Abstract
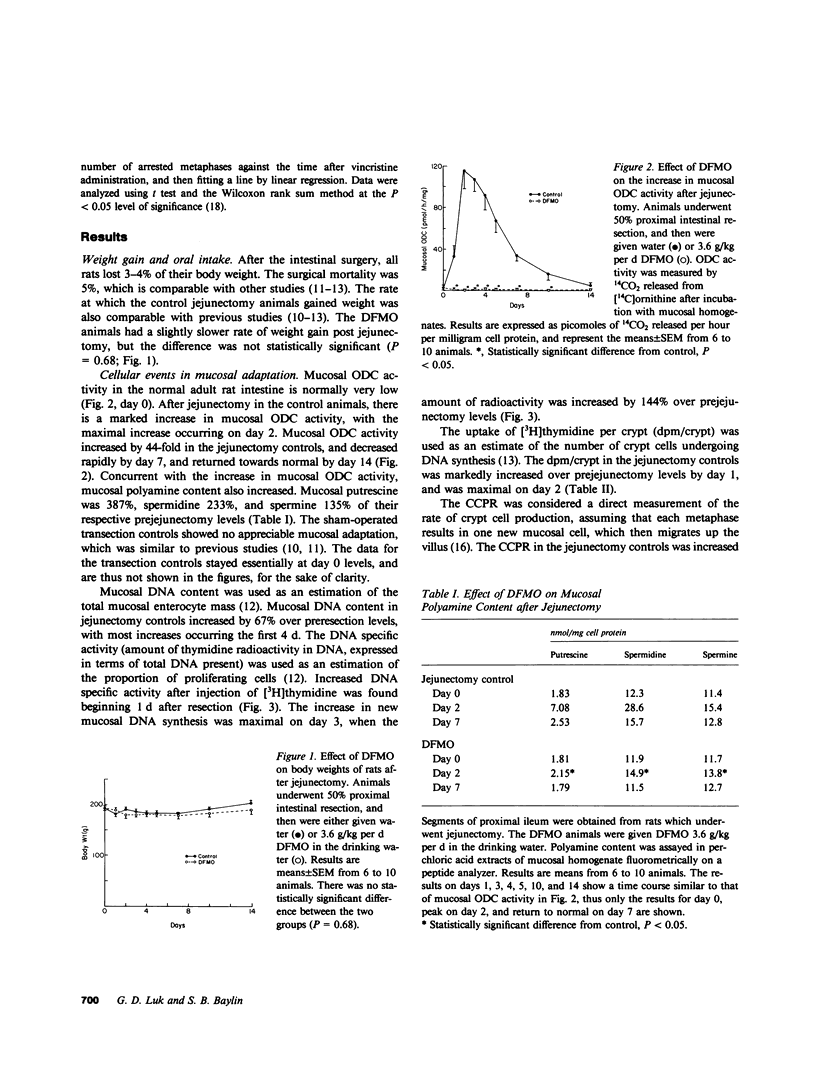
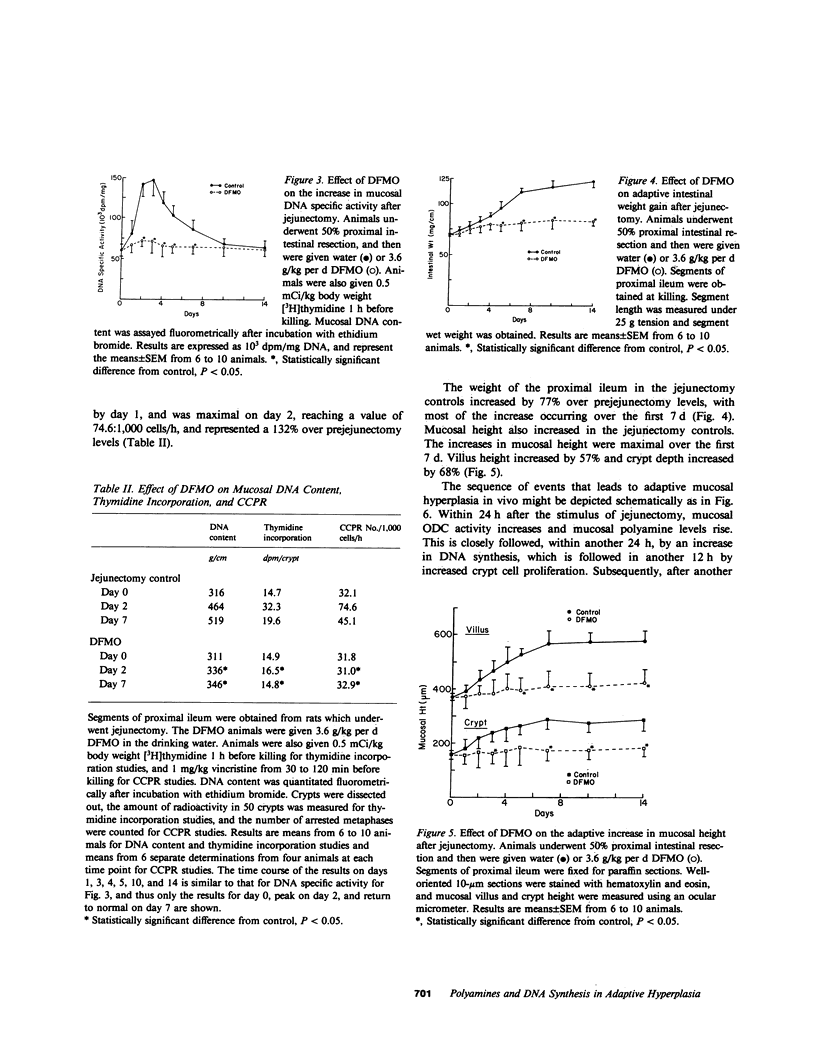
Transient increases in the activity of ornithine decarboxylase (ODC), the first and rate-limiting enzyme in polyamine biosynthesis, may be critical to initiation of cell growth. We previously reported such increases in ODC activity, and the polyamines, putrescine, and spermidine in rat ileal mucosa between days 1 and 4 after intestinal resection. During this time, there is initiation of mucosal cell hyperplasia, as measured morphologically and biochemically. Intestinal weight and mucosal thickness increase, as do mucosal DNA content and DNA synthesis. In the present study, we gave rats the specific irreversible ODC inhibitor, alpha-difluoromethyl ornithine (DFMO), beginning 3 d before jejunectomy. DFMO completely suppressed the increases in ODC activity and polyamine content in the intestinal mucosa. The suppression in ODC activity was associated with an 87% suppression of DNA synthesis, and resulted in a complete abolition of intestinal adaptation, as manifested by the absence of intestinal weight gain, increase in mucosal thickness, or increase in crypt cell production. Our results indicate that the increases in ODC activity and polyamine biosynthesis are critical for adaptive postresectional crypt cell proliferation in vivo, and that the critical step mediated by polyamines in this adaptive process is the onset of new DNA synthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boer G. J. A simplified microassay of DNA and RNA using ethidium bromide. Anal Biochem. 1975 May 12;65(1-2):225–231. doi: 10.1016/0003-2697(75)90507-2. [DOI] [PubMed] [Google Scholar]
- Clarke R. M. A comparison of metaphase arresting agents and tritiated thymidine. Autoradiography in measurement of the rate of entry of cells into mitosis in the crypts of Lieberkühn of the rat. Cell Tissue Kinet. 1971 May;4(3):263–272. doi: 10.1111/j.1365-2184.1971.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Saito R., DeRubertis F. R. Role of local prostaglandin synthesis in the modulation of proliferative activity of rat colonic epithelium. J Clin Invest. 1983 Oct;72(4):1365–1375. doi: 10.1172/JCI111093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman E. J., Aures D., Grossman M. I. Epidermal growth factor stimulates ornithine decarboxylase activity in the digestive tract of mouse. Proc Soc Exp Biol Med. 1978 Dec;159(3):400–402. doi: 10.3181/00379727-159-40357. [DOI] [PubMed] [Google Scholar]
- Hanson W. R., Osborne J. W., Sharp J. G. Compensation by the residual intestine after intestinal resection in the rat. I. Influence of amount of tissue removed. Gastroenterology. 1977 Apr;72(4 Pt 1):692–700. [PubMed] [Google Scholar]
- Jänne J., Pösö H., Raina A. Polyamines in rapid growth and cancer. Biochim Biophys Acta. 1978 Apr 6;473(3-4):241–293. doi: 10.1016/0304-419x(78)90015-x. [DOI] [PubMed] [Google Scholar]
- Lembach K. J. Regulation of growth in vitro. I. Control of ornithine decarboxylase levels in untransformed and transformed mouse fibroblasts by serum. Biochim Biophys Acta. 1974 Jun 20;354(1):88–100. doi: 10.1016/0304-4165(74)90056-7. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Baylin S. B. Polyamines and intestinal growth--increased polyamine biosynthesis after jejunectomy. Am J Physiol. 1983 Nov;245(5 Pt 1):G656–G660. doi: 10.1152/ajpgi.1983.245.5.G656. [DOI] [PubMed] [Google Scholar]
- Luk G. D., Civin C. I., Weissman R. M., Baylin S. B. Ornithine decarboxylase: essential in proliferation but not differentiation of human promyelocytic leukemia cells. Science. 1982 Apr 2;216(4541):75–77. doi: 10.1126/science.6950518. [DOI] [PubMed] [Google Scholar]
- Lux G. D., Marton L. J., Baylin S. B. Ornithine decarboxylase is important in intestinal mucosal maturation and recovery from injury in rats. Science. 1980 Oct 10;210(4466):195–198. doi: 10.1126/science.6774420. [DOI] [PubMed] [Google Scholar]
- Obertop H., Nundy S., Malamud D., Malt R. A. Onset of cell proliferation in the shortened gut. Rapid hyperplasia after jejunal resection. Gastroenterology. 1977 Feb;72(2):267–270. [PubMed] [Google Scholar]
- Oka T., Perry J. W. Studies on regulatory factors of ornithine decarboxylase activity during development of mouse mammary epithelium in vitro. J Biol Chem. 1976 Mar 25;251(6):1738–1744. [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Sjoerdsma A. Suicide enzyme inhibitors as potential drugs. Clin Pharmacol Ther. 1981 Jul;30(1):3–22. doi: 10.1038/clpt.1981.121. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Weser E., Hernandez M. H. Studies of small bowel adaptation after intestinal resection in the rat. Gastroenterology. 1971 Jan;60(1):69–75. [PubMed] [Google Scholar]
- Williams-Ashman H. G., Canellakis Z. N. Polyamines in mammalian biology and medicine. Perspect Biol Med. 1979 Spring;22(3):421–453. doi: 10.1353/pbm.1979.0013. [DOI] [PubMed] [Google Scholar]
- Williamson R. C. Intestinal adaptation (first of two parts). Structural, functional and cytokinetic changes. N Engl J Med. 1978 Jun 22;298(25):1393–1402. doi: 10.1056/NEJM197806222982505. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Appleton D. R. The metaphase arrest technique. A critical review. Cell Tissue Kinet. 1980 Nov;13(6):643–663. doi: 10.1111/j.1365-2184.1980.tb00503.x. [DOI] [PubMed] [Google Scholar]



