Abstract
Oxidative stress suppresses host immunity by generating oxidized lipid agonists of the platelet-activating factor receptor (PAF-R). Because many classical chemotherapeutic drugs induce reactive oxygen species (ROS), we investigated whether these drugs might subvert host immunity by activating PAF-R. Here we show that PAF-R agonists are produced in melanoma cells by chemotherapy that is administered in vitro, in vivo or in human subjects. Structural characterization of the PAF-R agonists induced revealed multiple oxidized glycerophosphocholines that are generated non-enzymatically. In a murine model of melanoma, chemotherapeutic administration could augment tumor growth by a PAF-R-dependent process that could be blocked by treatment with antioxidants or cyclooxygenase-2 inhibitors or by depletion of regulatory T cells. Our findings reveal how PAF-R agonists induced by chemotherapy treatment can promote treatment failure. Further, they offer new insights into how to improve the efficacy of chemotherapy by blocking its heretofore unknown impact on PAF-R activation.
Keywords: Chemotherapy, Oxidized glycerophosphocholines, Platelet-activating factor, antioxidants
INTRODUCTION
Though not the most common type of skin cancer, malignant melanoma is one of the most lethal (1). The American Cancer Society estimates over 76,000 new patients were diagnosed with melanoma of the skin, and 9480 died from malignant melanoma in 2013 (www.cancer.org/statistics). The treatment for early stage (non-metastatic) melanoma is surgical excision. The treatment of metastatic melanoma is unsatisfactory as this tumor type is relatively resistant to chemotherapy or radiotherapy, possibly in part through cellular resistance to these agents (2,3). However, regional chemotherapy such as isolated limb chemoperfusion (ILP) with high-dose melphalan (10 times more than standard chemotherapy) for localized disease has proven efficacious, with complete responses in up to 50% and overall response rates approaching 90% (4–6). Yet, most of these responses are short-lived. Immunotherapy strategies appear to have the most promise for cure as the immune response is critical for the eradication of this tumor type (7,8).
Chemotherapeutic agents are designed to selectively kill tumor cells, and spare their non-neoplastic counterparts. Several targets for chemotherapeutic agents exist, including DNA and/or DNA replication/repair machinery (9). One of the consequences of many chemotherapeutic agents is the generation of reactive oxygen species (ROS) (10–13). Several groups have demonstrated suppression of host immunity in the presence of various pro-oxidative stressors through a mechanism involving platelet-activating factor (1-alkyl-2-acetyl-glycerophosphocholine; PAF). Pro-oxidative stressors including aromatic hydrocarbons found in jet fuel, cigarette smoke, and ultraviolet B radiation (UVB) (14–18) generate ROS that can act directly on glycerophosphocholines (GPC) to produce oxidized GPC (Ox-GPC) which are potent PAF-receptor (PAF-R) agonists (18–21). Structural studies using mass spectrometry have identified more than a dozen Ox-GPC, including native PAF itself (20–24). There is evidence for hundreds of these biologically active compounds (22–25) that have by-passed the tightly controlled enzymatic process of PAF production (26,27). Like native PAF, these Ox-GPC are metabolically unstable and their half-lives in tissue/blood are measured in minutes (27,28).
In addition to the ability of pro-oxidative stressors (e.g., UVB) or exogenous PAF-R agonists to inhibit host immunity as measured by contact hypersensitivity (CHS) responses to either chemical antigens such as 2, 4-dinitrofluorobenzene (DNFB) or delayed type hypersensitivity responses to antigens such as Candida albicans (14–18,29), recent studies have indicated that systemic PAF-R activation can augment experimental tumor growth in a process involving the cytokine IL-10 and Tregs (30). PAF-R antagonists have also been demonstrated to protect against UVB-mediated photocarcinogenesis in mice (31). PAF-mediated systemic immunosuppression involves interleukin 10 (IL-10) and cyclooxygenase-2 (COX-2)-generated eicosanoids with mast cells and regulatory T cells (Tregs) as effectors.
Since chemotherapeutic agents can induce ROS, the present studies were designed to test whether chemotherapeutic agents can generate PAF-R agonists as well as their structural characterization. Finally, these studies sought to define whether ROS-generated PAF-R agonists impact chemotherapy effectiveness. These studies provide the first evidence that chemotherapeutic agents induce systemic immunosuppression via systemic PAF-R signaling in a process that can be ameliorated via antioxidants and COX-2 inhibitors.
MATERIALS AND METHODS
Reagents and cell lines
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless indicated otherwise. B16F10 and SK23MEL cells obtained from ATCC (Boston, MA) were grown in DMEM high glucose with 10%FCS as previously described (30). Cell lines were grown to approximately 80–90% confluence in 10 cm dishes, and washed three times with Hanks Balanced Salt Solution (HBSS) and then incubated with 2 ml of pre-warmed (37 °C) HBSS with 10mg/ml fatty acid-free BSA with 2 µM of the serine hydrolase inhibitor pefabloc. In some experiments, antioxidants were preincubated for 60 min before addition of chemotherapeutic agents or DMSO (0.5%) vehicle. The incubations were quenched by addition of 2 ml of ice-cold methanol followed by methylene chloride, and lipids extracted as described (17,18,20).
Mice
Female C57BL/6-wild type mice (PAF-R expressing; age 6–8 week) were purchased from The Charles River Laboratories. Age-matched female PAF-R-deficient (Ptafr−/−) mice on a C57BL/6 background, generated as described previously (32), were a kind gift of Professor Takao Shimizu (University of Tokyo Department of Biochemistry). FoxP3-EGFP reporter mice (33) obtained from JAX, and FoxP3EGFP mice crossed with Ptafr−/− mice were used in some experiments. It should be noted that similar effects were noted between Ptafr−/− and Ptafr−/− Foxp3EGFP mice. Immunodeficient NOD.CB17-PrkdcSCID/J (Common name: NOD SCID) mice were purchased from the Indiana University Simon Cancer Center Core facility. In some experiments mice were fed with vitamin C-enriched (10g/kg; Research Diets, Inc., New Brunswick, NJ) and 5 mM N-acetylcysteine (NAC) in water ad libitum for 10 days prior to intratumoral chemotherapy injection of tumor and until the termination of the experiment as per our previous studies (17,30). All mice were housed under specific pathogen-free conditions at the Indiana University School of Medicine. All procedures were approved by the Animal Care and Use Committee of Indiana University School of Medicine.
Measurement of PAF-R agonists
Calcium mobilization studies
The presence of systemic PAF-R agonists in lipid extracts derived from the chemotherapeutic agent-treated tumors/cell lines was measured by the ability of the lipid extracts to induce an intracellular Ca2+ mobilization response in PAF-R expressing KBP cells, but not in KBM cells lacking the PAF-R, as previously described (17,34). In brief, KBP and KBM cells were preloaded with the Ca2+-sensitive indicator, fura-2-AM (4 µM in Hanks' balanced salt solution without dye) at 37°C for 90 min, washed and resuspended in Hanks' balanced salt solution at room temperature before use. Lipid extracts from cells or weighed tumors obtained from groups of chemotherapy vs vehicle treated cells/tumors untreated (sham) exposed mice were added to an aliquot of these cells (1.0–1.5 × 106 cells/2 ml) in a cuvette at 37°C with constant stirring. The lipid extracts were normalized to cell number or mg wet tissue weight or 1/10th volume of perfusate. CPAF and endothelin-1 (ET-1) dissolved in ethanol (adjusted to 1µM) were used as positive controls. Fura-2-AM fluorescence was monitored in a Hitachi F-4010 spectrophotometer with excitation and emission wavelengths of 331 and 410 nm, respectively. The Ca2+ influx in suspensions was calculated as described (17,18,34) and shown as percentage of maximal peak calcium flux induced by either CPAF or ET-1.
Mass Spectrometry studies
Mass spectrometry was performed on cell lines and perfusion samples using the AB Sciex (Foster City, CA) triple quadrupole QTRAP® 5500 mass spectrometer, equipped with a CTC-PAL autosampler and a Shimadzu HPLC as previously described (24). Please see on-line Supplemental Methods for details of instrument settings and characterization of the various species monitored.
In vivo tumor growth studies
To determine the ability of intratumoral chemotherapy to modulate melanoma tumor growth, 0.5×106 B16F10 cells which lack functional PAF-R (30), were implanted subcutaneously into both shaved hind flanks of WT and Ptafr−/− mice to produce two tumors. Tumor growth (length and width) was monitored and measured at various times with digital calipers (Mitituyo), and tumor volume was calculated (major length × minor length2/2). On day 6 of tumor implantation and every three days afterwards, the left flank tumor was injected with 100 µl of either etoposide (36 mg/kg), melphalan (15 mg/kg), or PBS with 0.5% DMSO vehicle. The working dose of etoposide and melphalan was achieved by performing dose-dependent pilot studies in WT mice (n=3–5). To define the ability of COX-2 inhibitors to modulate the effects of chemotherapy, SC-236 (200 ng), NS-398 (5 µg), or 100ul PBS with 0.5% DMSO vehicle were injected i.p. at day 0 and every 3 days afterwards.
Human regional chemotherapy studies
Subjects undergoing regional chemotherapy with melphalan for melanoma were recruited for these studies. During the procedure, 8 ml of perfusate was removed at various times (after establishment of the perfusion, once the core limb temperature was 40 °C, and 15, 30, 45 and 60 min following melphalan treatment) from the circuit and placed into equal volumes of ice-cold methanol and methylene chloride, and lipids extracted. The human studies were approved by the Indiana University’s and Duke University’s School of Medicine Institutional Review Boards.
Statistical analysis
For all murine studies, individual experiments were performed using at least 4 mice per experimental group and repeated as necessary (at least once) to verify reproducibility and provide additional data for analysis. All statistical calculations were performed using SAS Version 9.3. Tumor volume was calculated as (major × minor2)/2.For the mice studies, the analysis focused on the end of the study, specifically on Days 14–18, where available. The normality of data and equal variances were checked by Shapiro-Wilk’s and Levene’s tests and was a reasonable assumption in all cases. For the mice data and in vitro data, we used equal or unequal variance t-tests to compare two groups. For comparing more than two groups, we used analysis of variance (with the Welch approximation if the variances were unequal) and post hoc Tukey-adjusted pair-wise tests. The data represent mean values with SE. Differences were considered statistically significant when the P value was less than 0.05 and marginally significant when the P value was less than 0.10.
RESULTS
Chemotherapeutic agents generate PAF-R agonists in a process blocked by antioxidants
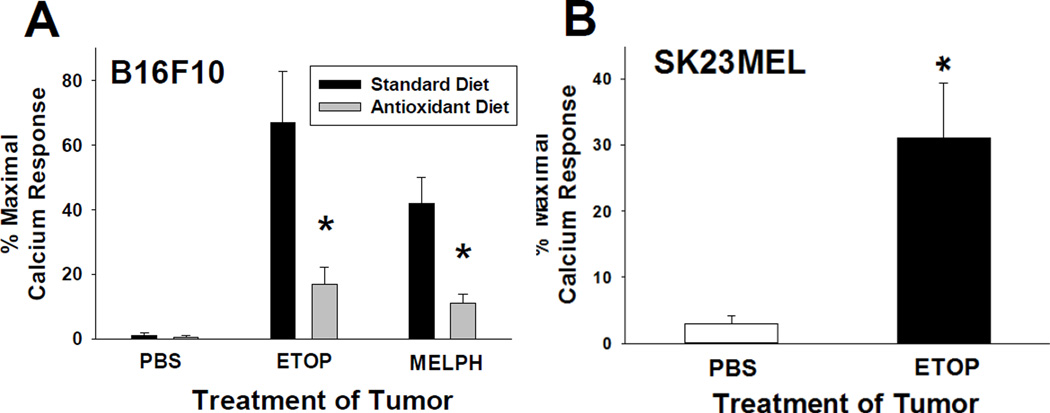
The first studies were designed to test whether chemotherapeutic agents can induce PAF-R agonists in melanoma cells. As multiple glycerophosphocholine species can act as PAF-R agonists, we quantified total PAF-R biochemical activity as measured by intracellular calcium mobilization responses in Fura-2-loaded PAF-R-expressing KBP cells (34) compared to excess (1 µM) of the metabolically stable PAF-R agonist carbamoyl-PAF (CPAF) in lipid extracts from murine B16F10 cells following treatment with the diverse agents etoposide, cisplatin or melphalan. Note that the amount of total PAF-R agonistic activity is defined as the Ca2+ mobilization peak height of the normalized lipid extract as a % of the peak height from excess CPAF (1 µM) response (17,18,34). Use of this semiquantitative biochemical assay allows all PAF-R activity to be measured. As shown in Fig. 1a, lipid extracts from B16F10 melanoma cells treated with chemotherapeutic agents induced PAF-R agonistic activity only in PAF-R-expressing KBP but not in PAF-R-negative KBM cells. Moreover, treatment of KBP but not KBM with these lipid extracts derived from chemotherapeutic agent-treated B16F10 cells resulted in the release of IL-8 in the supernatants using our published (18) methodology (data not shown). Time course studies revealed that chemotherapeutic agents triggered PAF agonistic activity in murine B16F10 cells that were similar to human SK23MEL cells (Fig. 1b and Supplementary Fig. S1). Murine B16F10 melanoma cells do not express functional PAF-Rs as measured by lack of Ca2+ response to CPAF, and lack of PAF-R mRNA by qPCR (39). To define whether the presence of the PAF-R (which has been reported to be expressed on many human melanomas (35)) can modulate chemotherapy-induced PAF agonistic activity, we transduced B16F10 cells with the PAF-R gene construct using MSCV2.1 retrovirus, generating B16F10PAF-R (PAF-R-expressing;B16P) and B16F10MSCV2.1 (control PAF-R-negative; B16M) cells (see ref 30 for characterization of these cell lines). Chemotherapeutic agent treatment resulted in significantly more PAF-R agonistic activity in the PAF-R-expressing B16P cells (Fig. 1c). Similar to our previously published findings examining PAF-R biochemical activity generated in human epithelial tumor cells or human skin in response to the pro-oxidative stressor UVB (20,24,36), preincubation of melanoma cells with antioxidants vitamin C or N-acetylcysteine (NAC) blocked this PAF agonistic activity (Fig. 1d).
Figure 1. Chemotherapy agents generate PAF-R agonist formation in melanoma cells.
A). Examples of PAF-R Ca2+ biochemical assays. PAF-R-expressing KBP or PAF-R-negative KBM cells were loaded with Fura-2 AM, and treated with lipid extracts derived from 5 × 106 B16F10 cells treated with 100 µg/ml etoposide or DMSO vehicle for 1 h, and intracellular Ca2+ levels monitored over time. Excess (1 µM CPAF or endothelin-1 [ET-1]) was added at the end of the assay to allow quantitation of the Ca2+ response. B). Time course of chemotherapy-generated PAF-R activity. Lipid extracts were obtained from 5 × 106 B16F10 cells treated with 100 µg/ml of chemotherapeutic agents, or 0.5% DMSO vehicle for various times, and tested for total PAF-R agonistic activity using PAF-R-positive KBP cells loaded with the calcium-specific dye Fura-2. The data are the Mean ± SE percentage of peak intracellular calcium response as a percentage of that induced by 1 µM CPAF from at least four separate experiments. C). Dose-responsiveness of chemotherapy-generated PAF-R agonists in PAF-R-negative vs B16F10 cells expressing PAF-Rs. Lipid extracts were obtained from PAF-R-expressing B16F10PAF-R (B16P) or control B16F10MSCV2.1 (B16M) cells treated with various doses of chemotherapeutic agents for 1 h, and tested for PAF-R agonistic activity as above. The data are the mean ± SE percentage of peak intracellular calcium response (normalized to CPAF) from 3–4 separate experiments. D). Chemotherapy agent-stimulated PAF-R agonist formation is inhibited by antioxidants. Lipid extracts were obtained from B16P and B16M cells preincubated for 1 h with antioxidants vitamin C (2.5 mM), NAC (5 mM), or 0.5% DMSO vehicle prior to a 1 h treatment with 100 µg/ml chemotherapeutic agents, and tested for total PAF-R agonistic activity. The data are the mean ± SE percentage of peak intracellular calcium response (normalized to CPAF) from at least three separate experiments. * Denotes statistically significant (P <0.05) changes in levels of PAF-R agonist activity from control values for figures (B) and (C), and differences between chemotherapy-treated B16P and B16M cells in (D). For (C) the significant changes were for cisplatin at 10 µg/ml and for cisplatin, and etoposide at 100 µg/ml vs 0 dose.
Mass spectrometry-based structural studies using deuterium-labeled internal standards revealed that there were significantly increased levels of not only native PAF, but also Ox-GPC PAF-R agonists produced non-enzymatically in B16F10 cells in response to etoposide treatment (Fig. 2). Levels of a large number of individual Ox-GPC were increased 2–3 fold over baseline, but no consistent changes in lyso-GPC species were noted (data not shown). The actual amounts, structures and methods used to measure the Ox-GPCs are shown in Supplementary Table I and the supplementary online methods. These studies indicate that chemotherapeutic agents can trigger the generation of Ox-GPCs with PAF-R activity in murine and human melanoma cells in vitro.
Figure 2. Structural characterization of chemotherapy-generated PAF-R agonists.
B16F10 cells were treated with 100 µg/ml of etoposide, or 0.5% DMSO vehicle for two hours. Lipid extracts were analyzed by HPLC/MS/MS using deuterium-labeled internal standards to quantify PAF and Ox-GPC species. The data are expressed as mean ± SE fold increase of etoposide over vehicle-treated from 5 separate experiments. Please see Supplementary Table I for structures and exact values for the GPC species. * Denotes statistically significant (P < 0.05) changes from vehicle-treated.
Intratumoral injection of chemotherapeutic agents generates PAF-R agonists
Though not commonly used, intratumoral injection of chemotherapeutic agents is a viable treatment for localized disease (37,38). To define whether chemotherapeutic agents can induce the production of PAF-R agonists in vivo, B16F10 tumors were implanted into syngeneic C57BL/6 murine hosts or human SK23MEL tumors implanted into immunodeficient SCID mice. When the tumors were approximately 10 mm in diameter, they were injected with either etoposide or melphalan or PBS vehicle control. Tumors were removed one hour post-injection, weighed, and lipids extracted and PAF-R biochemical activity assayed. Intratumoral injections of chemotherapy agents but not PBS vehicle resulted in the production of PAF-R agonists in both murine B16F10 and human SK23MEL tumors (Fig. 3). Of interest, injection of chemotherapeutic agents directly into skin without tumors did not result in the generation of significant levels of PAF-R agonistic activity (data not shown).
Figure 3. Intratumoral chemotherapy generates PAF-R agonist formation in vivo.
Lipid extracts were obtained from A). Murine B16F10 tumors implanted on WT mice fed 10 mg/kg vitamin C-enriched chow + 5 mM NAC in water ad libitum or standard diet/water for 10 days before tumor implantation or B) Human SK23MEL tumors implanted onto SCID mice fed standard diet, one hour following intratumoral injection with either 36mg/kg etoposide or 15mg/kg melphalan or 100 µl PBS vehicle, normalized to wet tumor weight (10 mg), and were tested for PAF-R agonistic activity as in Fig. 1. The data are the mean ± SE percentage of peak intracellular calcium response (normalized to CPAF) from 4–6 separate tumors. *Denotes statistically significant (P < 0.05) changes in levels of PAF-R agonists in comparison to vehicle-treated.
To assess the ability of antioxidants treatment to modulate chemotherapy-generated PAF-R agonists in vivo, C57BL/6 mice were fed with vitamin C-enriched chow and 5 mM NAC in water ad libitum for 10 days before tumor implantation and continued on antioxidant diet until the termination of the experiment. Tumors were treated with either etoposide, melphalan, or vehicle and PAF-R agonist activity determined from lipid extracts of 1 hour treated tumors. This antioxidant regimen, which we have previously demonstrated blocks both the inhibition of CHS and augmentation of tumor growth induced by UVB treatment (17,30) results in the decreased formation of PAF-R agonists by chemotherapy (Fig. 3a). These studies indicate that chemotherapeutic agents can generate PAF-R agonists in vivo, in a process partially blocked by antioxidants.
PAF-R activation diminishes experimental chemotherapy effectiveness via Tregs in a COX-2-dependent process
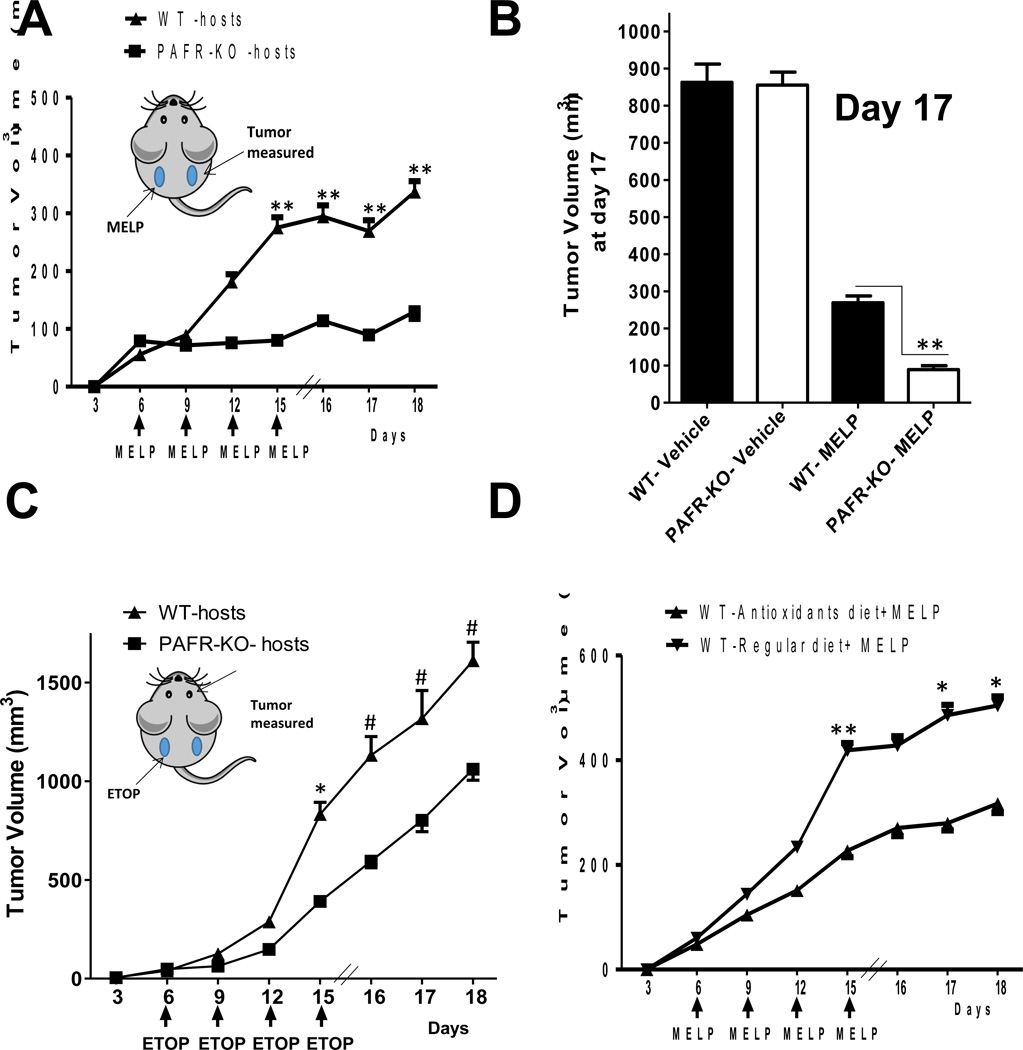
Given our findings that intratumoral chemotherapy generates PAF-R agonists in vivo and that systemic PAF-R activation augments experimental tumor growth via immunosuppression (30), we assessed whether intratumoral chemotherapy could generate enough PAF-R agonists to modulate tumor growth. Our protocol was modified in that WT and PAF-R-deficient (Ptafr−/−, PAFR-KO) mice underwent implantation with B16F10 tumors on both dorsal hindquarters (2 tumors/mouse). The left flanktumors were treated with intratumoral chemotherapy or PBS vehicle control starting at day 6 of tumor cell implantation and repeated every 3 days until the termination of the experiment, while the other (right flank) tumors were left undisturbed. Intratumoral chemotherapy of one tumor with melphalan (15 mg/kg) or etoposide (36 mg/kg) resulted in an enhanced growth of the second (undisturbed) tumor in PAF-R-positive WT compared with Ptafr−/− hosts (Fig. 4a–c). Though intratumoral chemotherapy resulted in growth inhibition of the chemotherapy-treated left flank tumors, loss of host PAF-R function exerted no perceptible effect on the left flank tumor growth characteristics (Supplementary Fig S2). Subjecting mice to antioxidant treatment had no effects on the untreated right flank tumor growth by itself, yet inhibited the melphalan-mediated augmentation of growth of the second tumor (Fig. 4d). Antioxidant diet also blocked etoposide-mediated augmentation of right flank tumor growth (Supplementary Fig. S3a). Importantly, antioxidant diet had no effect on the growth of right flank tumors in chemotherapy-treated PAF-R-deficient hosts (Supplementary Fig. S3). Of note, we did not detect differences in the growth of the chemotherapy-treated left flank tumors when WT or Ptafr−/− mice were placed on an antioxidant diet. Overall, these findings fit with the concept that chemotherapeutic agents generate Ox-GPCs via ROS which then inhibit tumor immunity resulting in an augmentation of tumor growth in a PAF-R-dependent manner.
Figure 4. Intratumoral chemotherapy treatment augments the growth of untreated B16F10 melanomas in a PAF-R-dependent manner.
WT and PAFR-KO (Ptafr−/−) mice were implanted with B16F10 tumors on both the dorsal hind flanks. Six days later (and q 3days afterwards) one of the tumors (on the left side) was treated with A, B). 15 mg/kg melphalan (n=6–7), C). 36 mg/kg etoposide (n=9–12), or vehicle (n=5–6), and the other tumor (on the right side) left undisturbed. The data depicted are the mean ± SE of tumor volume of untreated tumors over time. B). Data represent the volume of untreated tumors at day 17 from vehicle and melphalan-treated WT and PAFR-KO mice. D). Effect of antioxidants on the melphalan-mediated increased tumor growth. WT mice were placed on antioxidant diet as in Fig. 3 for 10 days before placement of dual B16F10 tumors, followed by intratumoral treatment with melphalan (n=10–11) every 3 days starting at day 6. The data depicted are the mean ± SE of tumor volume of untreated tumors over time. Between WT & PAFR-KO mice, there were no statistically significant differences in the growth of chemotherapy- or vehicle-injected tumors nor the growth of the undisturbed tumors in response to vehicle treatment of the contralateral tumors. Statistical significance of changes in tumor volumes denoted by **(P < 0.01) or * (P < 0.05) or #(P<0.1).
Previous reports have demonstrated that PAF-R-mediated inhibition of CHS is blocked by COX-2 inhibitors (14–18). To assess whether COX-2 is crucial for PAF-R mediated augmentation of experimental tumor growth, WT and Ptafr−/− mice were implanted with a single B16F10 tumor and then treated with COX-2 inhibitors (SC-236 & NS-398) alone or along with systemic CPAF. COX-2 inhibitors alone did not affect tumor growth, yet blocked CPAF-mediated augmentation of B16F10 tumor growth in WT mice (Fig. 5a,b) but had no effect on Ptafr−/− hosts (Supplementary Fig. S4). Pharmacologic inhibition of COX-2 also blocked the augmentation of tumor growth associated with chemotherapy (Fig. 5c for melphalan and Supplementary Fig. S5 for etoposide). These studies indicate that chemotherapy-generated PAF-R agonists augment experimental tumor growth in a process inhibited by antioxidants and COX-2 inhibitors.
Figure 5. Role of COX-2 and Tregs in chemotherapy-mediated PAF-R-dependent augmentation of tumor growth.
A,B). COX-2 inhibitor blocks PAF-R-augmentation of tumor growth. WT mice (n=6–7) implanted with a single tumor were treated at day 0 and every 6 days with ip injections of CPAF (250 ng) or vehicle, with or without COX-2 inhibitors A) SC-236 (200 ng), B) NS-398 (5 µg). C). COX-2 inhibitor blocks chemotherapy-mediated augmentation of tumor growth. WT mice implanted with two tumors were treated with SC-236 or vehicle at day 0 and every 3 days, and underwent intratumoral treatment with PBS vehicle (n=7) or melphalan (n=16) (C) every 3 days starting at day 6. Tumor growth was assessed over time as in Fig 4. The data depicted are the mean ± SE of tumor volume of untreated tumors over time in which the contralateral tumor was treated with chemotherapeutic agent. D). Depleting Tregs blocks etoposide-mediated enhanced growth of secondary tumors. WT mice (n=7–8) were treated with isotype control (IgG1 and IgM1) or depleting antibodies against CD25 (clones PC61.5.3 IgG1 and 7D4 IgM1, 1 mg each) two days before dual tumor implantation and etoposide treatment as outlined in Fig 4. Statistical significance of changes in tumor volumes denoted by **(P < 0.01) or * (P < 0.05) or #(P<0.1).
PAF-R-dependent inhibition of CHS reactions is due to an increase in Tregs (18). Our previous studies have also demonstrated that Tregs are necessary for UVB-mediated augmentation of B16F10 tumor growth (30). The next studies were designed to define the role of Tregs in chemotherapy-mediated augmentation of experimental tumor growth. Use of a Treg-depleting strategy (anti-CD25 antibodies) which we have previously reported blocked UVB-mediated upregulation of tumoral Tregs (30) inhibited the chemotherapy-mediated augmentation of second tumor growth (Fig. 5d). Using Foxp3EGFP reporter transgenic mice we measured increased numbers of Tregs in tumors in response to systemic PAF-R activation which was blocked by COX-2 inhibition (Supplementary Fig. S6). These studies demonstrate that systemic PAF-R-mediated augmentation of tumor growth is dependent upon Tregs and suggest that COX-2 is necessary for their formation.
Identification of PAF-R agonists produced in response to chemotherapy in humans
To test whether chemotherapy exposure results in the production of PAF-R agonists in humans, 8 ml aliquots of perfusate were removed at various times from human subjects undergoing regional chemotherapy for melanoma. In this procedure, the major artery and vein of an affected extremity is cannulated and blood along with melphalan is perfused for 30–60 minutes in the heated (40 °C) limb (39). Minimal amounts of PAF-R agonists were found in the circulating perfusate before addition of the melphalan (Fig. 6a). However, once the melphalan chemotherapy began perfusing in the heated limb, significant amounts of PAF-R agonistic activity was measured in the perfusates, with the highest amounts found at the conclusion of regional chemotherapy treatment. Structural characterization of perfusates using mass spectrometry identified PAF and several Ox-GPC species (Fig. 6b). These studies indicate that chemotherapy exposure generates systemic PAF-R agonists in humans.
Figure 6. PAF-R agonists are generated during regional chemotherapy.
A). Lipid extracts were obtained from 8 ml of perfusate drawn at various times (once the circuit was placed [Control], once the limb was at 40° C [Heat], or following addition of melphalan) from six separate subjects during isolated limb chemoperfusion and tested for PAF-R activity as in Figure 1. The data are the mean ± SE percentage of peak intracellular calcium response (normalized to 1 µM CPAF and 1/10th of blood volume) of duplicate samples. B). Structural characterization of Ox-GPCs in human subjects. Control and 30 min post chemotherapy perfusates from three subjects were subjected to mass spectrometry as outlined in Fig. 2. The data depicted are mean ± SE ng of GPC per 8 ml perfusate from three separate subjects. * Denotes statistically significant (P < 0.05) fold changes from values measured in control perfusates.
DISCUSSION
Chemotherapy is the most commonly used medical treatment for cancer. The present studies describe a previously unappreciated mechanism by which chemotherapy exposure results in the production of PAF-R agonists which are known to inhibit tumor immunity (30). These data support the model that Ox-GPC PAF-R agonists produced in part due to ROS from chemotherapeutic agents can exert systemic immunosuppressive effects. That chemotherapeutic agent-triggered PAF-R agonist formation and augmentation of tumor growth are partially inhibited by antioxidants suggests that antioxidants could have potential use in chemotherapy protocols. Of interest, systemic antioxidants have been championed for adjuvant use along with chemotherapy to decrease therapy side effects (40,41). Yet, use of antioxidants along with chemotherapy is considered controversial due to concerns about possible interference with chemotherapeutic agent-mediated direct killing of tumor cells (42,43). It should be noted that the present studies indicate that antioxidant treatment alone or in chemotherapy-treated PAF-R-deficient mice did not have any perceptible effects on tumor growth.
Oxidation of esterified fatty acyl residues introduces oxy functions, rearranges bonds and fragments carbon-carbon bonds by β-scission that generate a myriad of phospholipid reaction products including PAF-R agonists (19–25). In contrast to the tightly controlled enzymatic pathways for PAF biosynthesis, large amounts of numerous Ox-GPC PAF-R agonists can be produced non-enzymatically. The present studies not only demonstrate that chemotherapy-generated PAF-R agonistic activity is diminished by antioxidants, but structural characterization of this activity reveals Ox-GPCs known to be produced non-enzymatically. Consistent with the notion that chemotherapy is a potent pro-oxidative stressor, intratumoral injection of melphalan resulted in increased urine levels of immunoreactive 8-isoprostane (8-iso Prostaglandin F2α), an eicosanoid formed from free radical-catalyzed peroxidation of arachidonate, which was blunted in mice fed antioxidant diet (data not shown). It is likely that tumor cellular membranes serve as the source of oxidized phospholipids from the chemotherapeutic agent intratumoral injections and are thus the source of chemotherapy-mediated PAF-R agonist formation in the experimental murine models used. However, it is not clear whether the source of PAF-R agonists produced during human regional chemotherapy is derived from the tumors, or from normal tissue.
The present studies demonstrate that high levels of PAF-R agonist activity are measured during regional chemotherapy. In contrast to the poor responsiveness of metastatic melanoma to standard chemotherapy, regional chemotherapy appears to be one of the most successful anti-melanoma therapies as measured by percentage of clinical responders (4–6). Hyperthermic isolated limb perfusion, first reported in 1958, allows the regional delivery of chemotherapeutic agents (commonly melphalan) to patients with in-transit metastases localized to extremities which would not be approachable by surgical resection. The ability to use 10-fold higher than the standard chemotherapy doses along with heat provides a very potent combination to kill tumor cells. Another advantage of regional chemotherapy is the ability to treat the entire area at highest risk of reoccurrence by eliminating clinically occult microscopic tumor disease with minimal risk of systemic toxicity. We hypothesize that this combination of heat and high-dose melphalan also allows a rather unique environment that promotes ROS. Though it is possible that standard doses of systemic chemotherapy could generate immunosuppressive Ox-GPC PAF-R agonists which impede therapy effectiveness, this novel and previously unappreciated pathway would more likely play an important role in regional chemotherapy.
Tumoral resistance to chemotherapy is an important clinical problem and is an area of active study. In contrast to cellular resistance to the effects of chemotherapy, the present studies describe a novel mechanism by which chemotherapeutic agents can subvert anti-tumor immunity. Indeed, our previous studies using UVB irradiation of skin as the source for PAF-R agonists provide several lines of evidence implicating anti-tumor immunity, in particular Tregs in the PAF-mediated effects on experimental B16F10 tumor growth (30). First, PAF effects are not seen when tumors are placed in immunodeficient murine hosts. Second, use of PAF-R-negative B16F10 cells transduced with functional PAF-Rs implanted in WT vs Ptafr−/− hosts have confirmed that the PAF-R mediating the response is on the host, not tumor. Finally, use of neutralizing antibodies against IL-10 or depleting Tregs both block PAF-mediated augmentation of experimental tumor growth. It is possible that this previously unappreciated pathway could provide an explanation for why immunotherapy strategies tend to be more effective when given to patients who have not received prior chemotherapy, which according to our model could potentially tolerize the immune system to the tumors (44–46).
Exogenous pro-oxidative stressors ranging from aromatic hydrocarbons to cigarette smoke to UVB radiation have been shown to induce systemic immunosuppression via PAF-R signaling which is blocked by antioxidants (16–18). Apoptotic cells generate PAF and also contribute to melanoma tumor progression via PAF-R activation (47). The production of PAF-R agonists from these various agents begins a cascade of events leading to systemic immunosuppression. The cytokines which appear to be critical for the immunosuppression include IL-10 and COX-2-generated eicosanoids (14,15,18). Mast cells and regulatory T cells are also implicated in PAF-R-dependent systemic immunosuppression (18,30,48). The present studies demonstrating that COX-2 inhibitors block chemotherapy-mediated augmentation of experimental tumor growth are not only consistent with previous studies characterizing the role of this eicosanoid-generating enzyme in PAF-mediated systemic immunosuppression (14,15,18), they also provide the rationale for future studies testing the ability of COX-2 inhibitors to enhance the effectiveness of regional chemotherapy. It should be noted that COX-2 inhibition has been shown to exert not only protective properties on the host, but also direct antitumor effects in a variety of tumor types (49,50). In contrast, antioxidant use along with chemotherapy is associated with controversy due to possible concerns that these agents could interfere with the efficacy of chemotherapy (42,43).
In summary, the present studies provide the first evidence that PAF-R signaling can inhibit chemotherapy effectiveness. In contrast to chemotherapy resistance that is applicable at the tumor cell level, this process is due to the subversion of host tumor immunity. That chemotherapy generates PAF-R agonists in humans is suggestive that this novel pathway could have tremendous clinical significance. Since this process involves the pro-oxidative qualities of chemotherapeutic agents and is neutralized by antioxidants, with downstream effects susceptible to COX-2 inhibition, these studies could provide the impetus for future studies to define the clinical significance of this novel pathway in humans.
Supplementary Material
Acknowledgements
The authors wish to acknowledge the technical assistance of Ms. Qiaofang Yi. We also wish to acknowledge the helpful suggestions from Dr. Mark Kaplan. This research was supported in part by grants from the Riley Memorial Association, and the National Institutes of Health grant R01 HL062996 (JBT&RLK), R01 CA134014 (CET), R21 ES020965 (RLK), and Veteran’s Administration Merit Award 5I01BX000853 (JBT), American Institute for Cancer Research 09A062 (RPS), ACSIRG 4185607 (RPS), and Showalter Research Trust Fund 4485602 (RPS). MF was supported by an exchange scholarship grant from Fundacao de Amparo a Pesquisa do Estado de Sao Paulo 2013/00584-2. The mass spectrometers were maintained by NIH grant U54 HL117798.
Abbreviations used in this paper
- CHS
contact hypersensitivity
- COX-2
cyclooxygenase type 2
- CPAF
1-hexadecyl-2-N-methylcarbamoyl glycerophosphocholine
- DNFB
dinitrofluorobenzene
- GPC
glycerophosphocholine
- Ox-GPC
oxidized GPC
- IL-10
interleukin 10
- NAC
N-acetylcysteine
- PAF
platelet-activating factor
- PAF-R
PAF receptor
- PAF-AH
PAF acetylhydrolase
- ROS
reactive oxygen species
- Treg
regulatory T cells
Footnotes
None of the authors have a relevant conflict of interest
REFERENCES
- 1.Miller AJ, Mihm MC., Jr Melanoma. New Engl J Med. 2006;355(1):51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 2.Finn L, Markovic SN, Joseph RW. Therapy for metastatic melanoma: the past, present, and future. BMC Med. 2012;10:23–27. doi: 10.1186/1741-7015-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jilaveanu LB, Aziz SA, Kluger HM. Chemotherapy and biologic therapies for melanoma: do they work? Clinics in Dermatol. 2009;27(6):614–625. doi: 10.1016/j.clindermatol.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 4.Testori A, Faries MB, Thompson JF, et al. Treatment of melanoma metastases in a limb by isolated limb perfusion and isolated limb infusion. J Surg Oncol. 2011;104(4):397–404. doi: 10.1002/jso.22028. [DOI] [PubMed] [Google Scholar]
- 5.Deroose JP, Eggermont AM, van Geel AN, Verhoef C. Isolated limb perfusion for melanoma in-transit metastases: developments in recent years and the role of tumor necrosis factor alpha. Curr Opin Oncol. 2011;23(2):183–188. doi: 10.1097/CCO.0b013e3283424dbc. [DOI] [PubMed] [Google Scholar]
- 6.Turley RS, Raymond AK, Tyler DS. Regional treatment strategies for in-transit melanoma metastasis. Surg Oncol Clin N Amer. 2011;20(1):79–103. doi: 10.1016/j.soc.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guida M, Pisconte S, Colucci G. Metastatic melanoma: the new era of targeted therapy. Exp Opin Therap Targets. 2012;16(Suppl 2):S61–S70. doi: 10.1517/14728222.2011.645807. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4(127) doi: 10.1126/scitranslmed.3003634. 127ps8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang R, Niepel M, Mitchison TK, Sorger PK. Dissecting variability in responses to cancer chemotherapy through systems pharmacology. Clin Pharm & Therap. 2010;88(1):34–38. doi: 10.1038/clpt.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hug H, Strand S, Gambihler A, et al. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J Biol Chem. 1997;272:28191–28193. doi: 10.1074/jbc.272.45.28191. [DOI] [PubMed] [Google Scholar]
- 11.Jin SM, Cho HJ, Jung ES, Shim MY, Mook-Jung I. DNA damage-inducing agents elicit gamma-secretase activation mediated by oxidative stress. Cell Death & Diff. 2008;15(9):1375–1284. doi: 10.1038/cdd.2008.49. [DOI] [PubMed] [Google Scholar]
- 12.Li T, Southall MD, Yi Q, et al. The epidermal Platelet-activating factor receptor augments chemotherapy-induced apoptosis in human carcinoma cell lines. J Biol Chem. 2003;278:16614–16621. doi: 10.1074/jbc.M211287200. [DOI] [PubMed] [Google Scholar]
- 13.Darst M, Al-Hassani M, Li T, Yi Q, Travers JM, Lewis DA, Travers JB. Augmentation of chemotherapy-induced cytokine production by expression of the Platelet-activating factor receptor in a human epithelial carcinoma cell line. J Immunol. 2004;172:6330–6335. doi: 10.4049/jimmunol.172.10.6330. [DOI] [PubMed] [Google Scholar]
- 14.Walterscheid JP, Ullrich SE, Nghiem DX. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med. 2002;195:171–179. doi: 10.1084/jem.20011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Yao Y, Konger RL, et al. UVB radiation-mediated inhibition of contact hypersensitivity reactions is dependent on the platelet-activating factor system. J Invest Derm. 2008;128:1780–1787. doi: 10.1038/sj.jid.5701251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos G, Limon-Flores AY, Ullrich SE. Dermal exposure to jet fuel suppresses delayed-type hypersensitivity: a critical role for aromatic hydrocarbons. Toxicol Sci. 2007;100(2):415–422. doi: 10.1093/toxsci/kfm247. [DOI] [PubMed] [Google Scholar]
- 17.Yao Y, Wolverton JE, Zhang Q, et al. Ultraviolet B radiation generated Platelet-activating factor receptor agonist formation involves EGF-R-mediated reactive oxygen species. J Immunol. 2009;182(5):2842–2848. doi: 10.4049/jimmunol.0802689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahu RP, Petrache I, Vander Mark MJ, et al. Cigarette smoke exposure inhibits contact hypersensitivity via the generation of platelet activating factor agonists. J Immunol. 2013;190(5):2447–2454. doi: 10.4049/jimmunol.1202699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel KD, Zimmerman GA, Prescott SM, McIntyre TM. Novel leukocyte agonists are released by endothelial cells exposed to peroxide. J Biol Chem. 1992;267(21):15168–15175. [PubMed] [Google Scholar]
- 20.Marathe GK, Johnson C, Billings SD, et al. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J Biol Chem. 2005;280(42):35448–35457. doi: 10.1074/jbc.M503811200. [DOI] [PubMed] [Google Scholar]
- 21.Konger RL, Marathe GK, Yao Y, Zhang Q, Travers JB. Oxidized glycerophosphocholines as biologically active mediators for ultraviolet radiation-mediated effects. Prost Other Lipid Mediat. 2008;87(1–4):1–8. doi: 10.1016/j.prostaglandins.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen R, Chen X, Salomon RG, McIntyre TM. Platelet activation by low concentrations of intact oxidized LDL particles involves the PAF receptor. Arter Thromb Vasc Biol. 2009;29(3):363–371. doi: 10.1161/ATVBAHA.108.178731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldstein AE, Lopez R, Tamimi TA, et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010;51(10):3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao Y, Harrison KA, Al-Hassani M, et al. Platelet-activating factor agonists mediate Xeroderma Pigmentosum A photosensitivity. J Biol Chem. 2012;287:9311–9321. doi: 10.1074/jbc.M111.332395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gruber F, Bicker W, Oskolkova OV, Tschachler E, Bochkov VN. A simplified procedure for semi-targeted lipidomic analysis of oxidized phosphatidylcholines induced by UVA irradiation. J Lipid Res. 2012;53(6):1232–1242. doi: 10.1194/jlr.D025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braquet P, Touqui L, Shen TY, Vargaftig BB. Platelet-activating factor. Pharmacol Rev. 1987;9(2):97–145. [PubMed] [Google Scholar]
- 27.Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Ann Rev Pharmacol & Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- 28.Stafforini DM, McIntyre TM, Carter ME, Prescott SM. Human plasma platelet-activating factor acetylhydrolase: Association with lipoprotein particles and role in the degradation of platelet-activating factor. J Biol Chem. 1987;262:4215–4222. [PubMed] [Google Scholar]
- 29.Zhang Q, Mousdicas N, Yi Q, et al. Staphylococcal lipoteichoic acid inhibits delayed type hypersensitivity reactions via the Platelet-activating factor receptor. J Clin Invest. 2005;115:2855–2861. doi: 10.1172/JCI25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahu RP, Turner MJ, DaSilva SC, et al. The environmental stressor ultraviolet B radiation inhibits murine anti-tumor immunity through its ability to generate Platelet-activating factor agonists. Carcinogenesis. 2012;33:1360–1367. doi: 10.1093/carcin/bgs152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sreevidya CS, Khaskhely NM, Fukunaga A, Khaskina P, Ullrich SE. Inhibition of photocarcinogenesis by platelet-activating factor or serotonin receptor antagonists. Cancer Res. 2008;68(10):3978–84. doi: 10.1158/0008-5472.CAN-07-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishii S, Kuwaki T, Nagase T, et al. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 34.Pei Y, Barber LA, Murphy RC, et al. Activation of the epidermal platelet-activating factor receptor results in cytokine and cyclooxygenase-2 biosynthesis. J Immunol. 1998;161:1954–1961. [PubMed] [Google Scholar]
- 35.Melnikova VO, Mourad-Zeidan AA, Lev DC, Bar-Eli M. Platelet-activating factor mediates MMP-2 expression and activation via phosphorylation of cAMP-response element-binding protein and contributes to melanoma metastasis. J Biol Chem. 2006;281(5):2911–2922. doi: 10.1074/jbc.M508683200. [DOI] [PubMed] [Google Scholar]
- 36.Travers J, Al-Hassani M, Yao Y, Konger RL, Travers JB. Ultraviolet B radiation of human skin generates Platelet-activating factor receptor agonists. Photochem Photobiol. 2010;86:949–954. doi: 10.1111/j.1751-1097.2010.00743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brincker H. Direct intratumoral chemotherapy. Crit Rev Oncol Hematol. 1993;15:91–98. doi: 10.1016/1040-8428(93)90049-a. [DOI] [PubMed] [Google Scholar]
- 38.Duvillard C, Romanet P, Cosmidis A, Beaudouin N, Chauffert B. Phase 2 study of intratumoral cisplatin and epinephrine treatment for locally recurrent head and neck tumors. Ann Otol Rhinol Laryngol. 2004;113:229–233. doi: 10.1177/000348940411300312. [DOI] [PubMed] [Google Scholar]
- 39.Raymond AK, Beasley GM, Broadwater G, et al. Current trends in regional therapy for melanoma: Lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. J Amer Coll Surg. 2011;213(2):306–316. doi: 10.1016/j.jamcollsurg.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vera-Ramirez L, Sanchez-Rovira P, Ramirez-Tortusa MC, et al. Free radicals in breast carcinogenesis, breast cancer progression and cancer stem cells. Biological bases to develop oxidative-based therapies. Crit Rev Oncol-Hematol. 2011;80(3):347–368. doi: 10.1016/j.critrevonc.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C2008. Impact of antioxidant supplementation on chemotherapeutic toxicity: a systematic review of the evidence from randomized controlled trials. Int J Can. 2008;123(6):1227–1239. doi: 10.1002/ijc.23754. [DOI] [PubMed] [Google Scholar]
- 42.Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Nat Can Inst. 2008;100(11):773–783. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- 43.Nakayama A, Alladin KP, Igbokwe O, White JD. Systematic review: generating evidence-based guidelines on the concurrent use of dietary antioxidants and chemotherapy or radiotherapy. Cancer Invest. 2011;29(10):655–667. doi: 10.3109/07357907.2011.626479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwala SS. Novel immunotherapies as potential therapeutic partners for traditional or targeted agents: cytotoxic T-lymphocyte antigen-4 blockade in advanced melanoma. Melanoma Res. 2010;20(1):1–10. doi: 10.1097/CMR.0b013e328333bbc8. [DOI] [PubMed] [Google Scholar]
- 45.Kaehler KC, Sondak VK, Schadendorf D, Hauschild A. Pegylated interferons: prospects for the use in the adjuvant and palliative therapy of metastatic melanoma. Eur J Can. 2010;46(1):41–46. doi: 10.1016/j.ejca.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Fang L, Lonsdorf AS, Hwang ST. Immunotherapy for advanced melanoma. J Invest Derm. 2008;128(11):2596–605. doi: 10.1038/jid.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bachi AL, Dos Santos LC, Nonogaki S, Jancar S, Jasiulionis MG. Apoptotic cells contribute to melanoma progression and this effect is partially mediated by platelet-activating factor receptor. Mediators Inflam. 2012;2012:610371. doi: 10.1155/2012/610371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Byrne SN, Limón-Flores AY, Ullrich SE. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J Immunol. 2008;180(7):4648–4655. doi: 10.4049/jimmunol.180.7.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohammadianpanah M, Razmjou-Ghalei S, Shafizad A, et al. Efficacy and safety of concurrent chemoradiation with weekly cisplatin +/− low-dose celecoxib in locally advanced undifferentiated nasopharyngeal carcinoma: a phase II-III clinical trial. J Can Res Ther. 2011;7(4):442–447. doi: 10.4103/0973-1482.92013. [DOI] [PubMed] [Google Scholar]
- 50.Khan Z, Khan N, Tiwari RP, Sah NK, Prasad GB, Bisen PS. Biology of Cox-2: an application in cancer therapeutics. Curr Drug Targets. 2011;12(7):1082–1093. doi: 10.2174/138945011795677764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.