Abstract
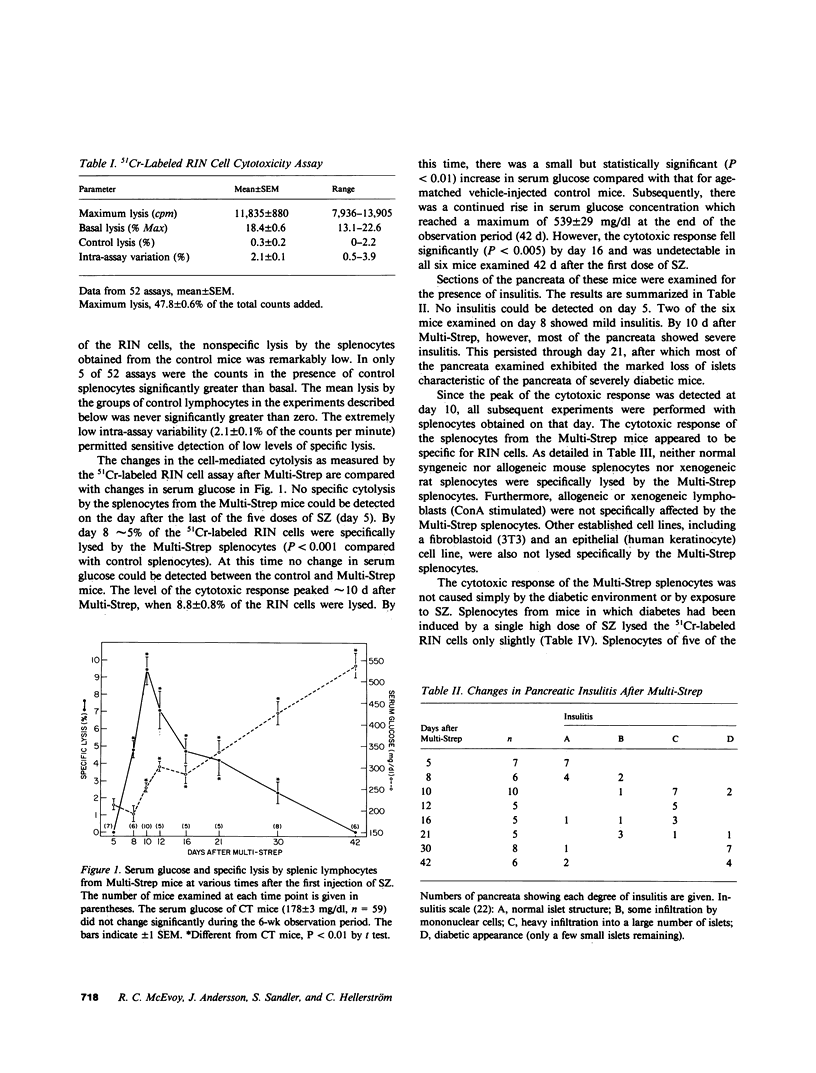
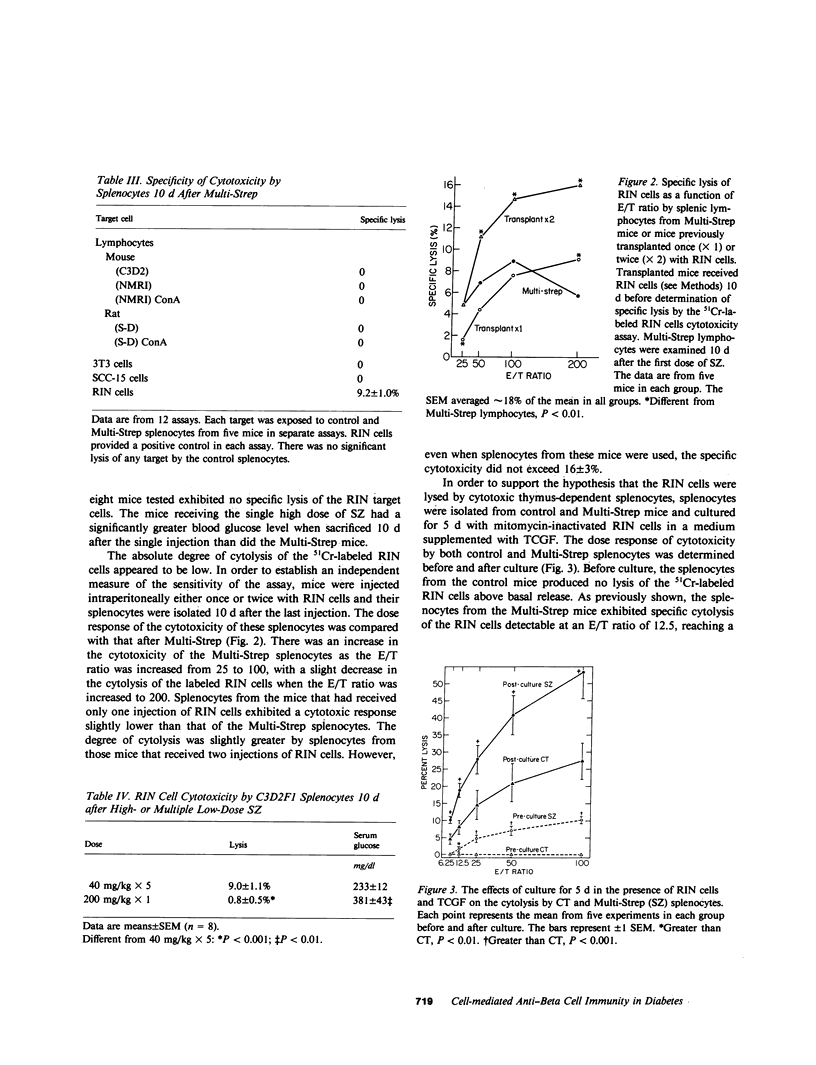
Mice were examined for the presence of splenocytes specifically cytotoxic for a rat insulinoma cell line (RIN) during the induction of diabetes by streptozotocin (SZ) in multiple low doses (Multi-Strep). Cytotoxicity was quantitated by the release of 51Cr from damaged cells. A low but statistically significant level of cytolysis (5%) by splenocytes was first detectable on day 8 after the first dose of SZ. The cytotoxicity reached a maximum of approximately 9% on day 10 and slowly decreased thereafter, becoming undetectable 42 d after SZ was first given. The time course of the in vitro cytotoxic response correlated with the degree of insulitis demonstrable in the pancreata of the Multi-Strep mice. The degree of cytotoxicity after Multi-Strep was related to the number of effector splenocytes to which the target RIN cells were exposed and was comparable to that detectable after immunization by intraperitoneal injection of RIN cells in normal mice. The cytotoxicity was specific for insulin-producing cells; syngeneic, allogeneic, and xenogeneic lymphocytes and lymphoblasts, 3T3 cells, and a human keratinocyte cell line were not specifically lysed by the splenocytes of the Multi-Strep mice. This phenomenon was limited to the Multi-Strep mice. Splenocytes from mice made diabetic by a single, high dose of SZ exhibited a very low level of cytotoxicity against the RIN cells. The cytotoxic response was also quantitated in splenocytes from control and Multi-Strep mice (10 d after the first dose of SZ) before and after culture with mitomycin-treated RIN cells in the presence of T cell growth factor (TCGF). The cytotoxicity of the Multi-Strep splenocytes was enhanced more than fivefold after such culture, suggesting the proliferation of an effector cell that could be stimulated and supported in vitro by TCGF. These results support the hypothesis that cell-mediated anti-beta cell autoimmunity may play a role in the destruction of the beta cells in this animal model. The stimulation of this response by TCGF may provide a tool by which enough cytotoxic effector cells could be obtained to establish their possible direct pathogenetic role in the induction of insulin-dependent diabetes. In addition, such cells will be a valuable tool to define the specific beta-cell antigens that may direct the highly selective cell-mediated destruction of these cells in experimental models and, perhaps, in human insulin-dependent diabetes mellitus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson A. Islet implantation normalises hyperglycaemia caused by streptozotocin-induced insulitis. Experiments in mice. Lancet. 1979 Mar 17;1(8116):581–584. doi: 10.1016/s0140-6736(79)91008-0. [DOI] [PubMed] [Google Scholar]
- Appel M. C., Rossini A. A., Williams R. M., Like A. A. Viral studies in streptozotocin-induced pancreatic insulitis. Diabetologia. 1978 Oct;15(4):327–336. doi: 10.1007/BF02573827. [DOI] [PubMed] [Google Scholar]
- Beattie G., Lannom R., Lipsick J., Kaplan N. O., Osler A. G. Streptozotocin-induced diabetes in athymic and conventional BALB/c mice. Diabetes. 1980 Feb;29(2):146–150. doi: 10.2337/diab.29.2.146. [DOI] [PubMed] [Google Scholar]
- Bonnevie-Nielsen V., Steffes M. W., Lernmark A. A major loss in islet mass and B-cell function precedes hyperglycemia in mice given multiple low doses of streptozotocin. Diabetes. 1981 May;30(5):424–429. doi: 10.2337/diab.30.5.424. [DOI] [PubMed] [Google Scholar]
- Brooks C. G. Reversible induction of natural killer cell activity in cloned murine cytotoxic T lymphocytes. Nature. 1983 Sep 8;305(5930):155–158. doi: 10.1038/305155a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschard K., Rygaard J. Is the diabetogenic effect of streptozotocin in part thymus-dependent? Acta Pathol Microbiol Scand C. 1978 Feb;86(1):23–27. doi: 10.1111/j.1699-0463.1978.tb02552.x. [DOI] [PubMed] [Google Scholar]
- Dyrberg T., Baekkeskov S., Lernmark A. Specific pancreatic beta-cell surface antigens recognized by a xenogenic antiserum. J Cell Biol. 1982 Aug;94(2):472–477. doi: 10.1083/jcb.94.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Morris M. A., Scearce R. M. Cytotoxic antibodies to cloned rat islet cells in serum of patients with diabetes mellitus. J Clin Invest. 1981 Feb;67(2):403–408. doi: 10.1172/JCI110048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G., Handwerger B. S., Yunis E. J., Brown D. M. Immune response in the mutant diabetic C57BL/Ks-dt+ mouse. Discrepancies between in vitro and in vivo immunological assays. J Clin Invest. 1978 Feb;61(2):243–250. doi: 10.1172/JCI108933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Chick W. L., Oie H. K., Sims H. L., King D. L., Weir G. C., Lauris V. Continuous, clonal, insulin- and somatostatin-secreting cell lines established from a transplantable rat islet cell tumor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3519–3523. doi: 10.1073/pnas.77.6.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Watson J. Interleukin-2 dependent culture of cytolytic T cell lines. Immunol Rev. 1981;54:81–109. doi: 10.1111/j.1600-065x.1981.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Grönvik K. O., Andersson J. The role of T cell growth stimulating factors in T cell triggering. Immunol Rev. 1980;51:35–59. doi: 10.1111/j.1600-065x.1980.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Huang S. W., Maclaren N. K. Insulin-dependent diabetes: a disease of autoaggression. Science. 1976 Apr 2;192(4234):64–66. doi: 10.1126/science.769160. [DOI] [PubMed] [Google Scholar]
- Kataoka S., Satoh J., Fujiya H., Toyota T., Suzuki R., Itoh K., Kumagai K. Immunologic aspects of the nonobese diabetic (NOD) mouse. Abnormalities of cellular immunity. Diabetes. 1983 Mar;32(3):247–253. doi: 10.2337/diab.32.3.247. [DOI] [PubMed] [Google Scholar]
- Kiesel U., Freytag G., Biener J., Kolb H. Transfer of experimental autoimmune insulitis by spleen cells in mice. Diabetologia. 1980;19(6):516–520. doi: 10.1007/BF00253178. [DOI] [PubMed] [Google Scholar]
- Kiesel U., Kolb H., Freytag G. Strain dependency of the transfer of experimental immune insulitis in mice. Clin Exp Immunol. 1981 Feb;43(2):430–433. [PMC free article] [PubMed] [Google Scholar]
- Kiesel U., Kolb H. Low-dose streptozotocin-induced autoimmune diabetes is under the genetic control of the major histocompatibility complex in mice. Diabetologia. 1982 Jul;23(1):69–71. doi: 10.1007/BF00257735. [DOI] [PubMed] [Google Scholar]
- Koevary S., Rossini A., Stoller W., Chick W., Williams R. M. Passive transfer of diabetes in the BB/W rat. Science. 1983 May 13;220(4598):727–728. doi: 10.1126/science.6836309. [DOI] [PubMed] [Google Scholar]
- Kromann H., Christy M., Egeberg J., Lernmark A., Nerup J. Absence of H-2 genetic influence on streptozotocin-induced diabetes in mice. Diabetologia. 1982 Aug;23(2):114–118. doi: 10.1007/BF01271171. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Beamer W. G., Shultz L. D. The effect of immunosuppression on streptozotocin-induced diabetes in C57BL/KsJ mice. Diabetes. 1983 Feb;32(2):148–155. doi: 10.2337/diab.32.2.148. [DOI] [PubMed] [Google Scholar]
- Leiter E. H. Multiple low-dose streptozotocin-induced hyperglycemia and insulitis in C57BL mice: influence of inbred background, sex, and thymus. Proc Natl Acad Sci U S A. 1982 Jan;79(2):630–634. doi: 10.1073/pnas.79.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Like A. A., Appel M. C., Williams R. M., Rossini A. A. Streptozotocin-induced pancreatic insulitis in mice. Morphologic and physiologic studies. Lab Invest. 1978 Apr;38(4):470–486. [PubMed] [Google Scholar]
- Like A. A., Rossini A. A. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976 Jul 30;193(4251):415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- MacCuish A. C., Urbaniak S. J., Campbell C. J., Duncan L. J., Irvine W. J. Phytohemagglutinin transformation and circulating lymphocyte subpopulations in insulin-dependent diabetic patients. Diabetes. 1974 Aug;23(8):708–712. doi: 10.2337/diab.23.8.708. [DOI] [PubMed] [Google Scholar]
- Maclaren N. K., Neufeld M., McLaughlin J. V., Taylor G. Androgen sensitization of streptozotocin-induced diabetes in mice. Diabetes. 1980 Sep;29(9):710–716. doi: 10.2337/diab.29.9.710. [DOI] [PubMed] [Google Scholar]
- Paik S. G., Blue M. L., Fleischer N., Shin S. Diabetes susceptibility of BALB/cBOM mice treated with streptozotocin. Inhibition by lethal irradiation and restoration by splenic lymphocytes. Diabetes. 1982 Sep;31(9):808–815. doi: 10.2337/diab.31.9.808. [DOI] [PubMed] [Google Scholar]
- Paik S. G., Fleischer N., Shin S. I. Insulin-dependent diabetes mellitus induced by subdiabetogenic doses of streptozotocin: obligatory role of cell-mediated autoimmune processes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6129–6133. doi: 10.1073/pnas.77.10.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik S. G., Michelis M. A., Kim Y. T., Shin S. Induction of insulin-dependent diabetes by streptozotocin. Inhibition by estrogens and potentiation by androgens. Diabetes. 1982 Aug;31(8 Pt 1):724–729. doi: 10.2337/diab.31.8.724. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981 May;41(5):1657–1663. [PubMed] [Google Scholar]
- Rossini A. A., Appel M. C., Williams R. M., Like A. A. Genetic influence of the streptozotocin-induced insulitis and hyperglycemia. Diabetes. 1977 Oct;26(10):916–920. doi: 10.2337/diab.26.10.916. [DOI] [PubMed] [Google Scholar]
- Rossini A. A., Like A. A., Chick W. L., Appel M. C., Cahill G. F., Jr Studies of streptozotocin-induced insulitis and diabetes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2485–2489. doi: 10.1073/pnas.74.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini A. A., Williams R. M., Appel M. C., Like A. A. Complete protection from low-dose streptozotocin-induced diabetes in mice. Nature. 1978 Nov 9;276(5684):182–184. doi: 10.1038/276182a0. [DOI] [PubMed] [Google Scholar]
- Rossini A. A., Williams R. M., Appel M. C., Like A. A. Sex differences in the multiple-dose streptozotocin model of diabetes. Endocrinology. 1978 Oct;103(4):1518–1520. doi: 10.1210/endo-103-4-1518. [DOI] [PubMed] [Google Scholar]
- Sandler S., Andersson A. Islet implantation into diabetic mice with pancreatic insulitis. Acta Pathol Microbiol Scand A. 1981 Mar;89(2):107–112. doi: 10.1111/j.1699-0463.1981.tb00196.x. [DOI] [PubMed] [Google Scholar]
- Sandler S., Andersson A. Survival of intrasplenically implanted islets in mice with experimental insulitis and hyperglycemia. Diabetes. 1982 Aug;31 (Suppl 4):78–83. doi: 10.2337/diab.31.4.s78. [DOI] [PubMed] [Google Scholar]
- Sandler S., Andersson A. The partial protective effect of the hydroxyl radical scavenger dimethyl urea on streptozotocin-induced diabetes in the mouse in vivo and in vitro. Diabetologia. 1982 Oct;23(4):374–378. doi: 10.1007/BF00253747. [DOI] [PubMed] [Google Scholar]
- Sandler S. Protection by dimethyl urea against hyperglycaemia, but not insulitis, in low-dose streptozotocin-induced diabetes in the mouse. Diabetologia. 1984 May;26(5):386–388. doi: 10.1007/BF00266042. [DOI] [PubMed] [Google Scholar]
- Waters S. J., Waksal S. D., Norton G. P., Bona C. A. Antigen recognition by a T cell clone outside the context of the major histocompatibility complex. A role for Mls in antigen presentation. J Exp Med. 1984 Jan 1;159(1):305–312. doi: 10.1084/jem.159.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]



