scand. 1968 Sep;57(5):417–424. doi: 10.1111/j.1651-2227.1968.tb07314.x. [DOI] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Darveniza P., Landon D. N., Land J. M., Clark J. B. A mitochondrial myopathy with a deficiency of respiratory chain NADH-CoQ reductase activity. J Neurol Sci. 1979 Sep;43(1):27–46. doi: 10.1016/0022-510x(79)90071-6. [DOI] [PubMed] [Google Scholar]
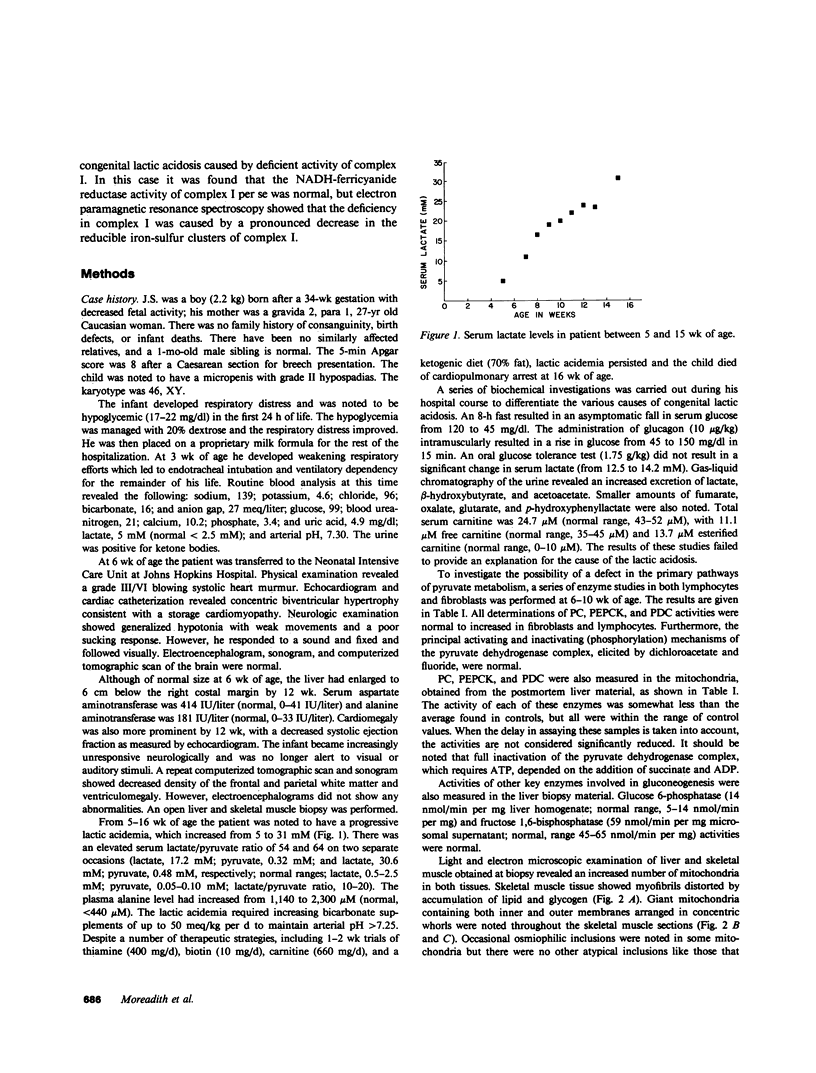
- Morgan-Hughes J. A., Hayes D. J., Clark J. B., Landon D. N., Swash M., Stark R. J., Rudge P. Mitochondrial encephalomyopathies: biochemical studies in two cases revealing defects in the respiratory chain. Brain. 1982 Sep;105(Pt 3):553–582. doi: 10.1093/brain/105.3.553. [DOI] [PubMed] [Google Scholar]
- Ohnishi T. Thermodynamic and EPR characterization of iron-sulfur centers in the NADH-ubiquinone segment of the mitochondrial respiratory chain in pigeon heart. Biochim Biophys Acta. 1975 Jun 17;387(3):475–490. doi: 10.1016/0005-2728(75)90087-0. [DOI] [PubMed] [Google Scholar]
- Onishi T., Asakura T., Yonetani T., Chance B. Electron paramagnetic resonance studies at temperatures below 77 degrees K on iron-sulfur proteins of yeast and bovine heart submitochondrial particles. J Biol Chem. 1971 Oct 10;246(19):5960–5964. [PubMed] [Google Scholar]
- Pagliara A. S., Karl I. E., Keating J. P., Brown B. I., Kipnis D. M. Hepatic fructose-1,6-diphosphatase deficiency. A cause of lactic acidosis and hypoglycemia in infancy. J Clin Invest. 1972 Aug;51(8):2115–2123. doi: 10.1172/JCI107018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen P. L., Greenawalt J. W., Reynafarje B., Hullihen J., Decker G. L., Soper J. W., Bustamente E. Preparation and characterization of mitochondria and submitochondrial particles of rat liver and liver-derived tissues. Methods Cell Biol. 1978;20:411–481. doi: 10.1016/s0091-679x(08)62030-0. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Galante Y. M., Hatefi Y. Purification of three iron-sulfur proteins from the iron-protein fragment of mitochondrial NADH-ubiquinone oxidoreductase. Biochemistry. 1982 May 11;21(10):2518–2524. doi: 10.1021/bi00539a035. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Sherwood W. G. Pyruvate dehydrogenase phosphatase deficiency: a cause of congenital chronic lactic acidosis in infancy. Pediatr Res. 1975 Dec;9(12):935–939. doi: 10.1203/00006450-197512000-00015. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Taylor J., Francois B., Beaudet A. L., Peterson D. F. Lactic acidosis, neurological deterioration and compromised cellular pyruvate oxidation due to a defect in the reoxidation of cytoplasmically generated NADH. Eur J Pediatr. 1983 Apr;140(2):98–101. doi: 10.1007/BF00441651. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Taylor J., Sherwood W. G. Deficiency of dihydrolipoyl dehydrogenase (a component of the pyruvate and alpha-ketoglutarate dehydrogenase complexes): a cause of congenital chronic lactic acidosis in infancy. Pediatr Res. 1977 Dec;11(12):1198–1202. doi: 10.1203/00006450-197712000-00006. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Taylor J., Sherwood W. G. The genetic heterogeneity of lactic acidosis: occurrence of recognizable inborn errors of metabolism in pediatric population with lactic acidosis. Pediatr Res. 1980 Aug;14(8):956–962. doi: 10.1203/00006450-198008000-00013. [DOI] [PubMed] [Google Scholar]
- Scholte H. R., Meijer A. E., van Wijngaarden G. K., Leenders K. L. Familial carnitine deficiency. A fatal case and subclinical state in a sister. J Neurol Sci. 1979 Jun;42(1):87–101. doi: 10.1016/0022-510x(79)90154-0. [DOI] [PubMed] [Google Scholar]
- Sheu K. F., Hu C. W., Utter M. F. Pyruvate dehydrogenase complex activity in normal and deficient fibroblasts. J Clin Invest. 1981 May;67(5):1463–1471. doi: 10.1172/JCI110176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel L. M. Quantitative determination of noncovalently bound flavins: types and methods of analysis. Methods Enzymol. 1978;53:419–429. doi: 10.1016/s0076-6879(78)53046-2. [DOI] [PubMed] [Google Scholar]
- Smith S., Cottingham I. R., Ragan C. I. Immunological assays of the NADH dehydrogenase content of bovine heart mitochondria and submitochondrial particles. FEBS Lett. 1980 Feb 11;110(2):279–282. doi: 10.1016/0014-5793(80)80092-5. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
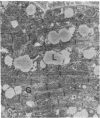
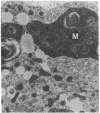
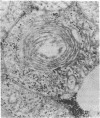
- Thayer W. S., Rubin E. Effects of chronic ethanol intoxication on oxidative phosphorylation in rat liver submitochondrial particles. J Biol Chem. 1979 Aug 25;254(16):7717–7723. [PubMed] [Google Scholar]
- Vercesi A., Reynafarje B., Lehninger A. L. Stoichiometry of H+ ejection and Ca2+ uptake coupled to electron transport in rat heart mitochondria. J Biol Chem. 1978 Sep 25;253(18):6379–6385. [PubMed] [Google Scholar]