Abstract
A detailed understanding of influenza movement in communities during yearly epidemics is needed to inform improved influenza control programs. We sought to determine the relative timing of influenza presentation and symptom onset by age group and influenza strain. Prospective, laboratory-confirmed surveillance was performed over three moderate influenza seasons in emergency departments and inpatient settings of both medical centers in Winston-Salem, NC. Influenza disease presented first in school age children through community epidemics of influenza A(H1N1)pdm09 and influenza B, and first in persons 5–49 years old for influenza A(H3N2). This finding indicates that influenza prevention in persons 5–49 years of age may be particularly important in influenza epidemic control.
Keywords: human influenza, influenza A virus, influenza B virus, inpatient, hospital emergency services, epidemiology
Influenza virus is an important cause of illness, outpatient visits and hospitalizations among persons of all ages.[1–7] Globally, influenza virus caused an estimated 508,000 deaths in 2010.[8] The importance of influenza epidemic control is recognized as an international public health priority.[9] An improved understanding of influenza spread within communities is needed to inform design of influenza prevention and control programs.
Despite the large annual influenza disease burden, a detailed understanding of how influenza spreads through communities is lacking. Literature suggests that children are important to the spread of influenza infection.[10–15] This important work has highlighted the need for additional studies that further examine the spread of influenza within communities.[16–18] This prospective, laboratory-confirmed influenza surveillance study was designed to address this gap by using prospective data to determine the relative timing of influenza disease by serotype and age group. We tested the hypothesis that influenza disease occurs first in school age children during annual influenza epidemics. Defining which age groups seek medical attention for influenza early in annual seasonal epidemics is important to inform the debate about universal influenza vaccination.[19–24]
Methods
Study Overview
Prospective, laboratory-confirmed surveillance for influenza was performed throughout each respiratory season among persons of all ages presenting to the emergency department or inpatient wards of the two large medical centers, including the children’s hospital, located in Winston-Salem, North Carolina. More than 94% of all Forsyth County residents that are seen in the emergency department or are hospitalized receive care at these surveillance hospitals.
Approval
Eligible persons were approached for enrollment, and written informed consent and assent, when appropriate, were provided. This study was approved by the Wake Forest School of Medicine Institutional Review Board and performed under an authorization agreement between the institutional review boards of Forsyth Medical Center and Wake Forest School of Medicine.
Study population
Patients of all ages who resided in Forsyth County or a contiguous North Carolina county and presented to any surveillance emergency department or inpatient setting with fever (by report or documentation) or any acute respiratory symptoms were study-eligible. Patients were eligible for enrollment if they presented with fever, cough, nasal congestion, difficulty breathing, ear pain, sore throat, and/or wheezing and if admitted were enrolled within 24 hours of hospitalization. Patients who presented with only fever were eligible unless they had an identified non-respiratory source of fever, i.e. cellulitis or urinary tract infection.
Influenza seasons
Surveillance was systematically conducted each year from November through April and began earlier or extended later if influenza was detected in the hospital laboratories or reported regionally. Enrollment was performed during daytime hours from Monday through Thursday during four consecutive influenza seasons from 2009–2010 through 2012–2013. Regional and national data from the CDC indicates three of these four seasons had moderate influenza circulation in the U.S and 2011–2012 was a mild influenza season; all three moderate influenza seasons had sufficient influenza-positive observations and were included in this analysis.[25] We enrolled fewer than half the number of patients with study-confirmed influenza in 2011–2012 than any other study years, and these 26 influenza-positive patients were divided among three serotypes. Thus, 2011–2012 was excluded given too few influenza-positive observations to compute an epidemic curve.
Regional data was evaluated to determine the circulating influenza viruses identified during each study season.[26] Influenza A(H1N1)pdm09 comprised 98% of all isolates from August 30, 2009 through April 17, 2010. From October 31, 2010 through April 30, 2011, the proportions of all typed influenza isolates were 21% influenza A(H1N1)pdm09, 58% influenza B, and 21% influenza A(H3N2) virus. From October 28, 2012 through April 27, 2013, the proportions of all typed influenza isolates were 59% influenza B, 37% seasonal influenza A (H3N2), and 2% influenza A(H1N1)pdm09. Hence, we included all study-confirmed influenza A(H1N1)pdm09 from 2009–2010 and 2010–2011 and both study-confirmed influenza B and influenza A(H3N2) in 2010–2011 and 2012–2013.
Patient/Parental Questionnaire
Patients or their guardians completed a standardized questionnaire to obtain demographic information and medical history. The date of birth was used to compute age at the time of enrollment. The age groups were determined a priori to be 0–4 years (preschool age), 5–17 years (school age), 18–49 years, and ≥50 years. We obtained the number of symptom days at enrollment from the patient or guardian with a cut-off maximum of 14 days. The date of symptom onset was computed by subtracting the number of symptom days from the date of enrollment.
High-risk medical conditions included all conditions with a specific CDC recommendation to receive the 2009–2010 influenza vaccine.[27] They include cardiopulmonary diseases, metabolic diseases, renal diseases, hemoglobinopathies, primary or secondary immunodeficiency, cognitive or neurologic conditions that can compromise respiratory functioning, pregnancy and children on long-term aspirin therapy. High-risk conditions were included because of their association with an increased risk of severe influenza disease.
Influenza vaccination status was obtained by patient/guardian report and verified in the North Carolina Immunization Registry or practice, when available. Most children ≥6 months (99.7%) and 83% adults ≥18 years had their vaccination status verified. Full vaccination was one of the following: receipt of one dose of influenza vaccine >14 days prior to enrollment for all persons ≥9 years of age, receipt of one dose of influenza vaccine >14 days prior to enrollment and history of influenza vaccination in prior year for children 1–8 years of age, and receipt of two doses of influenza vaccine one month apart with last dose >14 days prior to enrollment in children <9 years who had not received influenza vaccine in a prior year.
Nasal/Throat Swabs
A mid-turbinate nasal specimen and a throat specimen were obtained from enrolled subjects using flocked swabs. Both specimens were placed in one vial of viral transport media and transported on ice to the study laboratory.
Detection of Influenza
All nasal and throat swabs were cultured for influenza viruses on R-Mix Too™ cells (Diagnostic Hybrids). Detection of influenza culture was performed by direct fluorescence microscopy using type-specific antibodies (Diagnostic Hybrids). Reverse transcriptase polymerase chain reaction (RT-PCR) testing for influenza A(H3N2), influenza A(H1N1)pdm09, and influenza B without lineage delineation was performed using a RT-PCR analysis protocol developed at the CDC and kindly made available under a Material Transfer Agreement (Stephen Lindstrom, PhD, CDC). A human RNase P gene RNA was detected in parallel for each specimen as an internal control for human subject specimen adequacy.
A specimen was classified as influenza A(H1N1)pdm09 positive if the RT-PCR for influenza A(H1N1)pdm09 was positive. A specimen was classified as influenza B positive if type-specific RT-PCR or viral culture was positive. A specimen was classified as influenza A(H3N2) positive if the RT-PCR or viral culture was positive for influenza A(H3N2).
Statistical Analyses
By identifying the enrollment date of all influenza A(H1N1)pdm09 positive specimens obtained by prospective influenza surveillance for each season, we determined the influenza A(H1N1)pdm09 epidemic midpoint. The epidemic midpoint was defined as the date when the cumulative distribution of study-confirmed influenza A(H1N1)pdm09 infections reached 50% of the total for each season.[10] The demographic characteristics of persons enrolled on or before the epidemic midpoint were compared to those persons enrolled after the epidemic midpoint using the chi-square test. The cumulative proportion of study-confirmed influenza A(H1N1)pdm09 infections for each age group was plotted by time in weeks, relative to the overall epidemic midpoint for each season. The rank sum days of influenza A(H1N1)pdm09 infection for each age group were compared by Kruskal-Wallis test. These calculations and analysis were repeated using date of symptom onset instead of enrollment date to address the possibility that the timing of presentation to the hospital or emergency department varied by age group.
The analysis for both study-confirmed influenza B and influenza A(H3N2) used the same approach for each strain during the 2010–2011 and 2012–2013 seasons.
Results
Among 4083 approached and eligible patients presenting with fever or acute respiratory symptoms to the emergency department or inpatient setting in 2009–2010, 2010–2011 or 2012–2013, 3373 (83%) were enrolled. The gender and age group of persons who were approached and enrolled or not enrolled were similar. The study population comprised all 447 persons who had any of three study-confirmed influenza serotypes, 97% of all influenza positive persons from these study years. The study population comprised 447 (13%) persons who had any of three strains of study-confirmed influenza--93 persons with study-confirmed influenza A(H1N1)pdm09 in 2009–2010 or 2010–2011, 187 persons with study-confirmed influenza B in 2010–2011 or 2012–2013, and 177 persons with study confirmed influenza A(H3N2) in 2010–2011 or 2012–2013. Ten persons were co-infected with two influenza strains; 7 had influenza B and influenza A(H1N1)pdm09 and 3 had influenza B and influenza A(H3N2). By year for study-confirmed influenza A(H1N1)pdm09, 56 (6%) of 977 enrolled persons in 2009–2010 had influenza A(H1N1)pdm09 infection (epidemic midpoint 11/03/2009 by timing of enrollment) and 37 (3%) of 1020 enrolled persons in 2010–2011 had infection (epidemic midpoint 2/10/2011). For influenza B, 125 (12%) of 1020 enrolled persons in 2010–2011 had influenza B infection (epidemic midpoint 1/31/2011) and 62 (5%) of 1376 enrolled person in 2012–2013 (epidemic midpoint 3/6/2013). For influenza A(H3N2), 44 (4%) of 1020 enrolled persons in 2010–2011 had influenza A(H3N2) infection (epidemic midpoint 2/16/2011) and 133 (10%) of 1376 enrolled person in 2012–2013 (epidemic midpoint 12/10/2012).
For each influenza strain, the demographic characteristics of persons enrolled before or on the epidemic midpoint were compared to those enrolled after the epidemic midpoint (Table 1). For influenza A(H1N1)pdm09 and influenza A(H3N2), more children 5–17 years and adults 18–49 years were enrolled before or on the epidemic midpoint than after the epidemic midpoint. For influenza B, more children 0–4 years and 5–17 years were enrolled before or on the epidemic midpoint than after the epidemic midpoint. There were no significant differences before and after the midpoint by year, sex, high-risk conditions, insurance, full influenza vaccination status or hospitalization for any strain. Duration of symptom at time of presentation with influenza A(H1N1)pdm09 were similar for patients seen before and after the epidemic midpoint with an overall mean of 3.8 days for influenza A(H1N1)pdm09, 4.2 days for influenza B, and 4.3 days for influenza A(H3N2).
Table 1.
Demographic characteristics of persons by influenza strain and enrollment date relative to the epidemic midpoint
| Timing of influenza infection for each circulating strain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Influenza A(H1N1)pdm09 | Influenza B | Influenza A(H3) | |||||||
| Characteristics | At or before Midpoint | After Midpoint | P-value* | At or before Midpoint | After Midpoint | P-value* | At or before Midpoint | After Midpoint | P-value* |
| n (row%) | n (row %) | n (row%) | n (row %) | n (row%) | n (row %) | ||||
| Age group | 0.008 | 0.04 | 0.04 | ||||||
| 0–4 years | 6 (32%) | 13 (68%) | 21 (62%) | 13 (38%) | 16 (42%) | 22 (58%) | |||
| 5–17 years | 14 (82%) | 3 (18%) | 26 (72%) | 10 (28%) | 14 (58%) | 10 (42%) | |||
| 18–49 years | 24 (55%) | 20 (41%) | 34 (46%) | 40 (54%) | 38 (64%) | 21 (36%) | |||
| ≥50 years | 4 (31%) | 9 (69%) | 20 (47%) | 23 (53%) | 23 (41%) | 33 (59%) | |||
| Sex | 0.37 | 0.84 | 0.85 | ||||||
| Male | 17 (46%) | 20 (54%) | 52 (55%) | 43 (45%) | 41 (51%) | 40 (49%) | |||
| Female | 31 (55%) | 25 (45%) | 49 (53%) | 43 (47%) | 50 (52%) | 46 (48%) | |||
| Insurance | 0.07 | 0.09 | 0.46 | ||||||
| Public Only | 31 (61%) | 20 (39%) | 59 (61%) | 37 (39%) | 46 (51%) | 44 (49%) | |||
| Private | 9 (33%) | 18 (67%) | 29 (48%) | 31 (52%) | 29 (48%) | 32 (52%) | |||
| None | 8 (53%) | 7 (47%) | 13 (42%) | 18 (58%) | 15 (63%) | 9 (38%) | |||
| Year | 0.97 | 0.64 | 0.83 | ||||||
| 2009–2010 | 29 (52%) | 27 (48%) | NA | NA | NA | NA | |||
| 2010–2011 | 19 (51%) | 18 (49%) | 69 (55%) | 56 (45%) | 22 (50%) | 22 (50%) | |||
| 2012–2013 | NA | NA | 32 (52%) | 30 (48%) | 69 (52%) | 64 (48%) | |||
| High-risk condition | 0.94 | 0.88 | 0.39 | ||||||
| Yes | 22 (51%) | 21 (49%) | 40 (53%) | 35 (47%) | 46 (48%) | 49 (52%) | |||
| No | 26 (52%) | 24 (48%) | 61 (54%) | 51 (46%) | 45 (55%) | 37 (45%) | |||
| Full vaccination† | 0.48 | 0.63 | 0.87 | ||||||
| Yes | 3 (38%) | 5 (63%) | 15 (50%) | 15 (50%) | 15 (50%) | 15 (50%) | |||
| No | 45 (53%) | 40 (47%) | 86 (55%) | 71 (45%) | 76 (52%) | 71 (48%) | |||
| Hospitalized | 0.23 | 0.14 | 0.12 | ||||||
| Yes | 7 (39%) | 11 (61%) | 20 (44%) | 25 (56%) | 25 (43%) | 33 (57%) | |||
| No (ED only) | 41 (55%) | 34 (45%) | 81 (57%) | 61 (43%) | 66 (55%) | 53 (45%) | |||
| Symptoms | 0.17 | 0.81 | 1.00 | ||||||
| Fever only | 0 | 0 | 0 | 0 | 1 (50%) | 1 (50%) | |||
| Respiratory only†† | 9 (69%) | 4 (31%) | 19 (56%) | 15 (44%) | 11 (52%) | 10 (48%) | |||
| Both | 39 (49%) | 41 (51%) | 82 (54%) | 71 (46%) | 79 (51%) | 75 (49%) | |||
| Symptom Days | 0.64 | 0.80 | |||||||
| ≤2 days | 18 (49%) | 19 (51%) | 33 (65%) | 18 (35%) | 0.07 | 24 (53%) | 21 (47%) | ||
| >2 days | 30 (54%) | 24 (46%) | 68 (50%) | 68 (50%) | 67 (51%) | 64 (49%) | |||
| Mean (SD) | 3.9 (2.8) | 3.7 (2.4) | 4.0 (2.6) | 4.5 (3.0) | 3.7 (2.3) | 4.9 (3.6) | |||
| Range | 1–14 | 2–14 | 1–14 | 1–14 | 1–14 | 1–14 | |||
P-value for comparison of the characteristic between persons enrolled at or before the epidemic midpoint and those enrolled after the epidemic midpoint.
Full vaccination for H1N1 monovalent vaccine in 2009–2010 only and seasonal vaccine for all subsequent years.
Report of any respiratory symptoms--cough, nasal congestion, difficulty breathing, ear pain, sore throat or wheezing.
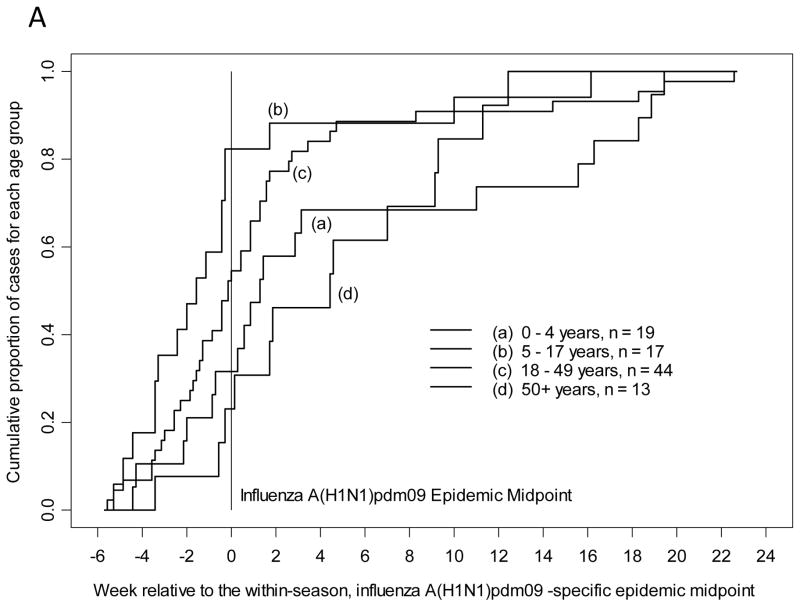
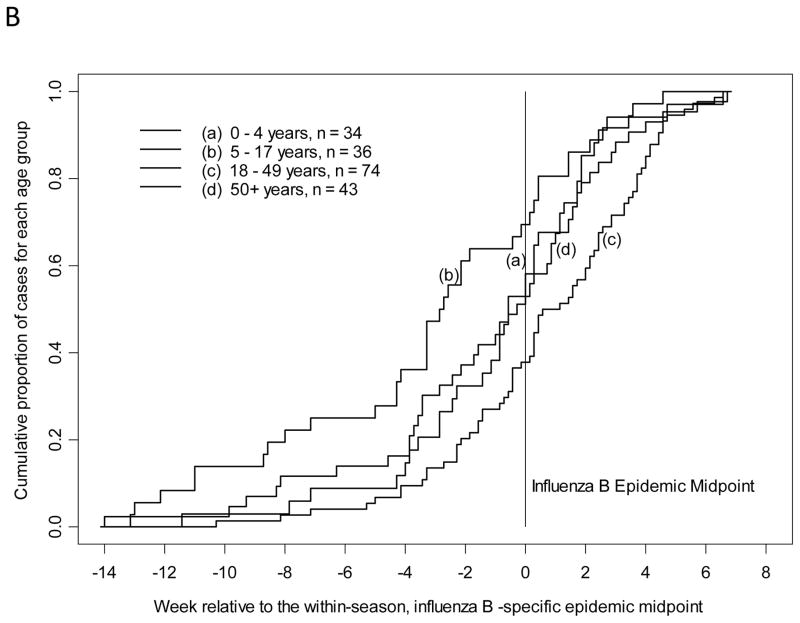
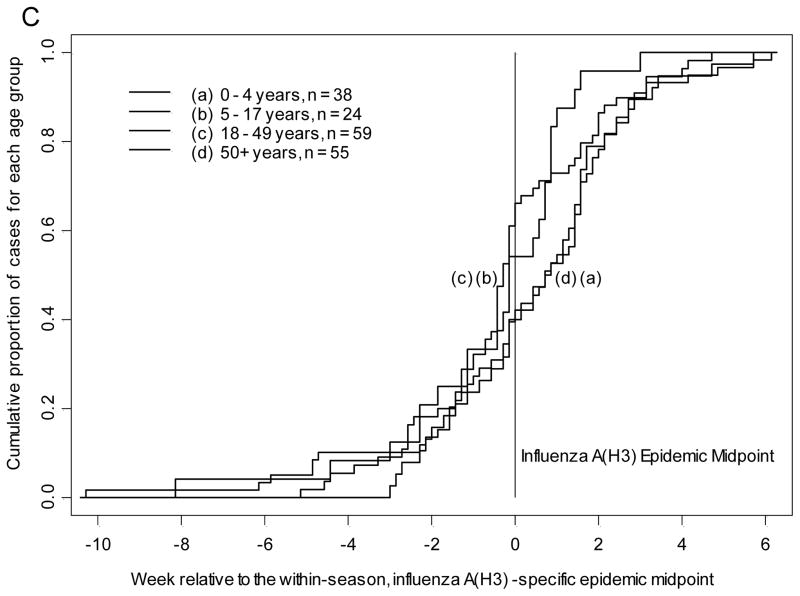
The cumulative proportion of all study-confirmed influenza A(H1N1)pdm09 infections from 2009–2010 and 2010–2011 was plotted by time in weeks, relative to the overall influenza A(H1N1)pdm09 epidemic midpoint for each season as determined by date of enrollment in prospective influenza surveillance. The median of influenza A(H1N1)pdm09 and influenza B infection (cumulative proportion of 0.5 or 50th percentile) was earliest for children 5–17 years of age. Children 5–17 years of age presented with study-confirmed influenza A(H1N1)pdm09 infection at least 10.5 days earlier all other age groups (Table 2, p=0.006 by Kruskal-Wallis test) and closely resembled Figure 1A. Also with influenza A(H1N1)pdm09, adults 18–49 years presented after school age children but prior to the epidemic midpoint. For study-confirmed influenza B, children 5–17 years presented at least 15 days earlier than all other age groups (Table 2, p=0.0003 by Kruskal-Wallis test) and closely resembled Figure 1B. Children 0–4 years of age also presented prior to the epidemic midpoint with influenza B and all adults presented after the epidemic midpoint. For study-confirmed influenza A(H3N2), both adults 18–49 years and children 5–17 years presented prior to the epidemic midpoint with adults presenting 1.5 days earlier than school age children (Table 2, p=0.04 by Kruskal-Wallis test) and closely resembled Figure 1C.
Table 2.
Median day of enrollment or symptom onset relative to epidemic midpoint by age group and strain.
| Influenza A(H1N1) pdm09 | Influenza B | Influenza A(H3) | |
|---|---|---|---|
| Median day of enrollment relative to epidemic midpoint† | |||
| 0–4 years | 6 days | −5 days | 3.5 days |
| 5–17 years | −11 days | −20 days | −3.5 days |
| 18–49 years | −0.5 days | 5.5 days | −5 days |
| ≥50 years | 32 days | 2 days | 7.5 days |
| p-value* | 0.006 | 0.0003 | 0.04 |
| Median day of symptom onset relative to epidemic midpoint† | |||
| 0–4 years | 9 days | −4 days | 5.5 days |
| 5–17 years | −11 days | −19.5 days | −1 days |
| 18–49 years | −1 days | 6 days | −2 days |
| ≥50 years | 31 days | −2 days | 5 days |
| p-value* | 0.008 | 0.0002 | 0.14 |
Negative numbers are medians before the epidemic midpoint, and positive numbers are medians after the epidemic midpoint.
P-value was computed using the Kruskal-Wallis Test.
Figure 1.
Timing of symptom onset for laboratory-confirmed influenza by age group and strain with A) influenza A(H1N1)pdm09, B) influenza B, and C) influenza A(H3N2).
These analyses were repeated by plotting the date of reported symptom onset among persons with each strain of study-confirmed influenza, relative to its epidemic midpoint for each season (as recalculated based on symptom onset to a maximum of 14 days). The results were similar to the analysis based on enrollment date. School age children had the earliest onset of symptoms with influenza A(H1N1)pdm09 and influenza B. Children 5–17 years of age reported onset of symptoms with study-confirmed influenza A(H1N1)pdm09 infection at least 10 days earlier than all other age groups (Table 2, p=0.008 by Kruskal-Wallis test, Figure 1A). Following the same pattern as with influenza A(H1N1)pdm09 enrollment, adults 18–49 years had onset of symptoms after school age children but prior to the epidemic midpoint. Children 5–17 years of age reported onset of symptoms with study-confirmed influenza B infection at least 15.5 days earlier than all other age groups (Table 2, p=0.0002 by Kruskal-Wallis test, Figure 1B). Similarly, children 0–4 years of age had onset of symptoms with influenza B prior to the epidemic midpoint. Symptom onset for study-confirmed influenza A(H3N2) followed the trend observed for presentation; adults 18–49 years reported onset of symptoms 1 day prior to children 5–17 years and both were before the epidemic midpoint (Table 2, p=0.14 by Kruskal-Wallis test, Figure 1C).
Discussion
In this study using prospective, laboratory-confirmed influenza surveillance data over three seasons, school age children 5–17 years of age presented to the emergency department or inpatient setting earlier with influenza A(H1N1)pdm09 and influenza B disease than patients 0–4, 18–49 or ≥50 years of age. School age children also presented early, but not first, with influenza A(H3N2) disease during these seasons, presenting 1.5 days after adults 18–49 years of age, and before younger children or older adults. Notably, the median time of presentation for school age children preceded the epidemic midpoint for all three influenza strains. These findings suggest that influenza disease transmission among school age children is an important determinant in the spread of influenza disease throughout the community. Annual influenza vaccination of school age children provides community-wide protection.[11;28–33] These findings support recommendations that all school age children receive the influenza vaccine each year.[34] Although there are many logistical challenges to vaccinating school age children,[35–37] programs that enhance the vaccination coverage of school age children may be especially successful in reducing influenza disease.
This study is novel in that it utilizes prospective surveillance with culture and RT-PCR to determine patients who are influenza-positive by serotype, avoiding the bias associated with physician-ordered testing and lack of test specificity for influenza A serotypes associated with rapid influenza tests. Study results using this distinct approach complement the literature suggesting that influenza community epidemics are disproportionately driven by disease transmission in school age children. Glezen et al. found that the large burden of influenza B infection in 1976–1977 among school age children in Houston, Texas preceded most of the identified cases among preschool children and adults.[38] By examining 11 years of Canadian national surveillance data from physician-ordered influenza tests, Schanzer et al. showed that children 10–19 years and adults 20–29 years had positive influenza tests 1 week earlier than other age groups; and that positive tests in children 10–19 years of age peaked 3 days earlier than in younger children and young adults during the influenza A(H1N1)pdm09 epidemic.[10]
Community adoption of programs to vaccinate school age children results in measurable benefits in disease reduction in both vaccine recipients and unvaccinated community members, as well as other indirect benefits including increased school attendance.[11;30;31;39;40] Reichert et al. showed that the population of Japan showed marked increases in rates of excess mortality following discontinuation of a countrywide program to promote influenza vaccination of schoolchildren.[32] Copeland et al. found that a Texas school district that closed all public schools for 8 days during the peak of the influenza A(H1N1)pdm09 epidemic had lower rates of self-reported acute respiratory illness or influenza-related emergency department visits than nearby communities with schools that remained mostly open.[33]
Another important finding in this study is that median time of influenza presentation and symptom onset in adults 18–49 years preceded the epidemic midpoint for both influenza A strains but not influenza B strains. Influenza A strains have been shown to evolve in humans approximately 2-fold to 3-fold faster than influenza B strains.[41–43] It may be that protective immunity following exposure to influenza B strains persists longer than that following influenza A exposure, given more rapid influenza A evolution. More adults than children enrolled in our study may have been exposed to influenza B strains of both Yamagata and Victoria lineages that have circulated since the early 1980s,[44] and consequently may have had more pre-existing immunity to circulating influenza B strains. It is interesting to speculate that prior strain-specific immunity may partially explain the relative timing of influenza A and influenza B to the epidemic midpoint for adults 18–49 years of age.
Since implementation of the universal childhood influenza vaccination recommendation in 2008,[45] influenza vaccination coverage for U.S. children 0.5–17 years of age was 55% for either seasonal or monovalent H1N1 vaccine in 2009–2010.[46] Influenza vaccine coverage was highest for young children and lowest for adolescents,[47] and these rates reflect the many logistical challenges in vaccinating school age children.[37] Barriers to increased vaccination of school age children include, but are not limited to, vaccine supply limitations, need for extra office visits to get the yearly vaccine each season, and challenges of communication between primary care providers, subspecialists, schools, health departments, pharmacists and other influenza vaccine providers.[35;37;48]
An important strength of our study is that prospective surveillance of persons of all ages was performed over multiple years, and influenza detection for circulating strains was not influenced by physician-ordered influenza testing patterns. This approach was particularly important during the 2009–2010 season given reports of poor rapid influenza diagnostic test detection of influenza A(H1N1)pdm09 and recommendations discouraging the use of rapid influenza diagnostic tests that season.[49;50] The majority of approached patients were enrolled with a range of mild to severe influenza disease. All major influenza serotypes circulating during these study years were available for analysis.
There are several limitations to this study. Surveillance was limited to one community, however our findings remain consistent over multiple seasons and is consistent with retrospective studies. There may be systematic differences between persons with influenza who were and were not enrolled. Enrolled patients sought medical care in the emergency department or were hospitalized with influenza infection, though most influenza disease is managed in the non-hospital setting.[4;51] Many pediatric and adult emergency department visits are for minor complaints;[52–54] approximately 10% of emergency department visits by persons <65 years in 2007 were non-urgent, with similar rates of non-urgent visits among uninsured, publicly insured and privately insured patients.[55] This study enrolled patients of all ages with a range of mild to severe influenza disease.
Conclusion
This study demonstrated that school age children acquired influenza A(H1N1)pdm09 and influenza B disease earlier than younger and older persons. The pattern for influenza A(H1N1)pdm09 and influenza A(H3N2) was children and adults 5–49 years of age presented prior to the epidemic midpoint. Each year our communities are besieged by influenza, relentless and lethal. Strongest defenses must stand before children; influenza strikes them first.
Highlights.
We prospectively measured relative timing of laboratory-confirmed influenza.
Influenza occurred earliest in children 5–17 years for influenza A(H1N1) and B.
Influenza occurred earliest in persons 5–49 years for influenza A(H3N2).
Yearly influenza occurs in school age children before the very young and elderly.
Acknowledgments
Funding Source: All phases of this study were supported by an NIH grant, R01 AI079226. The views expressed in this article are solely those of the authors and do not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the U.S. government. Dr. Poehling also received support from the Wachovia Research Foundation.
We thank all the patients and their families who generously participated in this surveillance as well as all the physicians and staff at Forsyth Medical Center and Wake Forest Baptist Health who made this study possible.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- RT-PCR
reverse transcriptase polymerase chain reaction
Footnotes
Components of this work were presented at the Pediatric Academic Societies meeting in Washington, DC in May 2013 and have been accepted for presentation at the Pediatric Academic Societies meeting in Vancouver, Canada in May 2014.
Conflicts of Interest Disclosure: Dr. Poehling, Dr. Peters, Ms. Blakeney and Ms. Vannoy have received research support from BD Diagnostics and MedImmune. No other authors have conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Timothy R. Peters, Email: tpeters@wakehealth.edu, Department of Pediatrics, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Beverly M. Snively, Email: bmellen@wakehealth.edu, Department of Biostatistical Sciences, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Cynthia K. Suerken, Email: csuerken@wakehealth.edu, Department of Biostatistical Sciences, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Elizabeth Blakeney, Email: eblakene@wakehealth.edu, Department of Pediatrics, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Lauren Vannoy, Email: lvannoy@wakehealth.edu, Department of Pediatrics, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Katherine A. Poehling, Email: kpoehlin@wakehealth.edu, Department of Pediatrics and Epidemiology and Prevention, Wake Forest School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
References
- 1.Poehling KA, Edwards KM, Griffin MR, et al. The burden of influenza in young children, 2004–2009. Pediatrics. 2013;131(2):207–16. doi: 10.1542/peds.2012-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubinson L, Mutter R, Viboud C, et al. Impact of the Fall 2009 Influenza A(H1N1)pdm09 Pandemic on US Hospitals. Med Care. 2013;51(3):259–65. doi: 10.1097/MLR.0b013e31827da8ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox CM, D’Mello T, Perez A, et al. Increase in rates of hospitalization due to laboratory-confirmed influenza among children and adults during the 2009–10 influenza pandemic. J Infect Dis. 2012;206(9):1350–8. doi: 10.1093/infdis/jis517. [DOI] [PubMed] [Google Scholar]
- 4.Fowlkes A, Dasgupta S, Chao E, et al. Estimating influenza incidence and rates of influenza-like illness in the outpatient setting. Influenza Other Respi Viruses. 2012;7(5):694–700. doi: 10.1111/irv.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuzil KM, Zhu Y, Griffin MR, et al. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J Infect Dis. 2002;185(2):147–52. doi: 10.1086/338363. [DOI] [PubMed] [Google Scholar]
- 6.Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory virus infections on persons with chronic underlying conditions. JAMA. 2000;283(4):499–505. doi: 10.1001/jama.283.4.499. [DOI] [PubMed] [Google Scholar]
- 7.Coffin SE, Zaoutis TE, Rosenquist AB, et al. Incidence, complications, and risk factors for prolonged stay in children hospitalized with community-acquired influenza. Pediatrics. 2007;119(4):740–8. doi: 10.1542/peds.2006-2679. [DOI] [PubMed] [Google Scholar]
- 8.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Disease Control Priorities in Developing Countries. Washington, DC: Oxford University Press and The World Bank; 2006. [PubMed] [Google Scholar]
- 10.Schanzer D, Vachon J, Pelletier L. Age-specific differences in influenza A epidemic curves: do children drive the spread of influenza epidemics? Am J Epidemiol. 2011;174(1):109–17. doi: 10.1093/aje/kwr037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monto AS, Davenport FM, Napier JA, Francis T., Jr Modification of an outbreak of influenza in Tecumseh, Michigan by vaccination of school children. J Infect Dis. 1970;122(1):16–25. doi: 10.1093/infdis/122.1-2.16. [DOI] [PubMed] [Google Scholar]
- 12.Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974–76. N Engl J Med. 1978;298(11):587–92. doi: 10.1056/NEJM197803162981103. [DOI] [PubMed] [Google Scholar]
- 13.Gaglani MJ, Piedra PA, Herschler GB, et al. Direct and total effectiveness of the intranasal, live-attenuated, trivalent cold-adapted influenza virus vaccine against the 2000–2001 influenza A(H1N1) and B epidemic in healthy children. Arch Pediatr Adolesc Med. 2004;158(1):65–73. doi: 10.1001/archpedi.158.1.65. [DOI] [PubMed] [Google Scholar]
- 14.Piedra PA, Gaglani MJ, Riggs M, et al. Live attenuated influenza vaccine, trivalent, is safe in healthy children 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in a community-based, nonrandomized, open-label trial. Pediatrics. 2005;116(3):e397–e407. doi: 10.1542/peds.2004-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prevention of influenza: recommendations for influenza immunization of children, 2008–2009. Pediatrics. 2008;122(5):1135–41. doi: 10.1542/peds.2008-2449. [DOI] [PubMed] [Google Scholar]
- 16.Meissner HC. Reducing the impact of viral respiratory infections in children. Pediatr Clin North Am. 2005;52(3):695–710. doi: 10.1016/j.pcl.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monto AS. Individual and community impact of influenza. Pharmacoeconomics. 1999;16 (Suppl 1):1–6. doi: 10.2165/00019053-199916001-00001. [DOI] [PubMed] [Google Scholar]
- 18.Poland GA, Hall CB. Influenza immunization of schoolchildren: can we interrupt community epidemics? Pediatrics. 1999;103(6 Pt 1):1280–2. doi: 10.1542/peds.103.6.1280. [DOI] [PubMed] [Google Scholar]
- 19.Ahout I, Ferwerda G, de GR. Influenza vaccination in kids, are you kidding me? J Infect. 2014;68 (Suppl 1):S100–S107. doi: 10.1016/j.jinf.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Doshi P, bi-Jaoude E, Lexchin J, Jefferson T, Thomas RE. Influenza vaccination of health care workers. CMAJ. 2013;185(2):150. doi: 10.1503/cmaj.113-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jefferson T, Rivetti A, Di PC, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2012;8:CD004879. doi: 10.1002/14651858.CD004879.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jefferson T, Di PC, Debalini MG, Rivetti A, Demicheli V. Inactivated influenza vaccines: methods, policies, and politics. J Clin Epidemiol. 2009;62(7):677–86. doi: 10.1016/j.jclinepi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Manzoli L, Ioannidis JP, Flacco ME, De VC, Villari P. Effectiveness and harms of seasonal and pandemic influenza vaccines in children, adults and elderly: a critical review and re-analysis of 15 meta-analyses. Hum Vaccin Immunother. 2012;8(7):851–62. doi: 10.4161/hv.19917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogan TJ. Issues in the economic evaluation of influenza vaccination by injection of healthy working adults in the US: a review and decision analysis of ten published studies. Pharmacoeconomics. 2012;30(5):355–71. doi: 10.2165/11596890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. FluView: National and Regional Level Outpatient Illness and Viral Surveillance. 2013 Available from URL: http://gis.cdc.gov/grasp/fluview/fluportaldashboard.html.
- 26.Centers for Disease Control and Prevention. 2009–2010 Influenza Season Summary. 2011 Available from URL: http://www.cdc.gov/flu/weekly/weeklyarchives2009-2010/09-10summary.htm.
- 27.Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1–52. [PubMed] [Google Scholar]
- 28.Uzicanin A, Thompson M, Smith P, et al. Effectiveness of 1 dose of influenza A (H1N1) 2009 monovalent vaccines in preventing reverse-transcription polymerase chain reaction-confirmed H1N1 infection among school-aged children in maine. J Infect Dis. 2012;206(7):1059–68. doi: 10.1093/infdis/jis441. [DOI] [PubMed] [Google Scholar]
- 29.Treanor JJ, Talbot HK, Ohmit SE, et al. Effectiveness of seasonal influenza vaccines in the United States during a season with circulation of all three vaccine strains. Clin Infect Dis. 2012;55(7):951–9. doi: 10.1093/cid/cis574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piedra PA, Gaglani MJ, Kozinetz CA, et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine. 2005;23(13):1540–8. doi: 10.1016/j.vaccine.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Loeb M, Russell ML, Moss L, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA. 2010;303(10):943–50. doi: 10.1001/jama.2010.250. [DOI] [PubMed] [Google Scholar]
- 32.Reichert TA, Sugaya N, Fedson DS, Glezen WP, Simonsen L, Tashiro M. The Japanese experience with vaccinating schoolchildren against influenza. N Engl J Med. 2001;344(12):889–96. doi: 10.1056/NEJM200103223441204. [DOI] [PubMed] [Google Scholar]
- 33.Copeland DL, Basurto-Davila R, Chung W, et al. Effectiveness of a School District Closure for Pandemic Influenza A (H1N1) on Acute Respiratory Illnesses in the Community: A Natural Experiment. Clin Infect Dis. 2013;56(4):509–16. doi: 10.1093/cid/cis890. [DOI] [PubMed] [Google Scholar]
- 34.Grohskopf L, Uyeki T, Bresee J, Cox N, Shimabukuro T. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)--United States, 2012–13 influenza season. MMWR. 2012;61(32):613–8. [PubMed] [Google Scholar]
- 35.Rand CM, Szilagyi PG, Yoo BK, Auinger P, Albertin C, Coleman MS. Additional visit burden for universal influenza vaccination of US school-aged children and adolescents. Arch Pediatr Adolesc Med. 2008;162(11):1048–55. doi: 10.1001/archpedi.162.11.1048. [DOI] [PubMed] [Google Scholar]
- 36.Kempe A, Barrow J, Stokley S, et al. Effectiveness and cost of immunization recall at school-based health centers. Pediatrics. 2012;129(6):e1446–e1452. doi: 10.1542/peds.2011-2921. [DOI] [PubMed] [Google Scholar]
- 37.Fiore AE, Epperson S, Perrotta D, Bernstein H, Neuzil K. Expanding the recommendations for annual influenza vaccination to school-age children in the United States. Pediatrics. 2012;129 (Suppl 2):S54–S62. doi: 10.1542/peds.2011-0737C. [DOI] [PubMed] [Google Scholar]
- 38.Glezen WP, Couch RB, Taber LH, et al. Epidemiologic observations of influenza B virus infections in Houston, Texas, 1976–1977. Am J Epidemiol. 1980;111(1):13–22. doi: 10.1093/oxfordjournals.aje.a112865. [DOI] [PubMed] [Google Scholar]
- 39.Glezen WP, Gaglani MJ, Kozinetz CA, Piedra PA. Direct and indirect effectiveness of influenza vaccination delivered to children at school preceding an epidemic caused by 3 new influenza virus variants. J Infect Dis. 2010;202(11):1626–33. doi: 10.1086/657089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis MM, King JC, Jr, Moag L, Cummings G, Magder LS. Countywide school-based influenza immunization: direct and indirect impact on student absenteeism. Pediatrics. 2008;122(1):e260–e265. doi: 10.1542/peds.2007-2963. [DOI] [PubMed] [Google Scholar]
- 41.Nobusawa E, Sato K. Comparison of the mutation rates of human influenza A and B viruses. J Virol. 2006;80(7):3675–8. doi: 10.1128/JVI.80.7.3675-3678.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita M, Krystal M, Fitch WM, Palese P. Influenza B virus evolution: co-circulating lineages and comparison of evolutionary pattern with those of influenza A and C viruses. Virology. 1988 Mar;163(1):112–22. doi: 10.1016/0042-6822(88)90238-3. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Holmes EC. The evolutionary dynamics of human influenza B virus. J Mol Evol. 2008 Jun;66(6):655–63. doi: 10.1007/s00239-008-9119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology. 1990 Mar;175(1):59–68. doi: 10.1016/0042-6822(90)90186-u. [DOI] [PubMed] [Google Scholar]
- 45.Fiore AE, Shay DK, Broder K, et al. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008 Aug 8;57(RR-7):1–60. [PubMed] [Google Scholar]
- 46.Setse RW, Euler GL, Gonzalez-Feliciano AG, et al. Influenza Vaccination Coverage--United States, 2000–2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):38–41. [PubMed] [Google Scholar]
- 47.Centers for Disease Control and Prevention. Final state-level influenza vaccination coverage estimates for the 2010–2011 season-United States. National Immunization Survey and Behavioral Risk Factro Surveillance System. August 2010 through May 2011 Available from URL: http://www.cdc.gov/flu/professionals/vaccination/coverage_1011estimates.htm.
- 48.Gargano LM, Pazol K, Sales JM, et al. Multicomponent interventions to enhance influenza vaccine delivery to adolescents. Pediatrics. 2011 Nov;128(5):e1092–e1099. doi: 10.1542/peds.2011-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hurt AC, Baas C, Deng YM, Roberts S, Kelso A, Barr IG. Performance of influenza rapid point-of-care tests in the detection of swine lineage A(H1N1) influenza viruses. Influenza Other Respi Viruses. 2009 Jul;3(4):171–6. doi: 10.1111/j.1750-2659.2009.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. Updated Interim Recommendations for the Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009–2010 Season. Available from URL: http://www.cdc.gov/H1N1flu/recommendations.htm.
- 51.Jackson ML, France AM, Hancock K, et al. Serologically confirmed household transmission of 2009 pandemic influenza A (H1N1) virus during the first pandemic wave--New York City, April-May 2009. Clin Infect Dis. 2011 Sep;53(5):455–62. doi: 10.1093/cid/cir437. [DOI] [PubMed] [Google Scholar]
- 52.Sarver JH, Cydulka RK, Baker DW. Usual source of care and nonurgent emergency department use. Acad Emerg Med. 2002 Sep;9(9):916–23. doi: 10.1111/j.1553-2712.2002.tb02193.x. [DOI] [PubMed] [Google Scholar]
- 53.Cunningham PJ, Clancy CM, Cohen JW, Wilets M. The use of hospital emergency departments for nonurgent health problems: a national perspective. Med Care Res Rev. 1995 Nov;52(4):453–74. doi: 10.1177/107755879505200402. [DOI] [PubMed] [Google Scholar]
- 54.Brousseau DC, Nimmer MR, Yunk NL, Nattinger AB, Greer A. Nonurgent emergency-department care: analysis of parent and primary physician perspectives. Pediatrics. 2011 Feb;127(2):e375–e381. doi: 10.1542/peds.2010-1723. [DOI] [PubMed] [Google Scholar]
- 55.Garcia TC, Bernstein AB, Bush MA. Emergency department visitors and visits: Who used the emergency room in 2007? Hyattsville, MD: National Center for Health Statistics; 2010. Report No.: 38. [PubMed] [Google Scholar]