1. Introduction
Cognitive symptoms are amongst the earliest in schizophrenia. They often develop in the prodromal period (Lencz, Smith, & McLaughlin, 2006; Kane & Lencz, 2008) and can be significant by the time of the first episode (Mesholam-Gately et al., 2009). Specific deficits have been found in all cognitive domains, including executive function, memory, and attention, and are between 0.5 and 1.5 standard deviations below matched control subjects (Velligan et al., 2000; Mohamed et al., 1999; Buchanan et al., 2005; Green, 2006; Zanelli et al., 2010; Bilder et al., 2000). Cognitive symptoms are highly disabling, having a strong correlation with functional outcome (Green, Kern, & Heaton, 2004; Green, Kern, & Braff, 2000; Bowie et al., 2008; Bowie et al., 2010). While already present during the first episode, the relationship between cognitive symptoms and functional outcome may increase with time (Verdoux et al. 2002), although cognitive deficits themselves may not worsen over the course of illness (Albus et al., 2006; Mesholam-Gately et al., 2009).
Although negative symptoms may modulate the effect of cognition on clinical outcome (Lin et al., 2013), cognition seems to be an independent, core symptom domain of schizophrenia that separately predicts long-term functional outcome and quality of life (Kane & Lencz, 2008; Keefe & Fenton, 2007; Green, Kern, & Braff, 2000). Despite the clinical and functional importance of cognitive symptoms, there are no currently approved and clearly effective pharmacologic treatments for these deficits (Harvey & Keefe, 2001; Coyle et al., 2010; Menniti et al., 2013; Choi, Wykes, & Kurtz, 2013). The small-to-moderate improvements with antipsychotics may reflect improvements of interfering hallucinations and thought disorganization or even negative symptoms (Harvey & Keefe, 2001). In a meta-analysis of medications targeting cholinergic, glutamatergic, or serotonergic receptors for cognitive impairment in schizophrenia, small-to-moderate effect sizes were found for some cholinergic medications in some aspects of cognition (Choi, Wykes, & Kurtz, 2013). However, these agents also improved negative and general symptoms, confounding the results. Although cognitive remediation has attracted considerable attention, it provides, at best, moderate benefits (Wykes et al., 2011), and patients need to be motivated and adhere to the training schedule. Finally, much of the improvement seen in schizophrenia cognition studies reflect practice effects (Goldberg et al., 2007), and the translation of improvements in isolated cognitive domains to enhanced real-world functioning is unclear.
Antidepressants are safe and used frequently in schizophrenia patients to address depressive and negative symptoms (Rummel et al. 2005; Singh et al. 2010; Hecht and Landy 2011). Theoretically, antidepressants could improve cognition via enhanced serotonergic, adrenergic, and dopaminergic transmission. These benefits may be anticipated to vary by antidepressant class, with, for example, those antidepressants showing marked anticholinergic activity (i.e., tricyclic antidepressants) expected to be less beneficial than other classes. While individual studies that used antidepressants to augment antipsychotics in schizophrenia have measured cognition, no meta-analysis has investigated the pooled efficacy of antidepressants for cognitive symptoms in schizophrenia. Therefore, we conducted a systematic review and meta-analysis to explore the effects of adjunctive antidepressants for cognition in patients with schizophrenia.
2. Methods
2.1. Search Strategy and Data Extraction
PubMed, Ovid (MEDLINE), PsycINFO, and Cochrane Library databases were searched (without time or language restriction) for randomized controlled trials (RCTs) comparing adjunctive antidepressants with placebo in the treatment of schizophrenia. The final search update was performed on 12/27/2013. Keywords included schizophrenia, random*, antidepressant, antidepressants, anti-depressant, anti-depressants, plus a list of all antidepressants ever approved for use in any country. This electronic search was supplemented by a hand search of references in review articles and articles pertinent to this meta- analysis. Article authors were contacted for additional data. Two of four authors (J.A.V., E.G., A.J.S., and M.S.V.) independently extracted study data. Two of three authors (J.A.V., E.G., and A.J.S.) independently entered and checked data entered into Review Manager Version 5.2.7 for Windows (Cochrane Collaboration, http://ims.cochrane.org/revman). Two authors (J.A.V. and C.U.C.) independently entered and checked data entered into Comprehensive Meta-Analysis V2 (Biostat, http://www.meta-analysis.com). Any discrepancies were resolved by consensus.
2.2. Inclusion Criteria
Eligible studies had to compare any antipsychotic plus any adjunctive antidepressant with any antipsychotic plus placebo and had to report on treatment effects on any cognitive domain. Agents with only theoretical antidepressant properties never approved in any country for depression were excluded from this meta-analysis. We also excluded studies whose sole cognition outcome was a scale that did not measure a specific cognitive function or domain, such as the Mini-Mental State Examination or Positive and Negative Syndrome Scale (PANSS)–cognitive scale.
2.3. Outcomes
Primary outcomes were test scores of any cognitive measure pooled on a study level to derive the following nine cognitive domain scores: executive function, attention, processing speed, visuospatial processing, auditory verbal long-term memory, visuospatial long-term memory, auditory verbal working memory, visuospatial working memory, and verbal fluency. Key secondary outcomes included higher-level cognitive domain scores (auditory verbal memory, visuospatial memory, long-term memory, working memory, memory) as well as a composite cognition score comprised of all included tests per study (see details below). Additional, secondary outcomes included all-cause discontinuation; discontinuation due to intolerability, inefficacy, and other reasons; total psychopathology; positive symptoms; negative symptoms; depressive symptoms; Parkinsonism; akathisia; dyskinesia; and other adverse events. For specific information about outcomes measured by each study see Supplementary Table 1.
2.4. Data Synthesis
For a detailed description of the data synthesis, see Supplementary Methods.
2.5. Statistical Analysis
Analyses were performed using Review Manager Version 5.2.7, except for the pooling of effect sizes of individual cognitive tests within a specific domain in order to obtain and pool domain sum scores across studies, which was done using Comprehensive Meta-Analysis V2. Analyses were carried out on outcomes for which data from ≥3 studies were available. We calculated the standardized mean difference (SMD)±95% confidence interval (CI) for continuous outcomes and the risk ratio (RR)±95%CI for categorical outcomes. Cognitive outcomes were standardized so that a positive SMD favors the antidepressant group. For all other continuous outcomes, a negative SMD (i.e., reduction in symptoms) favors the antidepressant group. When both change scores and endpoint scores were available, change scores were used preferentially unless they were significantly skewed (i.e., standard deviation more than double the mean), in which case endpoint scores were utilized, unless they, too, were skewed. Analyses for continuous outcomes were based on intention-to-treat (ITT; i.e., all randomized subjects receiving ≥1 dose of study medication) or modified ITT (i.e., all randomized subjects receiving ≥1 dose of study medication and having ≥1 post-baseline assessments) data, using last-observation-carried-forward or mixed models repeated measures analyses. Analyses for categorical outcomes were based on ITT data. All data were initially analyzed using a fixed effects model. Heterogeneity was studied using the I2 statistic, with I2≥50% indicating significant heterogeneity, as well as the chi square test for heterogeneity. All tests were two-sided, and alpha was set at 0.05.
In case of significant heterogeneity, the outcome was reanalyzed using a DerSirmonian and Laird (1986) random effects model. If the results remained significantly heterogeneous, preplanned subgroup analyses using the random effects model were conducted as follows when ≥3 studies were available for a given subanalysis: 1) not focusing on smoking cessation; 2) cognition as the primary outcome; 3) antipsychotic treatment – second-generation agents; 4) alpha-2 antagonist antidepressant (mirtazapine and mianserin) treatment; 5) mirtazapine treatment; 6) selective serotonin reuptake inhibitor (SSRI) treatment; 7) serotonergic antidepressant (SSRIs and duloxetine) treatment; and 8) noradrenergic antidepressant (duloxetine, reboxetine, and bupropion) treatment. Finally, for significant findings, pre-planned moderator analyses were conducted in studies: 1) not focusing on smoking cessation (as change in smoking status could affect outcomes) and 2) focusing on cognition as the primary outcome. Additionally, if the result for the cognitive composite was found to be significant, a third subanalysis was to be run utilizing only studies that measured ≥2 cognitive domains.
3. Results
3.1. Search
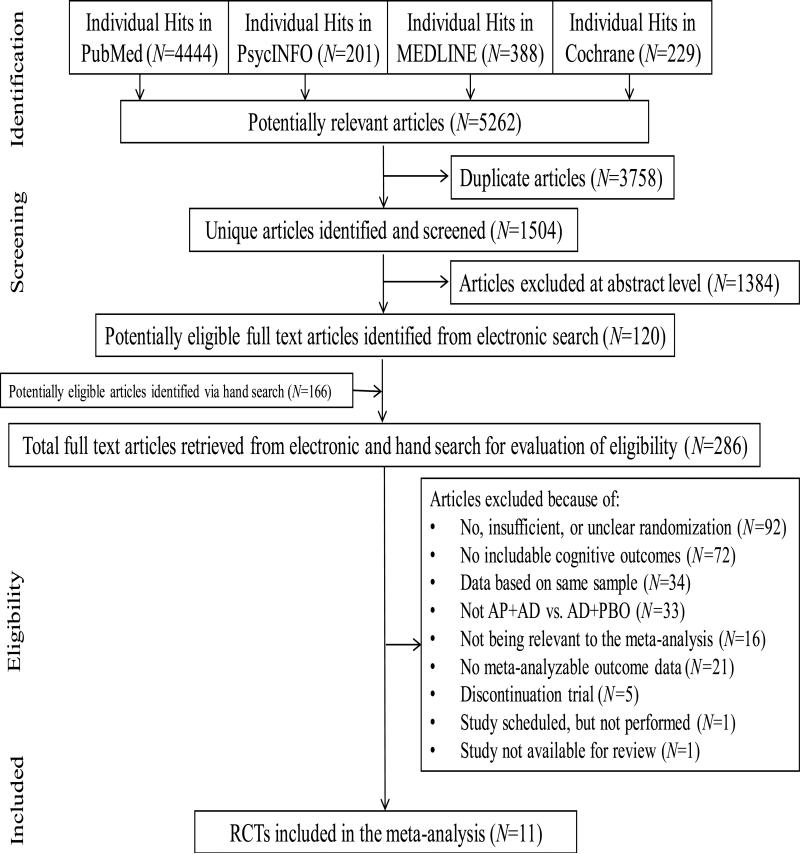
Our electronic search yielded 5,262 hits (Figure 1). After electronic filtering of duplicate records, 1,504 unique articles remained, of which 1,384 articles were excluded based on a review of titles/abstracts. The remaining 120 articles as well as 166 articles found via hand search underwent full-text inspection. Of these 286 articles, 16 were not relevant to the meta-analysis, 1 was not available for review, and—upon contacting the sponsoring agency— it was determined that 1 study had been scheduled but was not performed. Other reasons for exclusion were: no, insufficient, or unclear randomization (studies=92); no cognitive outcomes (studies=72); data based on a duplicate sample (studies=34); study not conducted in antipsychotic + antidepressant vs. antipsychotic + placebo format (studies=33); no meta-analyzable outcome data (studies=21); and discontinuation trial (studies=5). Ultimately, 11 studies were meta-analyzed (Table 1), including previously unpublished data from 8 studies (Acknowledgements).
Figure 1.
Flow diagram of article search and review process
Table 1.
Study, Patient, and Treatment Characteristics
| Study/ Sponsor |
Design | Total N |
Time (wks) |
Population | Mean Age |
Male Sex (%) |
Illness duration (years) |
Treatments | Mean Dose (mg/d) |
Primary Outcome(s) |
Secondary Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Selective Serotonin Reuptake Inhibitor | |||||||||||
| Dawes 2012/Zisook 2009a/Zisook 2010/Kasckow 2010 NIMH Department of Veterans Affairs | DBRPCT | 212 | 8 | Schizophrenia (n=117) or schizoaffective D/O (n=81) All subjects had “subsyndromal depression” Outpatients Baseline total PANSS or CGI-S score NR | 52.5 (n=198) | 78.3 (n=198) | NR | AP+citalopram AP+PBO |
AP doses not provided Citalopram = 28.9 |
Cognitive Tests; Psycho-pathology; Suicidality | EPS; Quality of Life; Metabolic side effects |
| Friedman 2005a Forest Laboratories | DBRPCT Cross overb | 19 | 12 | Chronic schizophrenia (n=17) or schizoaffective D/O (n=2) Stable Inpatients (42.1%) and outpatients (57.9%) Baseline total PANSS score = 78.90±14.46 Baseline CGI-S score = 4.00±0.69 | 45.0 | 68.4 | 25.6 | AP+citalopram SGA+PBO |
AP doses not provided Citalopram = 40 |
Cognitive Tests | Psycho-pathology; EPS |
| Niitsu 2012a No external funding | DBRPCT | 47 | 8 | Chronic schizophrenia Outpatients Baseline total PANSS score = 74.6 ± 10.7 | 37.4 | 61.7 | 11.5 | SGA+fluvoxamine SGA+PBO |
SGA = 257.9 CPZ equivalents Fluvoxamine = 150 |
Cognitive Tests | Psycho-pathology; EPS; Quality of Life |
| Serotonin-Norepinephrine Reuptake Inhibitor | |||||||||||
| Mico 2011 Funding source not specified | DBRPCT | 40 | 16 | Chronic schizophrenia Active positive and negative symptoms Outpatients Baseline total PANSS score = 65.7±12.6 | 35.0 | 60.0 | 6.5 | Clozapine+duloxetine Clozapine+PBO |
Clozapine = 518.3 (1036.6 CPZ equivalents) Duloxetine = 60 |
Total Psycho-pathology | Psycho-pathology; Cognitive Tests |
| Norepinephrine Reuptake Inhibitor | |||||||||||
| Poyurovsky 2009/Poyurovsky 2007 Stanley Medical Research Institute | DBRPCT | 33 | 6 | First-episode schizophrenia or schizophreniform D/O Remitted Inpatients Baseline CGI-S score = 4.18±0.64 | 31.1 | 63.6 | 3.6 | Olanzapine+reboxetine Olanzapine+PBO |
Olanzapine = 10 (200 CPZ equivalents) Reboxetine = 4 |
Cognitive Tests | Psycho-pathology; EPS |
| Dopamine-Norepinephrine Reuptake Inhibitor | |||||||||||
| Bloch 2010a National Alliance for Research on Schizophrenia and Depression Phillip Morris | DBRPCT | 61 | 14 | Schizophrenia (n=41), Schizoaffective D/O (n=19) or Diagnosis unclear (n=1) Smokers Stable Outpatients Baseline total PANSS = 72.90±21.63 (n=60) | 41.67 (n=60) | 75.4 | NR | AP+bupropion SR AP+PBO |
AP doses not provided Bupropion = 300 |
Smoking Cessation; Genetic Testing | Psycho-pathology; Cognitive Tests |
| Alpha 2 Antagonist | |||||||||||
| Berk 2009a Organon Australia | DBRPCT | 38 | 6 | Schizophrenia NR Inpatients (39.5%) or outpatients (39.5%) with unreported data for 21.1% of patients Baseline total PANSS score = 84.76±19.85 Baseline CGI-S score = 4.13±0.89 | 36.8 | 84.2 | NR | SGA+mirtazapine SGA+PBO |
SGA = 333.6 CPZ equivalents (n=27) Mirtazapine = 30 |
Total Psycho-pathology | Psycho-pathology; Cognitive Tests |
| Caforio 2013a Stanley Medical Research Institute Organon Italy (Schering Plough) | DBRPCT | 28 | 8 | Schizophrenia Recent exacerbation of psychotic symptoms requiring hospitalization Inpatients Baseline total PANSS score = 67.05±18.40 | 29.3 (n=20) | 75.0 (n=20) | 7.1 (n=20) | Olanzapine+mirtazapine Olanzapine+PBO |
Olanzapine = 17.3 (346.0 CPZ equivalents) (n=20) Mirtazapine = 30 (n=20) |
Negative symptoms; Cognitive Tests | Psycho-pathology |
| Cho 2011/Lee 2011 Funding source not specified | DBRPCT | 21 | 8 | Schizophrenia Stable Outpatients Baseline total PANSS score = 83.65±13.55 | 35.7 (n=20) | 50.0 (n=20) | 6.5 (n=20) | Risperidone+mirtazapine Risperidone+PBO |
Risperidone = 3.5 (175 CPZ equivalents) (n=20) Mirtazapine = 30 (n=20) |
Negative Symptoms; Cognitive Tests; EPS; Metabolic side effects | Cognitive Tests; Psycho-pathology; Adherence |
| Poyurovsky 2003a Funding source not specified but author states in a personal communication “This trial was not funded by any external sources.” | DBRPCT | 30 | 4 | Chronic schizophrenia Stable Inpatients Baseline CGI-S score = 3.5±0.6 (n=24) | 44.1 (n=24) | 70.8 (n=24) | 17.2 (n=24) | FGA+mianserin FGA+PBO |
FGA Defined Daily Dosage = 4.0 (n=24) Mianserin = 15 (n=24) |
Cognitive Tests | Psycho-pathology; EPS |
| Stenberg 2010a/Joffe 2009a/Terevnikov 2011 Stanley Medical Research Institute | DBRPCT | 39 | 6 | Chronic schizophrenia (n=38) or schizoaffective D/O, depressive type (n=1) Active positive and/or negative symptoms; at least moderate illness severity Inpatients (46.2%) and outpatients (53.8%) Baseline total PANSS score = 102.92±13.77 Baseline CGI-S score = 4.33±0.54 | 45.7 | 51.3 | 22.4 | FGA+mirtazapine FGA+PBO |
FGA = 323.8 CPZ equivalents Mirtazapine = 30 |
Psycho-pathology; Cognitive Tests | Psycho-pathology; Patient-rated Improvement |
| Total (unweighted means) | |||||||||||
| 11 Trials; Industry: N=4 Foundation: N=4 Government: N=1 Not reported/No external funding : N=4 | DBR PCT: N=11; Parallel: N=10 Cross-over: N=1 | 568 | 8.7±3.7 | SCZ:91.3% SZA:7.7% Chronic SCZ: 88.9%; first-episode SCZ or schizophreniform D/O: 5.8%; Outpatients: 60.1% Baseline total PANSS score = 78.5±12.1 (N=8) Baseline CGI-S score = 4.0±0.34 (N=5) | 39.5±6.9 | 67.2±10.9 | 12.5±8.0 (N=8) | Active: mirtazapine=4 mianserin=1 citalopram=2 duloxetine=1 fluvoxamine=1 reboxetine=1 bupropion=1 Comparator: SGA+PBO=6; FGA+PBO=2; CLO+PBO=1; OLA+PBO=2; RIS+PBO=1 |
Citalopram: 29.7 (weighted mean) Fluvoxamine: 150 Duloxetine: 60 Reboxetine: 4 Bupropion: 300 Mirtazapine: 30 Mianserin: 15
CPZ equivalents = 382.7±285.1 (N=7) |
Cognitive Tests: N=8; Total psycho-pathology: N=2; Psycho-pathology: N=2 Negative symptoms: N=2; Suicidality: N=1 Smoking cessation: N=1 Genetic testing : N=1 EPS:N=1 Metabolic side effects : N=1 | Psycho-pathology: N=10; EPS: N=5; Cognitive Tests: N=4; Patient rated improvement: N=1; Metabolic side effects: N=1; Adherence: N=1 Quality of Life = 2 |
additional, unpublished data were obtained from study author
pre-crossover data were obtained from study author
Abbreviations: AD=Antidepressant; AP=Antipsychotic; CGI-S=Clinical Global Impression–Severity Scale; CLO=Clozapine; CPZ=Chlorpromazine; DBRPCT=Double-blind, randomized, placebo-controlled trial; EPS=Extrapyramidal symptoms; FGA=First-generation antipsychotic; NR=Not reported; OLA=Olanzapine; PANSS=Positive and Negative Syndrome Scale; PBO=Placebo; RIS=Risperidone; SGA=Second-generation antipsychotic, SCZ=Schizophrenia; SZA=Schizoaffective disorder
3.2. Study, Patient, and Treatment Characteristics
All 11 studies were published in English and were randomized, double-blinded, and placebo-controlled. 10 studies (91%) were parallel studies; one was a crossover study, and pre-crossover data were obtained. Mean study duration was 8.7±3.7 weeks (range=4-16 weeks). Altogether, 568 subjects were included (sample size: n=19-212, age=39.5±6.9 years, male=67.2±10.9%). Only two studies reported ethnicity. All but one study (Poyurovsky et al., 2009), which included mostly first-episode schizophrenia patients, focused on patients with chronic schizophrenia (88.9% of patients). Mean illness duration was 12.5±8.0 years (range=3.6-25.6 years) (studies=8). Altogether, 60.1% were outpatients. The baseline total Positive and Negative Syndrome Scale (PANSS) score was 78.5±12.1 (studies=8), while the baseline Clinical Global Impression-Severity (CGI-S) score was 4.0±0.34 (studies=5).
6 studies used second-generation antipsychotics (SGAs; 1 using clozapine), 2 used only first-generation antipsychotics (FGAs), and 3 used both SGAs and FGAs. The average antipsychotic dose was 382.7±285.1 mg chlorpromazine (CPZ) equivalents (studies=7).
Add-on antidepressants included: the alpha-2 antagonists (studies=5) mirtazapine (studies=4; n=126; mean dose=30 mg/d) and mianserin (study=1; n=30; mean dose=15 mg/d); the SSRIs (studies=3) citalopram (studies=2; n=231; mean dose=29.7 mg/d) and fluvoxamine (study=1; n=47; mean dose=150 mg/d); the serotonin-norepinephrine reuptake inhibitor (SNRI; study=1) duloxetine (n=40; mean dose=60 mg/d); the norepinephrine reuptake inhibitor (NRI; study=1) reboxetine (n=33; mean dose=4 mg/d); and the dopamine-norepinephrine reuptake inhibitor (DNRI, study=1) bupropion (n=61; mean dose=300 mg/d).
3.3. Cognitive Outcomes
For specific cognitive outcomes that were analyzed per study and domain, see Supplemental Tables 1-3. Statistically significant, but clinically negligible, advantages were found for pooled antidepressants compared to placebo in executive function (Hedges’ g=0.17, 95%CI=0.025-0.31, p=0.02, I2=47%) and the cognitive composite (Hedges’ g=0.095, 95%CI=0.021-0.17, p=0.012, I2=45%) (Table 2). To explore possible moderating factors of significant results, pre-planned subanalyses were run for studies: 1) not focusing on smoking cessation (executive function=7 studies; cognitive composite=10 studies), and 2) using cognition as the primary outcome (executive function=5 studies; cognitive composite=8 studies). In these moderator analyses, results remained statistically significant, but all results became more heterogeneous. Moreover, results remained clinically negligible, except for executive function in studies where cognition was the primary outcome (Hedges’ g=0.25, 95%CI=0.06-0.43, p=0.01, I2=60%). Since this result was significantly heterogeneous, it was further explored using a random effects model, and the result remained heterogeneous but became statistically insignificant (Hedges’ g=0.27, 95%CI= -0.034 to 0.57, p=0.082, I2=60%). As the initial fixed effects analyses for executive function and composite cognition were not significantly heterogeneous, preplanned subgroup analyses based on medication class were not conducted.
Table 2.
Cognitive Test Domain Results
| Pooled Antidepressants vs. Placebo | ||||||
|---|---|---|---|---|---|---|
| Cognitive Domain | N Studies | N Participants | Hedges’ g | 95% CI | P | I2% |
| Executive function | 8 | 259 | 0.17 | 0.025,0.31 | 0.02 | 47 |
| Attention | 5 | 321 | 0.022 | −0.19,0.23 | 0.84 | 0 |
| Processing Speed | 6 | 344 | 0.09 | −0.031,−0.21 | 0.15 | 16 |
| Visuospatial Processinga | 3 | 94 | 0.14 | −0.73,1.00 | 0.76 | 78b |
| Auditory Verbal Long-Term Memory | 4 | 110 | 0.058 | −0.20,0.31 | 0.66 | 41 |
| Visuospatial Long-Term Memorya | 4 | 141 | 0.07 | −0.45,0.59 | 0.79 | 66b |
| Long-Term Memorya | 7 | 214 | 0.11 | −0.18,0.40 | 0.45 | 45 |
| Auditory Verbal Working Memory | 4 | 288 | 0.11 | −0.12,0.34 | 0.34 | 0 |
| Visuospatial Working Memory | 4 | 123 | 0.063 | −0.18,0.31 | 0.61 | 7 |
| Working Memory | 8 | 412 | 0.074 | −0.087,0.24 | 0.37 | 0 |
| Auditory Verbal Memory | 5 | 308 | 0.084 | −0.081,0.25 | 0.32 | 20 |
| Visuospatial Memorya | 5 | 160 | 0.065 | −0.16,0.29 | 0.57 | 0 |
| Memory | 9 | 432 | 0.077 | −0.038,0.19 | 0.19 | 46 |
| Verbal Fluency | 5 | 327 | 0.019 | −0.14,0.18 | 0.81 | 0 |
| Composite Cognition Score | 11 | 501 | 0.095 | 0.021,0.17 | 0.012 | 45 |
Positive Hedges’ g favors treatment group; fixed effects models, except where noted
Bolded p-values: p < 0.05
Random effects
< 3 studies available per subgroup so planned subanalyses not run
Antidepressants did not differ from placebo on any other cognitive domain scores for pooled antidepressants (Table 2). Significant heterogeneity (I2≥50%) was found for visuospatial processing, visuospatial long-term memory, long-term memory, and visuospatial memory. Therefore, these domains were re-analyzed using a random effects model. Visuospatial processing and visuospatial long-term memory remained significantly heterogeneous, but too few studies had data to conduct preplanned subgroup analyses.
3.4. Psychopathology Outcomes
Significant heterogeneity was found for total psychopathology, positive symptoms, negative symptoms, and depression. Therefore, these domains were explored using a random effects model. There were no significant differences between pooled antidepressants and placebo for total psychopathology (Hedges’ g=-0.27, 95%CI= -0.60 to 0.07, p=0.12, I2=65%), positive symptoms (Hedges’ g=-0.14, 95%CI= -0.45 to 0.17, p=0.38, I2=60%), or negative symptoms (Hedges’ g=-0.29, 95%CI= -0.62 to 0.05, p=0.09, I2=64%) (Table 3), but results were significantly heterogeneous. Therefore, subanalyses based on medication class were performed for SGAs (studies=6); alpha-2 antagonists (mirtazapine and mianserin, studies=5); mirtazapine (studies=4); serotonergic antidepressants (SSRIs and duloxetine; studies=4); SSRIs (studies=3); and noradrenergic antidepressants (duloxetine, reboxetine, and bupropion; studies=3). No significant differences were found (Table 4). Subanalyses excluding 1) 1 study focusing on smoking cessation and 2) 3 studies that did not use cognition as the primary outcome remained non-significant.
Table 3.
Psychopathology and Adverse Effects Outcomes
| Pooled Antidepressants vs. Placebo | ||||||
|---|---|---|---|---|---|---|
| Continuous Outcome | N Studies | N Participants | Hedges’ g | 95% CI | p | I2% |
| Total Psychopathologya | 11 | 491 | −0.27 | −0.60,0.07 | 0.12 | 65 |
| Positive Symptomsa | 11 | 500 | −0.14 | −0.45,0.17 | 0.38 | 60 |
| Negative Symptomsa | 11 | 500 | −0.29 | −0.62,0.05 | 0.09 | 64 |
| Depression (HAM-D-predominant)a | 9 | 455 | −0.14 | −0.47,0.18 | 0.39 | 58 |
| Depression (CDSS-predominant)a | 9 | 455 | −0.22 | −0.53,0.09 | 0.17 | 54 |
| EPS: Any | 8 | 407 | −0.13 | −0.33,0.06 | 0.18 | 13 |
| Parkinsonism | 7 | 360 | −0.11 | −0.32,0.10 | 0.30 | 21 |
| Akathisiaa | 4 | 265 | −0.64 | −1.71,0.43 | 0.24 | 85b |
| Dyskinesia | 3 | 230 | −0.13 | −0.39,0.13 | 0.32 | 39 |
| Categorical Outcome | N | n | RR | 95% CI | p | I2% |
|---|---|---|---|---|---|---|
| Discontinuation: All-cause | 11 | 568 | 1.16 | 0.85,1.59 | 0.36 | 0 |
| Discontinuation: Inefficacy | 10 | 540 | 0.39 | 0.12,1.33 | 0.13 | 0 |
| Discontinuation: Intolerability | 10 | 540 | 1.79 | 0.75,4.27 | 0.19 | 0 |
| Discontinuation: Other Reasons | 10 | 540 | 1.33 | 0.84,2.11 | 0.22 | 0 |
| <50% Decrease in PANSS Total Score | 7 | 442 | 1.00 | 0.98,1.03 | 0.71 | 0 |
| <20% Decrease in Any Negative Symptom Rating Scale | 7 | 236 | 0.96 | 0.87,1.06 | 0.47 | 0 |
| ≥20% Increase in PANSS Total Score | 4 | 163 | 2.70 | 0.47,15.32 | 0.26 | 0 |
| Study-defined Inefficacy (with HAM-D) | 3 | 272 | 0.78 | 0.68,0.91 | 0.0009 | 0 |
| Study-defined Inefficacy (with CDSS) | 3 | 272 | 0.76 | 0.65,0.90 | 0.0009 | 0 |
| Total Neuropsychiatric Adverse Events | 4 | 312 | 1.11 | 0.96,1.28 | 0.16 | 0 |
| Total Neurological Adverse Events | 4 | 312 | 1.24 | 0.83,1.85 | 0.30 | 12 |
| Headache | 4 | 312 | 1.06 | 0.56,2.00 | 0.86 | 16 |
| Total Psychiatric Adverse Events | 6 | 389 | 1.08 | 0.85,1.39 | 0.53 | 0 |
| Suicidal Ideation | 4 | 213 | 0.50 | 0.18,1.39 | 0.19 | N/A |
| Worsening of Psychosis | 5 | 168 | 3.08 | 0.65,14.54 | 0.16 | 0 |
| Psychiatric Hospitalization | 4 | 359 | 1.39 | 0.43,4.49 | 0.58 | 0 |
| Insomnia | 4 | 312 | 1.45 | 0.82,2.57 | 0.21 | 17 |
| Sedation | 4 | 118 | 2.91 | 1.03,8.17 | 0.04 | 0 |
| Weakness/Fatigue | 3 | 272 | 0.71 | 0.41,1.23 | 0.22 | 0 |
| Agitation/Irritability | 4 | 319 | 1.03 | 0.44,2.41 | 0.94 | 0 |
| Total GI Adverse Events | 4 | 312 | 1.17 | 0.99,1.39 | 0.06 | 0 |
| Total Metabolic Adverse Eventsa | 3 | 272 | 2.67 | 0.52,13.84 | 0.24 | 62b |
| Increase in Appetite | 3 | 272 | 1.34 | 0.59,3.05 | 0.48 | 0 |
| Weight Gain | 4 | 300 | 2.08 | 0.87,4.97 | 0.10 | 1 |
| Total Cardiorespiratory Adverse Events | 3 | 272 | 1.03 | 0.60,1.77 | 0.92 | 0 |
| Total Cardiac Adverse Events | 3 | 272 | 0.93 | 0.43,2.00 | 0.85 | 0 |
| Total Respiratory Adverse Events | 3 | 272 | 1.14 | 0.50,2.61 | 0.75 | 0 |
| Total Ophthalmological Adverse Events | 3 | 291 | 0.36 | 0.10,1.33 | 0.13 | 15 |
For continuous outcomes, negative Hedges’ g favors treatment group; for categorical outcomes values < 1 favor treatment group; fixed effects models, except where noted
Bolded p-value: p < 0.05
Random effects
< 3 studies available per subgroup so planned subanalyses not run
Abbreviations CDSS: Calgary Depression Scale for Schizophrenia; HAM-D: Hamilton Depression Rating Scale
Table 4.
Subanalyses of Psychopathology: Continuous Outcomes
| Subanalyses by Medication Class | ||||||
|---|---|---|---|---|---|---|
| Total Psychopathology | N Studies | N Participants | Hedges’ g | 95% CI | P | I2% |
| SGAs | 6 | 196 | −0.42 | −0.92,0.08 | 0.10 | 65 |
| Alpha 2 Antagonists | 5 | 139 | −0.37 | −0.94,0.20 | 0.20 | 62 |
| Mirtazapine | 4 | 115 | −0.50 | −1.17,0.16 | 0.14 | 66 |
| Serotonergic Ads | 4 | 285 | −0.40 | −1.02,0.23 | 0.21 | 79 |
| SSRIs | 3 | 245 | −0.08 | −0.47,0.31 | 0.67 | 38 |
| Noradrenergic ADs | 3 | 107 | −0.26 | −1.23,0.71 | 0.59 | 83 |
| Positive Symptoms | N | n | Hedges’ g | 95% CI | P | I2% |
|---|---|---|---|---|---|---|
| SGAs | 6 | 196 | −0.01 | −0.44,0.43 | 0.98 | 56 |
| Alpha 2 Antagonists | 5 | 139 | −0.50 | −1.12,0.12 | 0.12 | 68 |
| Mirtazapine | 4 | 115 | −0.53 | −1.33,0.27 | 0.19 | 76 |
| Serotonergic ADs | 4 | 285 | 0.07 | −0.30,0.43 | 0.72 | 44 |
| SSRIs | 3 | 245 | 0.09 | −0.44,0.61 | 0.75 | 62 |
| Noradrenergic ADs | 3 | 107 | 0.09 | −0.30,0.48 | 0.65 | 0 |
| Negative Symptoms | N | n | Hedges’ g | 95% CI | P | I2% |
|---|---|---|---|---|---|---|
| SGAs | 6 | 196 | −0.28 | −0.84,0.27 | 0.32 | 72 |
| Alpha 2 Antagonists | 5 | 139 | −0.42 | −0.96,0.12 | 0.13 | 58 |
| Mirtazapine | 4 | 115 | −0.55 | −1.17,0.07 | 0.08 | 61 |
| Serotonergic ADs | 4 | 285 | −0.32 | −0.96,0.32 | 0.32 | 80 |
| SSRIs | 3 | 245 | −0.03 | −0.55,0.48 | 0.90 | 61 |
| Noradrenergic ADs | 3 | 107 | −0.39 | −1.37,0.58 | 0.43 | 83 |
| Depression (HAM-D-predominant) | N | n | Hedges’ g | 95% CI | P | I2% |
|---|---|---|---|---|---|---|
| SGAs | 5 | 178 | 0.00 | −0.60,0.60 | 1.00 | 74 |
| Alpha 2 Antagonists | 4 | 118 | 0.05 | −0.46,0.55 | 0.85 | 46 |
| Mirtazapine | 3 | 94 | 0.01 | −0.68,0.71 | 0.97 | 63 |
| Serotonergic ADs | 4 | 304 | −0.39 | −0.61,−0.16 | 0.0009 | 0 |
| SSRIs | 3 | 264 | −0.33 | −0.57,−0.08 | 0.009 | 0 |
| Depression (CDSS-predominant) | N | n | Hedges’ g | 95% CI | P | I2% |
|---|---|---|---|---|---|---|
| SGAs | 5 | 178 | −0.08 | −0.62,0.47 | 0.78 | 68 |
| Alpha 2 Antagonists | 4 | 118 | −0.06 | −0.44,0.32 | 0.76 | 9 |
| Mirtazapine | 3 | 94 | −0.12 | −0.62,0.38 | 0.64 | 31 |
| Serotonergic ADs | 4 | 304 | −0.51 | −0.74,−0.28 | <0.0001 | 0 |
| SSRIs | 3 | 264 | −0.47 | −0.71,−0.22 | 0.0002 | 0 |
Negative Hedges’ g favors treatment group; random effects models
Bolded p-values: p < 0.05
Abbreviations: AD=Antidepressants; CDSS: Calgary Depression Scale for Schizophrenia; HAM-D: Hamilton Depression Rating Scale
Antidepressants did not differ from placebo for depression in either the HAM-D-predominant analysis (Hedges’ g=-0.14, 95%CI= -0.47 to 0.18, p=0.39, I2=58%) or the CDSS-predominant analysis (Hedges’ g=-0.22, 95%CI= -0.53 to 0.09, p=0.17, I2=54%) (Table 3). Due to significant heterogeneity, subgroup analyses based on medication class were performed for SGAs (studies=5); alpha-2 antagonists (studies=4); mirtazapine (studies=3); serotonergic antidepressants (studies=4); and SSRIs (studies=3). Depression improved with serotonergic antidepressants compared to placebo for both the HAM-D-predominant analysis (Hedges’ g=-0.39, 95%CI= -0.61 to -0.16, p=0.0009, I2=0%) and CDSS-predominant analysis (Hedges’ g=-0.51, 95%CI= -0.74 to -0.28, p<0.0001, I2=0%) (Table 4). Depression also improved with SSRIs compared to placebo for both the HAM-D-predominant analysis (Hedges’ g=- 0.33, 95%CI= -0.57 to -0.08, p=0.009, I2=0%) and the CDSS-predominant analysis (Hedges’ g=-0.47, 95%CI= -0.71 to -0.22, p=0.0002, I2=0%) (Table 4). Antidepressants outperformed placebo regarding study-defined inefficacy in both the HAM-D-included data condition (RR=0.78, 95%CI=0.68-0.91, p=0.0009, I2=0%) and the CDSS-included data condition (RR=0.76, 95%CI=0.65-0.90, p=0.0009, I2=0%) (Table 3). There were no statistically significant differences for any of the remaining psychopathology outcomes (Table 3).
3.5. Study Discontinuation
Antidepressants did not differ from placebo in all-cause discontinuation (RR=1.16, 95%CI=0.85-1.59, p=0.36, I2=0%) or in discontinuation due to inefficacy (RR=0.39, 95%CI=0.12-1.33, p=0.13, I2=0%), intolerability (RR=1.79, 95%CI=0.75-4.27, p=0.19, I2=0%), or other reasons (RR=1.33, 95%CI=0.84- 2.11, p=0.22, I2=0%) (Table 3).
3.6. Adverse Events
Sedation was more common with pooled antidepressants compared with placebo (RR=2.91, 95%CI=1.03-8.17, p=0.04, I2=0%) (Table 3), but this analysis was based entirely on alpha-2 antagonists. No other significant differences were found for any adverse event (Table 3).
4. Discussion
To our knowledge, this is the first meta-analysis of antidepressant augmentation of antipsychotics for the treatment of cognitive deficits in schizophrenia. Across 11 studies and 568 patients, no clinically meaningful improvement in any cognitive domain or the composite score was found for pooled antidepressants or any class of studied antidepressants compared with placebo. Though disappointing, the enhancement of serotonergic or noradrenergic neurotransmission on top of antipsychotic therapy does not appear to relevantly improve cognition in patients with chronic schizophrenia. The exact mechanism of cognitive dysfunction in schizophrenia is unclear, but glutamatergic, cholinergic, GABAergic, and histaminergic hypotheses have the most support (Abi-Dargham, 2004; Lisman et al., 2012; Nakazawa et al., 2012; Miyamoto et al., 2012; Jones et al., 2012; Foster et al., 2010; Vohora & Bhowmik, 2012). Since none of the studied antidepressants targets these neurotransmitter systems, it is not that surprising that they were ineffective for cognitive impairment in schizophrenia. While it is theoretically plausible that these neurotransmitter systems might be affected by the studied antidepressants via neurotransmitter cross-talk, the strength of such postulated indirect effects may be insufficient to significantly improve cognition.
Antidepressants have been found in some studies to significantly reduce depressive symptoms in schizophrenia patients with comorbid depression (Whitehead et al., 2003). However, in the schizophrenia patients included in this meta-analysis who were unselected for depression, antidepressants did not significantly improve depression. One exception was significant antidepressant efficacy in the 4 and 3 studies with serotonergic agents and SSRIs, respectively. Nevertheless, this non-significant effect on depression reduces the potential bias of a pseudo-specific finding of cognitive improvement secondary to improved depression.
Moreover, at least in chronic patients with schizophrenia unselected for any specific symptomatology or severity, antidepressant augmentation of antipsychotics was not associated with benefits in positive, negative, or general psychopathology symptoms. Likewise, except for a higher incidence of sedation confined to alpha-2 antagonists, antidepressants were not associated with higher drop-out rates or specific adverse effects. The lack of efficacy of antidepressants on schizophrenia psychopathology is in contrast to several meta-analyses that found antidepressant augmentation to significantly reduce negative symptoms (Rummel-Kluge et al., 2006; Sepehry et al., 2007; Singh et al., 2010; Hecht & Landy, 2012). However, these meta-analyses either focused on predominant negative symptom patients (Rummel-Kluge et al., 2006) or included many more studies measuring negative symptoms, whereas we only included studies with cognitive data.
There are several limitations of this study. The number of included studies and subjects was small. Data from five studies utilizing bupropion that tested cognition (Culhane et al., 2008; Evins et al., 2007; Evins et al., 2005a; Evins et al., 2005b; George et al., 2002; George et al., 2008; George et al., 2006; Moss et al., 2009; Weiner et al., 2012) were not meta-analyzable as presented in the published papers and were not obtainable from the authors. Notably, however, all bupropion studies targeted smoking cessation, and cognition was only a secondary outcome. Moreover, since bupropion has been found to significantly reduce smoking in schizophrenia (Tsoi et al., 2013), and since nicotine has pro-cognitive effects (Herman & Sofuoglu, 2010; Barr et al., 2008), results of these studies might have been confounded by change in smoking status.
Statistically significant but likely clinically irrelevant effects on executive function and composite cognition were found for pooled antidepressants. It is possible that subgroups of patients may have a more robust, clinically meaningful response to treatment, and a search for relevant biomarkers to identify such subgroups may prove fruitful. Antidepressant doses were also low- to mid-range; thus, higher doses could possibly produce greater effects, which should be explored in future studies of non-depressed patients with schizophrenia. Additionally, studied antidepressants were heterogeneous, as were the cognitive tests and outcomes. No study contained a complete cognitive battery or a complete subscale of a cognitive battery. Future studies should comprehensively measure a broad range of cognitive domains using complete neurocognitive batteries. Further, baseline antipsychotics and degree of patient stability varied. Studies were also short-term, yet even with these short intervention periods statistically significant effects were found on some aspects of cognition. It is possible longer durations of treatment might lead to clinically relevant effects. Finally, the majority of analyzed studies used chronic patients with a long duration of illness. It may be that earlier intervention with add-on antidepressants has a greater chance of success in the treatment of cognitive symptoms, and future studies should investigate the use of antidepressants in people with first-episode or early-phase schizophrenia. However, the one study in first-episode patients did not find significant effects either, although it was small (n=33).
Despite its limitations, this meta-analysis of adjunctive antidepressant treatment for cognition in schizophrenia provides important suggestive information about lack of efficacy. Additionally, results can guide the design of future studies of adjunctive antidepressants for cognitive impairment in schizophrenia.
Supplementary Material
Acknowledgements
We thank the following authors for providing us with additional, unpublished data: Alessandro Bertolino, MD, PhD, Grazia Caforio, MD, PhD, Seetal Dodd, MD, PhD, Richard P. Ebstein, PhD, Joseph I. Friedman, MD, Shahrokh Golshan, MD, Kenji Hashimoto, PhD, Salomon Israel, PhD, Ilana Kremer, MD, Tomihisa Niitsu, MD, PhD, Michael Poyurovsky, MD, Viatcheslav Terevnikov, MD, and Sidney Zisook, MD
Funding
This work was partially supported by the National Institute of Mental Health Advanced Center for Services and Intervention Research, The Zucker Hillside Hospital (P30MH090590); the National Institutes of Health (NIH) P50 Centers for Intervention Development and Applied Research (P50MH080173); and the Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1 RR024989 provided by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH.
Financial Disclosure:
Drs. Vernon, Grundnikoff, Vemulapalli, Pareek, and Goldberg and Mr. Seidman have nothing to disclose.
Dr. Frazier has received federal funding or research support from, acted as a consultant to, received travel support from, and/or received a speaker's honorarium from the Simons Foundation, Ingalls Foundation, Forest Laboratories, Ecoeos, IntegraGen, Shire Development, Bristol-Myers Squibb, National Institutes of Health, and the Brain and Behavior Research Foundation.
Dr. Kane has been a consultant to Alkermes, Amgen, Astra-Zeneca, Janssen, Pfizer, Eli Lilly, Bristol-Myers Squibb, Dainippon Sumitomo/Sepracor/Sunovion, Johnson & Johnson, Otsuka, Pierre Fabre. Vanda, Proteus, Takeda, Targacept, IntraCellular Therapies, Merck, Lundbeck, Novartis, Roche, Rules Based Medicine, Sunovion and has received honoraria for lectures from Otsuka, Eli Lilly, Esai, Boehringer-Ingelheim, Bristol-Myers Squibb, Merck and Janssen. He is a shareholder of MedAvante.
Dr. Correll has been a consultant and/or advisor to or has received honoraria from: Actelion, Alexza; Bristol-Myers Squibb, Cephalon, Eli Lilly, Genentech, Gerson Lehrman Group, IntraCellular Therapies, Janssen/J&J, Lundbeck, Medavante, Medscape, Merck, Otsuka, Pfizer, ProPhase, Roche, Sunovion, Supernus, Takeda, Teva, and Vanda. He has received grant support from BMS, Janssen/J&J, Novo Nordisk A/S and Otsuka.
Role of the Funding Source
The National Institute of Mental Health and National Institutes of Health had no role in the study design; collection, analysis, or interpretation of data; writing of the report; or decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. The Int J Neuropsychopharmacol Suppl. 2004;1:S1–5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- Albus M, Hubmann W, Mohr F, Hecht S, Hinterberger-Weber P, Seitz NN, Küchenhoff H. Neurocognitive functioning in patients with first-episode schizophrenia: results of a prospective 5-year follow-up study. Eur Arch Psychiatry Clin Neurosci. 2006;256(7):442–51. doi: 10.1007/s00406-006-0667-1. [DOI] [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008;33(3):480–90. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Berk M, Gama CS, Sundram S, Hustig H, Koopowitz L, D'Souza R, Malloy H, Rowland C, Monkhouse A, Monkhouse A, Bole F, Sathiyamoorthy S, Piskulic D, Dodd S. Mirtazapine add-on therapy in the treatment of schizophrenia with atypical antipsychotics: a double-blind, randomised, placebo-controlled clinical trial. Hum Psychopharmacol. 2009;24:233–8. doi: 10.1002/hup.1017. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JM, Woerner MG, Geisler S, Kane JM, Lieberman JA. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157(4):549–59. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Bloch B, Reshef A, Cohen T, Tafla A, Gathas S, Israel S, Gritsenko I, Kremer I, Ebstein RP. Preliminary effects of bupropion and the promoter region (HTTLPR) serotonin transporter (SLC6A4) polymorphism on smoking behavior in schizophrenia. Psychiatry Res. 2010;175(1-2):38–42. doi: 10.1016/j.psychres.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Leung WW, Reichenberg A, McClure MM, Patterson TL, Heaton RK, Harvey PD. Predicting schizophrenia patients' real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63(5):505–11. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie CR, Depp C, McGrath JA, Wolyniec P, Mausbach BT, Thornquist MH, Luke J, Patterson TL, Harvey PD, Pulver AE. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. Am J Psychiatry. 2010;167(9):1116–24. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan RW, Davis M, Goff D, Green MF, Keefe RS, Leon AC, Nuechterlein KH, Laughren T, Levin R, Stover E, Fenton W, Marder SR. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31(1):5–19. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- Caforio G, DiGiorgio A, Rampino A, Rizzo M, Romano R, Taurisano P, Fazio L, DeSimeis G, Ursini G, Blasi G, Nardini M, Mancini M, Bertolino A. Mirtazapine add-on improves olanzapine effect on negative symptoms of schizophrenia. J Clin Psychopharmacol. 2013;33(6):810–2. doi: 10.1097/JCP.0b013e3182a4ec77. [DOI] [PubMed] [Google Scholar]
- Cho SJ, Yook K, Kim B, Choi TK, Lee KS, Kim YW, Lee JE, Suh S, Yook KH, Lee SH. Mirtazapine augmentation enhances cognitive and reduces negative symptoms in schizophrenia patients treated with risperidone: a randomized controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):208–11. doi: 10.1016/j.pnpbp.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Choi KH, Wykes T, Kurtz MM. Adjunctive pharmacotherapy for cognitive deficits in schizophrenia: meta-analytical investigation of efficacy. Br J Psychiatry. 2013;203:172–8. doi: 10.1192/bjp.bp.111.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle JT, Balu D, Benneyworth M, Basu A, Roseman A. Beyond the dopamine receptor: novel therapeutic targets for treating schizophrenia. Dialogues Clin Neurosci. 2010;12(3):359–82. doi: 10.31887/DCNS.2010.12.3/jcoyle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhane MA, Schoenfeld DA, Barr RS, Cather C, Deckersbach T, Freudenreich O, Goff DC, Rigotti NA, Evins AE. Predictors of early abstinence in smokers with schizophrenia. J Clin Psychiatry. 2008;69:1743–50. doi: 10.4088/jcp.v69n1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes SE, Palmer BW, Meeks T, Golshan S, Kasckow J, Mohamed S, Zisook S. Does antidepressant treatment improve cognition in older people with schizophrenia or schizoaffective disorder and comorbid subsyndromal depression? Neuropsychobiology. 2012;65:168–72. doi: 10.1159/000331141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, Freudenreich O, Henderson DC, Schoenfeld DA, Rigotti NA, Goff DC. A 12-week double-blind, placebo-controlled study of bupropion SR added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol. 2007;27(4):380–6. doi: 10.1097/01.jcp.0b013e3180ca86fa. [DOI] [PubMed] [Google Scholar]
- Evins AE, Cather C, Deckersbach T, Freudenreich O, Culhane MA, Olm-Shipman CS, Henderson DC, Schoenfeld DA, Goff DC, Rigotti NA. A double-blind placebo-controlled trial of bupropionsustained-release for smoking cessation in schizophrenia. J Clin Psychopharmacol. 2005;25:218–25. doi: 10.1097/01.jcp.0000162802.54076.18. [DOI] [PubMed] [Google Scholar]
- Evins AE, Deckersbach T, Cather C, Freudenreich O, Culhane MA, Henderson DC, Green MF, Schoenfeld DA, Rigotti NA, Goff DC. Independent effects of tobacco abstinence and bupropion on cognitive function in schizophrenia. J Clin Psychiatry. 2005;66:1184–90. doi: 10.4088/jcp.v66n0915. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Jones CK, Conn PJ. Emerging approaches for treatment of schizophrenia: modulation of cholinergic signaling. Discov Med. 2012;14(79):413–20. [PMC free article] [PubMed] [Google Scholar]
- Friedman JI, Ocampo R, Elbaz Z, Parrella M, White L, Bowler S, Davis KL, Harvey PD. The effect of citalopram adjunctive treatment added to atypical antipsychotic medications for cognitive performance in patients with schizophrenia. J Clin Psychopharmacol. 2005;25(3):237–42. doi: 10.1097/01.jcp.0000161499.58266.51. [DOI] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, Kosten TR, Wexler BE. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology. 2002;26(1):75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- George TP, Vessicchio JC, Sacco KA, Weinberger AH, Dudas MM, Allen TM, Creeden CL, Potenza MN, Feingold A, Jatlow PI. A placebo-controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia. Biol Psychiatry. 2008;63:1092–6. doi: 10.1016/j.biopsych.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Vessicchio J, Allen T, Weinberger A, Sacco KA. A randomized, double-blind, placebo-controlled trial of sustained-release bupropion combined with transdermal nicotine patch for smoking cessation in schizophrenia: neuropsychological predictors of treatment outcome. ACNP Annual Meeting. 2006:S254–5. [Google Scholar]
- Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, Woerner MG, Schooler NR, Kane JM, Robinson DG. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch Gen Psychiatry. 2007;64(10):1115–22. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67(Suppl 9):3–8. [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Keefe RSE. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry. 2001;158:176–84. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- Hecht EM, Landy DC. Alpha-2 receptor antagonist add-on therapy in the treatment of schizophrenia: a meta-analysis. Schizophr Res. 2012;134(2-3):202–6. doi: 10.1016/j.schres.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Herman AI, Sofuoglu M. Cognitive effects of nicotine: genetic moderators. Addict Biol. 2010;15(3):250–65. doi: 10.1111/j.1369-1600.2010.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydebrand G, Weiser M, Rabinowitz J, Hoff AL, DeLisi LE, Csernansky JG. Correlates of cognitive deficits in first episode schizophrenia. Schizophr Res. 2004;68:1–9. doi: 10.1016/S0920-9964(03)00097-5. [DOI] [PubMed] [Google Scholar]
- Joffe G, Terevnikov V, Joffe M, Stenberg JH, Burkin M, Tiihonen J. Add-on mirtazapine enhances antipsychotic effect of first generation antipsychotics in schizophrenia: a double-blind, randomized, placebo-controlled trial. Schizophr Res. 2009;108:245–51. doi: 10.1016/j.schres.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Jones CK, Byun N, Bubser M. Muscarinic and nicotinic acetylcholine receptor agonists and allosteric modulators for the treatment of schizophrenia. Neuropsychopharmacology. 2012;37(1):16–42. doi: 10.1038/npp.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JM, Lencz T. Cognitive deficits in schizophrenia: short-term and long-term. World Psychiatry. 2008;7(1):29–30. doi: 10.1002/j.2051-5545.2008.tb00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasckow J, Lanouette N, Patterson T, Fellows I, Golshan S, Solorzano E, Zisook S. Treatment of subsyndromal depressive symptoms in middle-aged and older adults with schizophrenia: effect on functioning. Int J Geriatr Psychiatry. 2010;25:183–90. doi: 10.1002/gps.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe RSE, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull. 2007;33(4):912–20. doi: 10.1093/schbul/sbm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Cho SJ, Lee KS, Yook KH, Choe AY, Lee S, Kim B, Kim KH, Choi TK, Lee SH. The tolerability of mirtazapine augmentation in schizophrenic patients treated with risperidone: a preliminary randomized placebo-controlled trial. Clin Psychopharmacol Neurosci. 2011;9(2):73–7. doi: 10.9758/cpn.2011.9.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencz T, Smith CW, McLaughlin D. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863–71. doi: 10.1016/j.biopsych.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Lin CH, Huang CL, Chang YC, Chen PW, Lin CY, Tsai GE, Lane HY. Clinical symptoms, mainly negative symptoms, mediate the influence of neurocognition and social cognition on functional outcome of schizophrenia. Schizophr Res. 2013;146(1-3):231–7. doi: 10.1016/j.schres.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13(3):169–82. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti FS, Lindsley CW, Conn PJ, Pandit J, Zagouras P, Volkmann RA. Allosteric modulators for the treatment of schizophrenia: targeting glutamatergic networks. Curr Top Med Chem. 2013;13(1):26–54. doi: 10.2174/1568026611313010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315–36. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Micò U, Bruno A, Pandolfo G, Maria Romeo V, Mallamace D, D'Arrigo C, Spina E, Zoccali RA, Muscatello MRA. Duloxetine as adjunctive treatment to clozapine in patients with schizophrenia: a randomized, placebo-controlled trial. Int Clin Psychopharmacol. 2011;26(6):303–10. doi: 10.1097/YIC.0b013e32834bbc0d. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Miyake N, Jarskog LF, Fleischhacker WW, Lieberman JA. Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol Psychiatry. 2012;17(12):1206–27. doi: 10.1038/mp.2012.47. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Paulsen JS, O'Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry. 1999;56(8):749–54. doi: 10.1001/archpsyc.56.8.749. [DOI] [PubMed] [Google Scholar]
- Moss TG, Sacco KA, Allen TM, Weinberger AH, Vessicchio JC, George TP. Prefrontal cognitive dysfunction is associated with tobacco dependence treatment failure in smokers with schizophrenia. Drug Alcohol Depend. 2009;104:94–9. doi: 10.1016/j.drugalcdep.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62(3):1574–83. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niitsu T, Fujisaki M, Shiina A, Yoshida T, Hasegawa T, Kanahara N, Hashimoto T, Shiraishi T, Fukami G, Nakazato M, Shirayama Y, Hashimoto K, Iyo M. A randomized, double-blind, placebo-controlled trial of fluvoxamine in patients with schizophrenia: a preliminary study. J Clin Psychopharmacol. 2012;32(5):593–601. doi: 10.1097/JCP.0b013e3182664cfc. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M, Fuchs C, Pashinian A, Levi A, Faragian S, Maayan R, Gil-Ad I. Attenuating effect of reboxetine on appetite and weight gain in olanzapine-treated schizophrenia patients: a double- blind placebo-controlled study. Psychopharmacology (Berl) 2007;192:441–8. doi: 10.1007/s00213-007-0731-1. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M, Koren D, Gonopolsky I, Schneidman M, Fuchs C, Weizman A, Weizman R. Effect of the 5-HT2 antagonist mianserin on cognitive dysfunction in chronic schizophrenia patients: an add-on, double-blind placebo-controlled study. Eur Neuropsychopharmacol. 2003;13:123–8. doi: 10.1016/s0924-977x(02)00155-4. [DOI] [PubMed] [Google Scholar]
- Poyurovsky M, Faragian S, Fuchs C, Pashinian A. Effect of the selective norepinephrine reuptake inhibitor reboxetine on cognitive dysfunction in schizophrenia patients: an add-on, double-blind placebo-controlled study. Isr J Psychiatry Relat Sci. 2009;46(3):213–20. [PubMed] [Google Scholar]
- Rummel C, Kissling W, Leucht S. Antidepressants for the negative symptoms of schizophrenia. Cochrane Database of Syst Rev. 2006;3:CD005581. doi: 10.1002/14651858.CD005581.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehry AA, Potvin S, Elie R, Stip E. Selective serotonin reuptake inhibitor (SSRI) add-on therapy for the negative symptoms of schizophrenia: a meta-analysis. J Clin Psychiatry. 2007;68:604–10. doi: 10.4088/jcp.v68n0417. [DOI] [PubMed] [Google Scholar]
- Singh SP, Singh V, Kar N, Chan K. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry. 2010;197:174–9. doi: 10.1192/bjp.bp.109.067710. [DOI] [PubMed] [Google Scholar]
- Stenberg JH, Terevnikov V, Joffe M, Tiihonen J, Tchoukhine E, Burkin M, Joffe G. Effects of add-on mirtazapine on neurocognition in schizophrenia: a double-blind, randomized, placebo-controlled study. Int J Neuropsychopharmacol. 2010;13:433–41. doi: 10.1017/S1461145709990897. [DOI] [PubMed] [Google Scholar]
- Terevnikov V, Stenberg JH, Tiihonen J, Joffe M, Burkin M, Tchoukhine E, Joffe G. Add-on mirtazapine improves depressive symptoms in schizophrenia: a double-blind randomized placebo-controlled study with an open-label extension phase. Hum Psychopharmacol. 2011;26:188–93. doi: 10.1002/hup.1189. [DOI] [PubMed] [Google Scholar]
- Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;2:CD007253. doi: 10.1002/14651858.CD007253.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Bow-Thomas CC, Mahurin RK, Miller AL, Halgunseth LC. Do specific neurocognitive deficits predict specific domains of community function in schizophrenia? J Nerv Ment Dis. 2000;188(8):518–24. doi: 10.1097/00005053-200008000-00007. [DOI] [PubMed] [Google Scholar]
- Verdoux H, Liraud F, Assens F, Abalan F, van Os J. Social and clinical consequences of cognitive deficits in early psychosis: a two-year follow-up study of first-admitted patients. Schizophr Res. 2002;56:149–59. doi: 10.1016/s0920-9964(01)00225-0. [DOI] [PubMed] [Google Scholar]
- Vohora D, Bhowmik M. Histamine H3 receptor antagonists/inverse agonists on cognitive and motor processes: relevance to Alzheimer's disease, ADHD, schizophrenia, and drug abuse. Front Syst Neurosci. 2012;6(72):1–10. doi: 10.3389/fnsys.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner E, Ball MP, Buchholz AS, Gold JM, Evins AE, McMahon RP, Buchanan RW. Bupropion sustained release added to group support for smoking cessation in schizophrenia: a new randomized trial and a meta-analysis. J Clin Psychiatry. 2012;73(1):95–102. doi: 10.4088/JCP.10m06143gre. [DOI] [PubMed] [Google Scholar]
- Whitehead C, Moss S, Cardno A, Lewis G. Antidepressants for the treatment of depression in people with schizophrenia: a systematic review. Psychol Med. 2003;33:589–99. doi: 10.1017/s0033291703007645. [DOI] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–85. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- Zanelli J, Reichenberg A, Morgan K, Fearon P, Kravariti E, Dazzan P, Morgan C, Zanelli C, Demjaha A, Jones PB, Doody GA, Kapur S, Murray RM. Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. Am J Psychiatry. 2010;167(1):78–85. doi: 10.1176/appi.ajp.2009.09010118. [DOI] [PubMed] [Google Scholar]
- Zisook S, Kasckow JW, Lanouette NM, Golshan S, Fellows I, Vahia I, Mohamed S, Rao S. Augmentation with citalopram for suicidal ideation in middle-aged and older outpatients with schizophrenia and schizoaffective disorder who have subthreshold depressive symptoms: a randomized controlled trial. J Clin Psychiatry. 2010;71(7):915–22. doi: 10.4088/JCP.09m05699gre. [DOI] [PubMed] [Google Scholar]
- Zisook S, Kasckow JW, Golshan S, Fellows I, Solorzano E, Lehman D, Mohamed S, Jeste DV. Citalopram augmentation for subsyndromal symptoms of depression in middle-aged and older outpatients with schizophrenia and schizoaffective disorder: a randomized controlled trial. J Clin Psychiatry. 2009;70(4):562–71. doi: 10.4088/jcp.08m04261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.