Abstract
Purpose
To evaluate the risk of cataract in the setting of AIDS.
Design
Prospective cohort study.
Participants
Subjects with AIDS free of ocular opportunistic infections throughout catamnesis.
Methods
During 1998–2008 inclusive, subjects ≥13 years of age were enrolled. Demographic characteristics and clinical characteristics were documented at enrollment and semiannually.
Main Outcome Measures
Cataract was defined as high-grade lens opacity observed by biomicroscopy and judged to be the cause of a best-corrected visual acuity worse than 20/40. Eyes that underwent cataract surgery during follow-up were considered to have developed cataract prior to the first visit when pseudophakia or aphakia was observed.
Results
Among 1,606 participants (3,212 eyes), at enrollment 1.9% (95% confidence interval (CI): 1.3%−2.7%) were observed to have cataract or prior cataract surgery. Among the 2,812 eyes initially free of cataract, and followed longitudinally (median follow-up=4.6 years), the incidence of cataract was 0.37%/eye-year (95% CI: 0.26%– 0.53%). In addition to age, significant cataract risk factors included prior cataract in the contralateral eye (adjusted hazard ratio (aHR)=21.6, 95% CI: 10.4–44.8), anterior segment inflammation (aHR=4.40, 95% CI: 1.64–11.9), prior retinal detachment (aHR=4.94, 95% CI: 2.21–11.0), and vitreous inflammation (aHR=7.12, 95% CI: 2.02– 25.0), each studied as a time-updated characteristic. Detectable HIV RNA in peripheral blood was associated with lower risk of cataract at enrollment (adjusted odds ratio=0.32, 95% CI: 0.12–0.80) but not of incident cataract (aHR=1.58, 95% CI: 0.90–2.76). After adjustment for other factors, neither the then current absolute CD4+ T cell count nor antiretroviral therapy status showed consistent association with cataract risk, nor did an additive diagnosis of other other co-morbidities. Compared to the available population-based studies that used similar definitions of cataract, the age-specific prevalence of cataract in our cohort was higher than in one of two such studies, and the age-specific incidence of cataract surgery was higher.
Conclusions
Our results suggest cataract may occur earlier among patients with AIDS free of ocular opportunistic infections than in the general population. Cataract risk was associated most strongly with age and with other ocular morbidity in this population. With improved survival, the burden of cataract likely will increase for persons with HIV/AIDS.
Cataract is the leading cause of visual impairment in the United States1 and worldwide2 and is the leading cause of legal blindness among African-Americans.1 Historically, cataract has not been viewed as one of the significant causes of ocular morbidity in patients with the Acquired Immune Deficiency Syndrome (AIDS), because such morbidity has been dominated by ocular opportunistic complications of immunodeficiency.3 Furthermore, cataract is typically a disorder of older adults,4 whereas the AIDS epidemic began predominantly among younger and middle aged adults.5;6
In the era of highly active antiretroviral therapy (HAART), the incidence of opportunistic complications of AIDS has declined substantially,7;8 including the incidence of ocular opportunistic complications.8–13 Among persons who have a long-term favorable response to HAART, AIDS has evolved into a chronic disease wherein long-term survival is expected. This context provides an opportunity for individuals with AIDS to develop ocular diseases of aging, including cataract. In addition, there are indications that these individuals may experience accelerated aging,14;15 of which cataract, and earlier onset of cataract, are potential indicators.16
In our investigations of the long-term ocular complications of AIDS, we found that patients with both AIDS and CMV retinitis are at high risk for cataract.17 We also documented that cataract was the second leading cause of visual impairment in the Longitudinal Study of Ocular Complications of AIDS (LSOCA) cohort among subjects who did not have CMV retinitis at the time of enrollment.18 To characterize better the risk of cataract among these subjects with AIDS, we report now analyses of the prevalence and incidence of cataract in LSOCA subjects who were free of CMV retinitis and other intraocular opportunistic infections at the time of enrollment.
Methods
The methods of the Longitudinal Study of Ocular Complications of AIDS (LSOCA) have been described extensively.18–27 Briefly, patients with AIDS ages 13 years and older were enrolled at 19 United States centers specializing in ocular complications of AIDS, beginning in 1998—entirely within the HAART era. The study respects the principles of the Declaration of Helsinki at all centers, operating under the ongoing approval of each site’s governing institutional review board.
From the beginning of the study on 2 September, 1998, through 27 March, 2008, the period of the observations reported here, subjects free of intraocular opportunistic infections were evaluated at semiannual study visits. Demographic and clinical data about AIDS diagnosis, current and nadir CD4+ T cell count, current and zenith HIV load in peripheral blood, anemia, the presence systemic opportunistic complications of AIDS, past and present use of medications, and the presence of co-morbidities were obtained at enrollment and updated at each study visit. A complete ophthalmological examination including both binocular biomicroscopy and dilated ophthalmoscopy were performed by a study-certified ophthalmologist at each visit, including grading of lens opacities as: normal or trivial opacities (less than Grade 1); peripheral vacuoles (Grade 1); peripheral opacity (Grade 2); central opacity (Grade 3); central opacity affecting vision (Grade 4); or surgical aphakia or pseudophakia. A total of 158 examiners at 20 clinics evaluated cataract status. The median number of assessments per examiner was 13 (range: 1 to 1670, Interquartile range (IQR)w: 3 to 50). The median number of examiners per clinic was 10 (range: 1 to 23, IQR: 7 to 14). Best-corrected visual acuity with a logarithmic chart was measured at every visit by gold standard methods.28 For purposes of these analyses, eyes were defined as having a cataract if they met the following three criteria: 1) graded as having a lens opacity; 2) best-corrected visual acuity worse than 20/40; and 3) reduction of best-corrected visual acuity attributed by the examining ophthalmologist to cataract. Eyes that had undergone cataract surgery prior to the first study visit also were considered to have had a cataract. Among phakic eyes free of cataract at the time of enrollment, the incidence of cataract was counted as occurring on the first follow-up visit at which either cataract, pseudophakia, or aphakia was noted. Anterior segment inflammation was defined as present for eyes with anterior chamber cells, anterior chamber flare, a diagnosis of anterior uveitis or keratitis, and/or the presence of posterior synechiae. Posterior segment inflammation was defined as present for eyes with vitreous cells and/or vitreous haze; eyes with a diagnosis with intermediate uveitis, posterior uveitis, or panuveitis, or endophthalmitis also were considered to have posterior segment inflammation.
Most population-based studies of cataract in both the United States and Australia have diagnosed cataract based on masked gradings of lens photographs by a reading center, independent of the effects of any opacities on visual acuity.29–32 However, slit lamp biomicroscopy-based grading methods comparable to those used here have been applied to estimate the prevalence of cataract in two population-based studies in the United States, each of which studied Hispanics predominantly of Mexican origin.33;34 One of these, the Los Angeles Latino Eye Study (LALES),35 provided data on the incidence of individual-level cataract surgery for comparison to the data from LSOCA cohort in this analysis.
Sensitivity analyses were performed to determine whether risk factor associations with incident cataract were consistent across two possible alternative definitions of cataract: 1) presence of a “central opacity affecting vision (grade 4)”; and 2) the ophthalmologist’s indication that “based solely on lens status, …[the eye would] be a candidate for cataract surgery”.
The prevalence of either present or prior cataract was evaluated at the time of enrollment in LSOCA. Logistic regression models were fit via generalized estimating equations to estimate the prevalence of cataract and to compare the prevalence and the four-year incidence in the LSOCA cohort to the population-based studies. Staggered entry Kaplan-Meier curves were constructed to show the cumulative probability of incident cataract over time. Cox proportional hazards models evaluated risk factors for incident cataract. All time-to-event analysis were anchored on age to account for this potent cataract risk factor and clustered by individual to account for inter-eye correlation. Subscripts before and after estimates of adjusted odds ratios (ORs) and hazard ratios (HRs) indicate the upper and lower bounds of 95% confidence intervals. Statistical analyses were performed with SAS software version 9.1 (SAS Inc, Cary, NC), Stata software release 10 (StataCorp, College Station, TX), and R software version 2.11.1 (The R Project for Statistical Computing, http://www.r-project.org/, last accessed April 14, 2014).
Results
Two thousand one hundred twenty-one subjects with AIDS were enrolled into the LSOCA cohort between 2 September 1998 and 28 March 2008. Twenty-six subjects were excluded from the analysis for the following reasons: 1) they had been diagnosed with a non-CMV herpetic retinitis, toxoplasmosis involving the retina, or syphilitic eye disease (n = 21) prior to enrollment or during follow-up; 2) they did not complete the enrollment visit (n = 3); or 3) they had unknown cataract status (1) or unknown CMV retinitis status (1) at the time of enrollment. Among the remaining 2,095 subjects with complete cataract and CMV retinitis data, 1,606 (88%) were free of intraocular opportunistic infections in both eyes at enrollment and did not develop intraocular opportunistic infections during follow-up. The prevalence and incidence of cataract were evaluated in the 3,212 eyes of these 1,606 patients.
Characteristics of the analyzed population at enrollment are given as Table 1, available at http://aaojournal.org. Most patients were young and middle-aged male adults, 46% of whom were white, 36% African-American, 15% Hispanic, and the rest of another race/ethnicity. By inclusion criteria of LSOCA, all met the then current CDC definition of AIDS,36 63.2% based on a systemic opportunistic infection and the remainder based on CD4+ T lymphopenia. As of the enrollment visit, the median time since AIDS diagnosis was 4.2 years. Although 84% were receiving HAART, 56% had a detectable HIV load in peripheral blood at enrollment. While for 53% the nadir CD4+ T cell count at or prior to enrollment was less than 50 cells/µL, at enrollment 82% had a CD4+ T cell count of 50 cells/µL or more. A substantial proportion of subjects were ill at enrollment, including about 31.3% with anemia, 9% with diagnosed diabetes mellitus, 20% with systemic hypertension, and 21% with hyperlipidemia; 49% had a Karnofsky score37 of 80 or less.
Table 1.
Characteristics of individuals and eyes at enrollment for individuals in the Longitudinal Study of Ocular Complications of AIDS without cytomegalovirus retinitis.
| Characteristics of patients at enrollment | Participants (N = 1606) |
|
|---|---|---|
| Demographic characteristics | ||
| Age at enrollment, years | ||
| Median (interquartile range) | 43 | (38 , 49) |
| Gender | ||
| Female | 317 | (20%) |
| Male | 1289 | (80%) |
| Race/Ethnicity | ||
| White | 739 | (46%) |
| Black | 577 | (36%) |
| Hispanic | 236 | (15%) |
| Other | 54 | (3%) |
| Education | ||
| High school or less | 652 | (41%) |
| Some college | 490 | (31%) |
| College graduate | 460 | (29%) |
| Missing, N(%) | 4 | (0%) |
| Associated morbidities | ||
| Karnofsky score | ||
| 90–100 | 826 | (52%) |
| ≤ 80 | 779 | (49%) |
| Missing, N(%) | 1 | (0%) |
| Anemia | ||
| No | 1104 | (69%) |
| Yes | 492 | (31%) |
| Missing, N(%) | 10 | (1%) |
| Diabetes | ||
| No | 1462 | (91%) |
| Yes | 144 | (9%) |
| Hypertension | ||
| No | 1282 | (80%) |
| Yes | 323 | (20%) |
| Missing, N(%) | 1 | (0%) |
| Hyperlipidemia | ||
| No | 1256 | (78%) |
| Yes | 346 | (22%) |
| Missing, N(%) | 4 | (0%) |
| Human Immunodeficiency Virus (HIV)/Acquired Immune Deficiency Syndrome (AIDS) History | ||
| Years since AIDS diagnosis (years) | ||
| Median (interquartile range) | 4.2 | (1.6 , 7.1) |
| Absolute CD4+ T cell count, cells/µL | ||
| Median (interquartile range) | 189 | (78 , 338) |
| Cells/uL, N(%) | ||
| ≥ 50 cells/uL | 1306 | (82%) |
| < 50 cells/uL | 283 | (18%) |
| Missing, N(%) | 17 | (1%) |
| Nadir CD4+ T cells (cells/uL) | ||
| ≥ 50 cells/uL | 742 | (47%) |
| < 50 cells/uL | 839 | (53%) |
| Missing, N(%) | 25 | (2%) |
| (HIV) viral load, log10(copies/mL) | ||
| Median (interquartile range) | 2.9 (2.0, 4.7) | |
| Log 10 (copies/mL) | ||
| < 2.6 (undetectable) | 677 | (44%) |
| 2.6 or higher | 848 | (56%) |
| Missing, N(%) | 81 | (5%) |
| Highly active antiretroviral therapy | ||
| No | 261 | (16%) |
| Yes | 1344 | (84%) |
| Missing, N(%) | 1 | (0%) |
| Co-infections | ||
| Cerebral toxoplasmosis | ||
| No | 1593 | (99%) |
| Yes | 13 | (1%) |
| Hepatitis B | ||
| No | 1488 | (93%) |
| Yes | 117 | (7%) |
| Missing, N(%) | 1 | (0%) |
| Hepatitis C | ||
| No | 1487 | (93%) |
| Yes | 118 | (7%) |
| Missing, N(%) | 1 | (0%) |
| Ocular characteristics |
Eyes (E = 3212) |
|
| Anterior inflammation* | ||
| No | 3121 | (97%) |
| Yes | 91 | (3%) |
| Vitreous inflammation† | ||
| No | 3151 | (98%) |
| Yes | 61 | (2%) |
| History of retinal detachment‡ | ||
| No | 3188 | (99%) |
| Yes | 24 | (1%) |
Anterior chamber cells or flare, diagnosis with anterior uveitis or keratitis, presence of posterior synechiae, or a combination thereof.
Vitreous haze, anterior vitreous cells, intermediate uveitis, endophthalmitis.
Retinal detachment includes individuals with a documented history of retinal detachment and/or the presence of silicone oil.
Prevalence of Cataract
At enrollment, 30 individuals (1.21.92.7%) had a visually significant cataract (as defined above) or previously had undergone cataract surgery (“cataract”) in at least one eye. Of these, 23 (77%) had cataract in one eye and 7 (23%) had cataract in each eye. In comparison to the population-based studies that assessed cataract prevalence by a comparable method (see Table 2),33;34 the prevalence of cataract was similar among Proyecto VER participants (Hispanics living in Arizona) and LSOCA participants without CMV retinitis or other ocular opportunistic infections, with the exception that the prevalence of cataract was significantly higher in the 40–49 year old age group in LSOCA (1.6%) than Proyecto VER (0.3%; interaction [age group-study cohort] p=0.042; see Table 2). In contrast, after adjustment for age, the LSOCA cohort had a higher overall prevalence of cataract than the Los Angeles Latino Eye Study (LALES) cohort (adjusted OR (aOR) = 1.322.153.49 , p = 0.002, Table 2). Non-Latino population-based studies did not assess cataract in a manner comparable to that in LSOCA.
Table 2.
Comparison of the prevalence of at least one eye with a cataract in individuals from the ProyectoVER, LSOCA (patients without CMV retinitis or other ocular opportunistic infections), and LALES cohorts.*
| Age cate- gory |
Proyecto VER Cohort |
LSOCA Cohort No CMV Retinitis |
LALES Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | (95% CI) | N | % | (95% CI) | N | % | (95% CI) | |
| < 40 | 0 | n/a | 518 | 0.6% | (0.1% – 1.7%) | 0 | n/a | ||
| 40–49 | 1594 | 0.3% | (0.06% – 6.5%) | 736 | 1.6%** | (0.8% – 2.9%) | 2364 | 0.9% | (0.6% – 1.4%) |
| 50–59 | 1362 | 2.0% | (1.3% – 28.8%) | 313 | 2.2% | (0.9% – 4.6%) | 1853 | 1.8% | (1.3%–2.6%) |
| 60–69 | 984 | 8.6% | (6.9% – 10.6%) | 63 | 9.5% | (3.5% – 19.6%) | 1195 | 7.3% | (5.9% – 8.9%) |
| 70–79 | 636 | 23.3% | (20.0% – 26.8%) | 10 | 20.0% | (2.5% – 55.7%) | 584 | 24.1% | (20.7% – 27.8%) |
| 80+ | 196 | 58.2% | (50.9% – 65.2%) | 0 | n/a | 146 | 52.1% | (43.6% – 60.4%) | |
N = number of individuals; % = percent; 95% CI = exact binomial 95% confidence interval LSOCA = Longitudinal study of the ocular complications of AIDS; CMV = cytomegalovirus; LALES = Los Angeles Latino Eye Study; Proyecto VER data derive from reference 28, and LALES data from reference 29.
The prevalence of 1.6% in the LSOCA Cohort 40–49 year-old age category was statistically significantly different than the same age group in the Proyecto VER Cohort (age-group/study cohort interaction p=0.042). No other statistically significant age-group/study cohort interactions were observed.
In the LSOCA cohort, older age was associated with a markedly higher risk of cataract (aOR = 1.682.614.05 per 10-year increase in age) (see Table 3), similar to the pattern universally observed in population studies,4 including those that evaluated cataract similarly.33;34 The other factors strongly associated with a greater prevalence of cataract reflected ocular diseases, including a history of retinal detachment (adjusted OR (aOR)=13.848.7171.4) and of anterior segment inflammation (aOR=1.545.3118.3). Detectable HIV in peripheral blood was associated with a reduced prevalence of cataract (aOR=0.120.320.80), unlike the pattern observed in the incidence analysis (see below). Neither time since diagnosis with AIDS, current CD4+ T cell count, current HIV load in peripheral blood, nor use vs. non-use of highly active antiretroviral therapy (HAART) was associated with altered cataract risk (see Table 4, available at http://aaojournal.org ). Subjects diagnosed with diabetes mellitus and hypertension had an increased crude prevalence of cataract, which was attributable to confounding by age. Hepatitis B infection also was associated with increased crude prevalence of cataract, which was attributable to confounding by HIV load.
Table 3.
Risk factors for Cataract (or Prior Cataract Surgery) at the Time of Cohort Entry in Eyes without an Opportunistic Ocular Infection during follow-up in the Longitudinal Studies of the Complications of AIDS, Final Logistic Regression Model
| Characteristics* | No Cataract | Cataract | Unadjusted Odds Ratio (95% Confidence Interval) |
Unadjusted P-value |
Adjusted Odds Ratio (95% Confidence Interval) |
Adjusted P-value |
|---|---|---|---|---|---|---|
| Age (per 10 years) | NA | NA | 2.83 (1.88, 4.25) | < 0.0001 | 2.61 (1.68, 4.05) | < 0.0001 |
| HIV viral load [Log10(copies/mL)] | ||||||
| < 2.6 (undetectable) | 1329 (98%) | 25 (2%) | 1.00 | 1.00 | ||
| 2.6 or higher | 1686 (99%) | 10 (1%) | 0.32 (0.13, 0.72) | 0.0061 | 0.32 (0.12, 0.80) | 0.0141 |
| Anterior inflammation** | ||||||
| No | 3088 (99%) | 33 (1%) | 1.00 | 1.00 | ||
| Yes | 87 (96%) | 4 (4%) | 5.99 (2.1, 17.03) | 0.0008 | 5.31 (1.54, 18.24) | 0.0079 |
| History of retinal detachment*** | ||||||
| No | 3158 (99%) | 30 (1%) | 1.00 | 1.00 | ||
| Yes | 17 (71%) | 7 (29%) | 28.08 (6.89, 1114.29) | < 0.0001 | 48.66 (13.81, 171.40) | < 0.0001 |
HIV = human immunodeficiency virus; NA = not applicable (age is a numerical variable).
The following characteristics were evaluated but not included in the final logistic regression model (summarized in Table 4, available at: http://aaojournal.org) since they were not significantly associated with cataract at the time of cohort entry: gender, race/ethnicity, education, Karnofsky score, anemia, hyperlipidemia, time since AIDS diagnosis, current CD4+ T-cell count, nadir CD4+ T-cell count, current use of highly active antiretroviral therapy, and hepatitis C. African American race and vitreous inflammation had no crude association with cataract at the time of cohort entry, but were associated after adjustment for the factors above. Hepatitis B, diabetes, and hypertension were associated with increased risk of prevalent cataract that was attributable to counfounding by the variables included in the final multiple logistic regression model above.
Anterior chamber cells or flare, diagnosis with anterior uveitis or keratitis, presence of posterior synechiae, or a combination thereof.
Retinal detachment includes individuals with a documented history of retinal detachment and/or the presence of silicone oil.
Table 4.
Risk factors for Cataract (or Prior Cataract Surgery) at the Time of Cohort Entry in Eyes without an Opportunistic Ocular Infection during follow-up in the Longitudinal Study of Ocular Complications of AIDS
| Characteristics | No Cataract | Cataract | Unadjusted Odds Ratio (95% Confidence Interval) |
Unadjusted P-value |
Adjusted Odds Ratio (95% Confidence Interval) |
Adjusted P-value |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Age (per 10 years) | NA | NA | 2.83 (1.88, 4.25) | < 0.0001 | 2.61 (1.68, 4.05) | < 0.0001 |
| Gender | ||||||
| Female | 623 (98%) | 11 (2%) | 1.00 | 1.00 | ||
| Male | 2552 (99%) | 26 (1%) | 0.58 (0.24, 1.37) | 0.2104 | 0.52 (0.19, 1.41) | 0.1939 |
| Race/Ethnicity | ||||||
| Not black | 2040 (99%) | 18 (1%) | 1.00 | 1.00 | ||
| Black | 1135 (98%) | 19 (2%) | 1.9 (0.88, 4.06) | 0.0982 | 2.97 (1.41, 6.22) | 0.0039 |
| Education | ||||||
| High school or less | 1291 (99%) | 13 (1%) | 1.00 | 1.00 | ||
| Some college | 967 (99%) | 13 (1%) | 1.34 (0.53, 3.31) | 0.5327 | 1.18 (0.46, 3.01) | 0.7330 |
| College graduate | 909 (99%) | 11 (1%) | 1.2 (0.48, 2.98) | 0.6910 | 0.77 (0.29, 1.96) | 0.5777 |
| Missing | 8 (100%) | |||||
| Associated morbidities | ||||||
| Karnofsky score (at enrollment) | ||||||
| 90–100 | 1639 (99%) | 13 (1%) | 1.00 | 1.00 | ||
| ≤ 80 | 1534 (98%) | 24 (2%) | 1.97 (0.89, 4.33) | 0.0900 | 1.79 (0.8, 3.99) | 0.1546 |
| Missing | 2 (100%) | |||||
| Anemia | ||||||
| No | 2179 (99%) | 29 (1%) | 1.00 | 1.00 | ||
| Yes | 976 (99%) | 8 (1%) | 0.62 (0.23, 1.6) | 0.3176 | 0.78 (0.24, 2.47) | 0.6779 |
| Missing | 20 (100%) | |||||
| Diabetes | ||||||
| No | 2897 (99%) | 27 (1%) | 1.00 | 1.00 | ||
| Yes | 278 (97%) | 10 (3%) | 3.86 (1.56, 9.53) | 0.0034 | 2.92 (0.96, 8.85) | 0.0582 |
| Hypertension | ||||||
| No | 2543 (99%) | 21 (1%) | 1.00 | 1.00 | ||
| Yes | 630 (98%) | 16 (2%) | 3.08 (1.41, 6.69) | 0.0045 | 1.92 (0.86, 4.25) | 0.1086 |
| Missing | 2 (100%) | |||||
| Hyperlipidemia | ||||||
| No | 2482 (99%) | 30 (1%) | 1.00 | 1.00 | ||
| Yes | 685 (99%) | 7 (1%) | 0.85 (0.32, 2.18) | 0.7271 | 0.39 (0.14, 1.05) | 0.0603 |
| Missing | 8 (100%) | |||||
| Human Immunodeficiency Virus (HIV)/Acquired Immune Deficiency Syndrome (AIDS) History | ||||||
| Time since AIDS diagnosis (years) | ||||||
| < 2 years | 906 (99%) | 10 (1%) | 1.00 | 1.00 | ||
| 2–4 years | 593 (99%) | 5 (1%) | 0.76 (0.2, 2.82) | 0.6856 | 0.53 (0.13, 2.11) | 0.3668 |
| 4–6 years | 570 (99%) | 8 (1%) | 1.27 (0.42, 3.85) | 0.6703 | 1.08 (0.32, 3.62) | 0.8974 |
| ≥6 years | 1058 (99%) | 14 (1%) | 1.2 (0.44, 3.22) | 0.7188 | 0.79 (0.25, 2.45) | 0.6788 |
| Missing | 48 (100%) | |||||
| CD4+ T cells, N(%) | ||||||
| ≥ 50 cells/uL | 2580 (99%) | 32 (1%) | 1.00 | 1.00 | ||
| < 50 cells/uL | 563 (99%) | 3 (1%) | 0.43 (0.12, 1.44) | 0.1686 | 0.80 (0.28, 2.03) | 0.6807 |
| Missing | 32 (94%) | 2 (6%) | ||||
| Nadir CD4+ T cells (cells/uL) | ||||||
| ≥50 cells/uL | 1465 (99%) | 19 (1%) | 1.00 | 1.00 | ||
| < 50 cells/uL | 1661 (99%) | 17 (1%) | 0.79 (0.36, 1.71) | 0.5480 | 1.01 (0.45, 2.22) | 0.9848 |
| Missing | 49 (98%) | 1 (2%) | ||||
| HIV viral load [Log10(copies/mL)] | ||||||
| < 2.6 (undetectable) | 1329 (98%) | 25 (2%) | 1.00 | 1.00 | ||
| 2.6 or higher | 1686 (99%) | 10 (1%) | 0.32 (0.13, 0.72) | 0.0061 | 0.32 (0.12, 0.80) | 0.0141 |
| Missing | 160 (99%) | 2 (1%) | ||||
| Highly active antiretroviral therapy | ||||||
| No | 515 (99%) | 7 (1%) | 1.00 | 1.00 | ||
| Yes | 2658 (99%) | 30 (1%) | 0.83 (0.29, 2.31) | 0.7212 | 0.43 (0.12, 1.41) | 0.1605 |
| Missing | 2 (100%) | |||||
| Co-infections | ||||||
| Hepatitis B | ||||||
| No | 2947 (99%) | 29 (1%) | 1.00 | 1.00 | ||
| Yes | 226 (97%) | 8 (3%) | 3.6 (1.38, 9.37) | 0.0088 | 1.63 (0.62, 4.27) | 0.3199 |
| Missing | 2 (100%) | |||||
| Hepatitis C | ||||||
| No | 2939 (99%) | 35 (1%) | 1.00 | 1.00 | ||
| Yes | 234 (99%) | 2 (1%) | 0.72 (0.16, 3.04) | 0.6518 | 0.60 (0.14, 2.54) | 0.4846 |
| Missing | 2 (100%) | |||||
| Ocular characteristics | ||||||
| Anterior inflammation* | ||||||
| No | 3088 (99%) | 33 (1%) | 1.00 | 1.00 | ||
| Yes | 87 (96%) | 4 (4%) | 5.99 (2.1, 17.03) | 0.0008 | 5.31 (1.54, 18.24) | 0.0079 |
| Vitreous inflammation** | ||||||
| No | 3116 (99%) | 35 (1%) | 1.00 | 1.00 | ||
| Yes | 59 (97%) | 2 (3%) | 2.13 (0.63, 7.08) | 0.2183 | 0.10 (0.03, 0.32) | 0.0001 |
| History of retinal detachment*** | ||||||
| No | 3158 (99%) | 30 (1%) | 1.00 | 1.00 | ||
| Yes | 17 (71%) | 7 (29%) | 28.08 (6.89, 1114.29) | < 0.0001 | 48.66 (13.81, 171.40) | < 0.0001 |
Anterior chamber cells or flare, diagnosis with anterior uveitis or keratitis, presence of posterior synechiae, or a combination thereof.
Vitreous haze, anterior vitreous cells, intermediate uveitis, endophthalmitis.
Retinal detachment includes individuals with a documented history of retinal detachment and/or the presence of silicone oil.
In sensitivity analyses with the alternative definitions of cataract, results were similar, except that diagnosis with diabetes mellitus remained associated significantly with increased risk of cataract after adjustment for other variables (aOR=1.032.636.70) in the analysis defining cataract as a “central opacity affecting vision”, but not with the alternative definition (aOR= 0.842.195.73)
Incidence of Cataract
Three thousand one hundred seventy-five phakic eyes (of 1,599 patients) were free of cataract at enrollment. Of these, 2,812 (89%) completed at least one follow-up visit. The median follow-up time was 4.6 years (range: 0.37 – 9.12). Out of 25,377 expected visits over the period of follow-up, 22,251 (88%) study visits were completed. During 12,707 eye-years of risk for cataract, 56 new cataracts were observed (incidence rate = 0.260.370.53/100 eye-years).
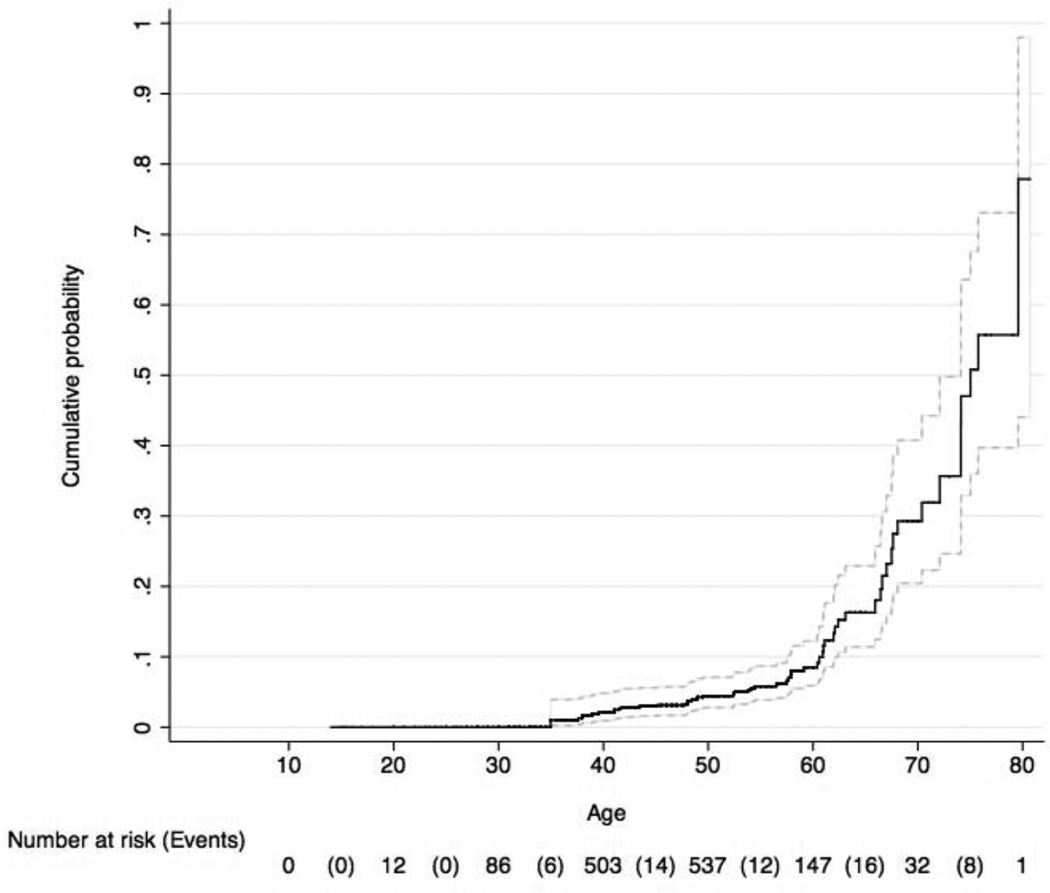
Just as for prevalent cataract, the risk of incident cataract increased with age (see Figure), following a trajectory similar to population-based reports, with the higher risk in the group ages 60 years and older.4;33;34 To account for this known predictive factor, the remaining risk analyses of time-to-cataract are anchored to age; thus, age is not included as a covariate in these models.
Figure.
Cumulative incidence of cataract as a function of age of diagnosis among participants with AIDS enrolled in the Longitudinal Study of Ocular Complications of AIDS (95% confidence interval indicated by hatched lines).
As in the prevalence analyses, characteristics (other than age) associated with increased incidence of cataract (see Table 5) were primarily ocular, including a history of cataract in the opposite eye (adjusted hazard ratio (aHR)=10.421.644.8), anterior segment inflammation (aHR1.644.4011.9), vitreous inflammation (aHR2.027.1225.0), and a history of retinal detachment (aHR2.214.9411.0); all these were measured as time-updated characteristics. Karnofsky score ≤80 and infection with hepatitis B each were associated with marginally significantly increased crude incidence of cataract, but the associations were attributable to confounding by contralateral cataract (see Table 6, available at http://aaojournal.org). Detectable HIV RNA in peripheral blood (aHR=0.901.582.76), CD4+ T cell count, and use of HAART were not associated significantly with incidence of cataract, although a modest increase in risk with low CD4+ T cell count and with non-use of HAART could not be excluded with the available power. No other demographic and clinical characteristics were associated with altered incidence of cataract.
Table 5.
Assessment of Risk Factors for Incident Cataract in Eyes without an Opportunistic Ocular Infection (participants in the Longitudinal Study of Ocular Complications of AIDS), Final Cox Regression Model.
| Characteristics* | Rate per 100 Eye-years |
Count of Cataracts per Eye-year at Risk |
Unadjusted Hazard Ratio (95% Confidence Interval [CI]) |
Unadjusted P-value |
Adjusted Hazard Ratio (95% CI) |
Adjusted P- value |
|---|---|---|---|---|---|---|
| Human Immunodeficiency Virus (HIV)/Acquired Immune Deficiency Syndrome (AIDS) History | ||||||
| CD4+ T cells (time-varying) | ||||||
| ≥50 cells/uL | 0.43 | (50 / 11633) | 1.00 | 1.00 | ||
| < 50 cells/uL | 0.55 | (6 / 1091.3) | 2.16 (0.88, 5.25) | 0.090 | 1.64 (0.77, 3.45) | 0.19 |
| 0 | (0 / 135.7) | |||||
| Nadir CD4+ T cells (at enrollment) | ||||||
| ≥50 cells/uL | 0.64 | (39 / 6132.4) | 1.00 | 1.00 | ||
| < 50 cells/uL | 0.26 | (17 / 6458.2) | 0.62 (0.33, 1.16) | 0.13 | 0.55 (0.31, 0.95) | 0.031 |
| 0 | (0 / 111.6) | |||||
| HIV viral load (time-varying) | ||||||
| < 2.6 (undetectable) | 0.45 | (35 / 7764.9) | 1.00 | 1.00 | ||
| 2.6 or higher | 0.43 | (21 / 4849.8) | 1.58 (0.9, 2.76) | 0.11 | 1.51 (0.86, 2.64) | 0.1485 |
| Highly active antiretroviral therapy (time-varying) | ||||||
| No | 0.81 | (8 / 982.8) | 1.00 | 1.00 | ||
| Yes | 0.41 | (48 / 11743.6) | 0.5 (0.22, 1.1) | 0.082 | 0.54 (0.26, 1.11) | 0.093 |
| Ocular Characteristics | ||||||
| History of cataract in contralateral eye | ||||||
| No | 0.27 | 34 / 12570.3 | 1.00 | 1.00 | ||
| Yes | 14.1 | 22 / 156 | 21.16 (10.05, 44.53) | < 0.0001 | 21.58 (10.39, 44.81) | < 0.0001 |
| Anterior inflammation (time-varying)† | ||||||
| No | 0.4 | 50 / 12570.3 | 1.00 | 1.00 | ||
| Yes | 3.85 | 6 / 156 | 6 (1.67, 21.52) | 0.0060 | 4.40 (1.64, 11.78) | 0.0032 |
| Vitreous inflammation (time-varying)‡ | ||||||
| No | 0.41 | 52 / 12661.8 | 1.00 | 1.00 | ||
| Yes | 6.2 | 4 / 64.5 | 11.31 (3.6, 35.52) | < 0.0001 | 7.12 (2.02, 25.02) | 0.0022 |
| History of retinal detachment§ | ||||||
| No | 0.39 | 48 / 12387.4 | 1.00 | 1.00 | ||
| Yes | 2.36 | 8 / 338.9 | 5.30 (2.51, 11.16) | < 0.0001 | 4.94 (2.21, 11.02) | < 0.0001 |
Additional variables that were not associated with increased incidence of cataract included: gender, race, education, anemia (time-varying), diabetes, hypertension, hyperlipidemia, current CD4+ T-cells, current human immunodeficiency virus load, current use of highly active antiretroviral therapy, and hepatitis C. Nadir CD4+ T-cell count had no crude association with incident cataract, but was associated with incident cataract after adjustment for the factors above. Several variables were associated significantly with incident cataract in the crude analysis, but were omitted from the adjusted analysis because they were confounded by 1 or more of the variables in the final model and were not associated with incident cataract after adjustment. These include: Karnofsky score and hepatitis B.
Anterior chamber cells or flare, diagnosis with anterior uveitis or keratitis, presence of posterior synechiae, or a combination thereof.
Vitreous haze, anterior vitreous cells, intermediate uveitis, endophthalmitis.
Retinal detachment includes individuals with a documented history of retinal detachment and/or the presence of silicone oil.
Table 6.
Assessment of Risk Factors for Incident Cataract in Eyes without an Opportunistic Ocular Infection (participants in the Longitudinal Study of Ocular Complications of AIDS)
| Characteristics* | Rate per 100 Eye-years |
Count of Cataracts per Eye-years at Risk |
Ratio (95% Confi- | Unadjusted P-value |
Adjusted Hazard Ratio (95% CI) |
Adjusted P-value |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Gender | ||||||
| Female | 0.49 | (12 / 2438.4) | 1.00 | 1.00 | ||
| Male | 0.43 | (44 / 10288) | 0.86 (0.45, 1.65) | 0.6538 | 0.98 (0.5, 1.92) | 0.9535 |
| Race/Ethnicity | ||||||
| Not black | 0.44 | (37 / 8366) | 1.00 | 1.00 | ||
| Black | 0.44 | (19 / 4360.3) | 1.43 (0.78, 2.61) | 0.2468 | 1.20 (0.71, 2.05) | 0.4898 |
| 4.15 | (1 / 24.1) | |||||
| Education (at enrollment) | ||||||
| High school or less | 0.45 | (21 / 4646.8) | 1.00 | 1.00 | ||
| Some college | 0.25 | (10 / 3926.3) | 0.54 (0.25, 1.14) | 0.1025 | 0.72 (0.34, 1.52) | 0.3891 |
| College graduate | 0.58 | (24 / 4129.1) | 0.82 (0.43, 1.53) | 0.5269 | 1.03 (0.57, 1.84) | 0.9300 |
| Associated morbidities | ||||||
| Karnofsky score (at enrollment) | ||||||
| 90–100 | 0.36 | (26 / 7285.9) | 1.00 | 1.00 | ||
| <80 | 0.55 | (30 / 5440.4) | 1.73 (1.01, 2.96) | 0.0457 | 1.43 (0.85, 2.39) | 0.1740 |
| 0 | (0 / 3.8) | |||||
| Anemia (time-varying) | ||||||
| No | 0.43 | (37 / 8645.3) | 1.00 | 1.00 | ||
| Yes | 0.47 | (19 / 4077.3) | 1.37 (0.77, 2.42) | 0.2819 | 1.35 (0.82, 2.21) | 0.2378 |
| Diabetes (at enrollment) | ||||||
| No | 0.44 | (51 / 11664.1) | 1.00 | 1.00 | ||
| Yes | 0.47 | (5 / 1062.2) | 0.89 (0.35, 2.21) | 0.7940 | 0.91 (0.35, 2.35) | 0.8484 |
| Hypertension (at enrollment) | ||||||
| No | 0.37 | (39 / 10408.6) | 1.00 | 1.00 | ||
| Yes | 0.73 | (17 / 2317.7) | 1.36 (0.77, 2.39) | 0.2815 | 1.41 (0.77, 2.57) | 0.2604 |
| 0 | (0 / 10.3) | |||||
| Hyperlipidemia (at enrollment) | ||||||
| No | 0.41 | (40 / 9757.9) | 1.00 | 1.00 | ||
| Yes | 0.54 | (16 / 2958.1) | 1.12 (0.61, 2.04) | 0.7202 | 1.16 (0.6, 2.22) | 0.6494 |
| Human Immunodeficiency Virus (HIV)/Acquired Immune Deficiency Syndrome (AIDS) History | ||||||
| CD4+ T cells (time-varying) | ||||||
| ≥50 cells/uL | 0.43 | (50 / 11633) | 1.00 | 1.00 | ||
| < 50 cells/uL | 0.55 | (6 / 1091.3) | 2.16 (0.88, 5.25) | 0.0896 | 1.64 (0.77, 3.45) | 0.1925 |
| 0 | (0 / 135.7) | |||||
| Nadir CD4+ T cells (at enrollment) | ||||||
| ≥50 cells/uL | 0.64 | (39 / 6132.4) | 1.00 | 1.00 | ||
| < 50 cells/uL | 0.26 | (17 / 6458.2) | 0.62 (0.33, 1.16) | 0.1312 | 0.55 (0.31, 0.95) | 0.0307 |
| 0 | (0 / 111.6) | |||||
| HIV viral load (time-varying) [Log10(copies/mL)] | ||||||
| < 2.6 (undetectable) | 0.45 | (35 / 7764.9) | 1.00 | 1.00 | ||
| 2.6 or higher | 0.43 | (21 / 4849.8) | 1.58 (0.9, 2.76) | 0.1050 | 1.51 (0.86, 2.64) | 0.1485 |
| Highly active antiretroviral therapy (time-varying) | ||||||
| No | 0.81 | (8 / 982.8) | 1.00 | 1.00 | ||
| Yes | 0.41 | (48 / 11743.6) | 0.5 (0.22, 1.1) | 0.0819 | 0.54 (0.26, 1.11) | 0.0930 |
| Co-infections | ||||||
| Hepatitis B (at enrollment) | ||||||
| No | 0.42 | (51 / 12206.9) | 1.00 | 1.00 | ||
| Yes | 0.97 | (5 / 516.3) | 2.7 (1.04, 7.01) | 0.0406 | 2.36 (0.93, 5.98) | 0.0705 |
| 0 | (0 / 3.1) | |||||
| Hepatitis C (at enrollment) | ||||||
| No | 0.45 | (55 / 12171.1) | 1.00 | 1.00 | ||
| Yes | 0.18 | (1 / 552.1) | 0.45 (0.06, 3.28) | 0.4306 | 0.56 (0.08, 3.95) | 0.5640 |
| Ocular characteristics | ||||||
| History of cataract in contralateral eye | ||||||
| No | 0.27 | (34 / 12570.3) | 1.00 | 1.00 | ||
| Yes | 14.1 | (22 / 156) | 21.16 (10.05, 44.53) | < 0.0001 | 21.58 (10.39, 44.81) | < 0.0001 |
| Anterior inflammation (time-varying)† | ||||||
| No | 0.4 | (50 / 12570.3) | 1.00 | 1.00 | ||
| Yes | 3.85 | (6 / 156) | 6 (1.67, 21.52) | 0.0060 | 4.40 (1.64, 11.78) | 0.0032 |
| Vitreous inflammation (time-varying)‡ | ||||||
| No | 0.41 | (52 / 12661.8) | 1.00 | 1.00 | ||
| Yes | 6.2 | (4 / 64.5) | 11.31 (3.6, 35.52) | < 0.0001 | 7.12 (2.02, 25.02) | 0.0022 |
| History of retinal detachment§ | ||||||
| No | 0.39 | (48 / 12387.4) | 1.00 | 1.00 | ||
| Yes | 2.36 | (8 / 338.9) | 5.30 (2.51, 11.16) | < 0.0001 | 4.94 (2.21, 11.02) | < 0.0001 |
Anterior chamber cells or flare, diagnosis with anterior uveitis or keratitis, presence of posterior synechiae, or a combination thereof.
Vitreous haze, anterior vitreous cells, intermediate uveitis, endophthalmitis.
Retinal detachment includes individuals with a documented history of retinal detachment and/or the presence of silicone oil.
In the two sensitivity analyses, results were similar, with a few exceptions. For cataract definition 2, vitreous inflammation was not significant in the adjusted model due to confounding with cataract in the fellow eye (aHR= 0.572.6412.2). For cataract definition 3, neither anterior inflammation (aHR= 0.802.8810.3) nor a history of retinal detachment (aHR= 0.891.954.25) was significant in the adjusted model due to confounding with vitreous inflammation. Hepatitis B infection, which was associated with a non-significant increase in the main model (aHR= 0.932.365.98), passed the threshold of significance with a similar aHR in the two sensitivity analyses. Detectable HIV in peripheral blood was associated with a significantly increased risk under cataract definition 3 (aHR= 1.091.913.33) but not in the main model or the alternative sensitivity analysis.
The incidence of cataract surgery was greater in the LSOCA cohort than the LALES cohort after adjustment for age (aOR=1.0061.843.36, , p = 0.048), the differences being most apparent in the older age groups.
Discussion
In this large cohort of subjects with AIDS who were free of ocular opportunistic infections, age and ocular diseases were the primary drivers of cataract risk. The relationship between cataract risk and age was qualitatively similar to that observed in samples of the general population. However, there appeared to be an increase in cataract prevalence when compared in an age-matched fashion to the HIV-uninfected populations, with higher risk than observed in the LALES cohort, and earlier onset than observed in the Proyecto VER cohort. Also, the age-adjusted risk of cataract surgery was higher in the AIDS subjects compared to a general population sample. While the latter observation could be argued to reflect the more frequent contact between subjects and ophthalmologists38 mandated in the LSOCA protocol than the in LALES protocol, a similar result was observed in a Danish nationwide hospital registry study, suggesting the result is real.39 While markers of severity of HIV/AIDS were not statistically significantly associated with cataract risk, association may have been mitigated by competing risk of death in patients with unfavorable markers, and incomplete information regarding patients’ entire clinical course. Better current CD4+ T cell count, HIV load and HAART status all tended to be associated with lower risk of cataract, though not to a statistically significant degree. Lower nadir CD4+ T cell count tended to be associated with lower risk of cataract, which also might reflect a survivor bias, whereby otherwise more healthy patients with the same low CD4+ T cell count nadir may have survived to be enrolled and likely would have had lower cataract risk. Thus, the results suggest an overall higher risk of cataract in the setting of AIDS, absent opportunistic ocular complication, otherwise with a pattern of risk factors otherwise similar to that of the general population. This result could be taken as supporting the concept that HIV/AIDS results in faster aging.
Our findings of increased cataract risk and ocular inflammation or prior retinal detachment reflect well-known associations. Many techniques for repair of retinal detachment (e.g., vitrectomy and the injection of vitreous substitutes—particularly silicone oil40) increase the risk of cataract, and both ocular inflammation itself and treatment with corticosteroids have been known to contribute to a higher incidence of cataract .41;42 These associations are not necessarily unique to HIV/AIDS, except that HIV/AIDS, its associated conditions, and the management of these may increase the risk of ocular inflammation43 and/or retinal detachment.44 Likewise, genetic and environmental risk factors for cataract are well-known. The strong concordance in cataract risk between eyes of the same patient observed here also may reflect these factors.
In these subjects without ocular opportunistic infections, most indicators of HIV disease status did not predict cataract, including current and previous absolute CD4+ T cell count and HIV load in peripheral blood. The exception was that detectable HIV in peripheral blood was associated with a significantly lower risk of cataract in the prevalence, but not the incidence, analysis, in which the risk pattern tended in the opposite direction. This inconsistency might be explained because viremic subjects may have been more likely to be referred to the participating ophthalmology centers for CMV retinitis screening, whereas others may have been less likely referred unless they had visual complaints (in some cases due to cataract), which may have led to differential enrollment that balanced out over follow-up time (affecting prevalence more than incidence). Use of HAART tended to be associated with a lower risk of cataract but not to a statistically significant level with the available power; a 1/2- to 1/3-fold difference in risk associated with HAART could not be excluded with the available information. Conditions such as diabetes and hyperlipidemia—the risks and severities of which may be influenced by some HIV-related treatments—were not associated with large differences in the risk of cataract in this analysis. However, diabetes is a well-established risk factor for cataract,45;46 which could be relevant in the setting of HIV/AIDS given the higher risk of diabetes that has been associated with at least some forms of antiretroviral therapy and with AIDS.47
Limitations of these analyses include the possibility that the risk of cataract in the LSOCA cohort could be overestimated because subjects were recruited when they presented for eye care, a limitation that would have affected prevalence more than incidence. Likewise, because several participating clinics are centers also focusing on the care of uveitis, ocular inflammation may be over-represented in the cohort, although some have proposed a higher incidence of uveitis in cases of HIV/AIDS.43 While incidence is a methodologically superior measurement than prevalence for evaluating possibly associated risk factors, the moderate duration of follow-up for a long-term outcome like cataract provides limitations on the power of our incidence study to detect associations, even though the scale of this study was large. Thus, additional data would be needed to identify factors associated with mild to moderately increased risk of cataract. The lack of an internal non-AIDS comparison group also is a limitation, which we attempted to overcome here by comparing results to well-established population-based samples that had used similar methods of cataract ascertainment and documentation. Hispanic population studies were the only US studies available that had used similar cataract definitions; whether the risk of cataract differs among Hispanics from other racial/ethnic groups is uncertain but possible.48 However, the risk of cataract within the LSOCA cohort did not differ by racial/ethnic status. Standardization of cataract grading across sites was not feasible, which is a limitation, but the outcome observed was supported across two sensitivity analyses.
Strengths of the study include: 1) considerably greater study power to address the question of cataract risk with AIDS than previously was available; 2) reliable information regarding diagnosis of AIDS and its associated clinical status and complications; and 3) the estimation of prevalence and incidence under a common protocol that was enforced and monitored rigorously across all participating centers. The LSOCA cohort also is similar in its distribution of demographic characteristics to the general population of individuals with AIDS in the United States.
In summary, our data suggest that the risk of cataract among subjects with AIDS free of intraocular opportunistic infection may be higher than that observed in the general population, consistent with the hypothesis of accelerated aging in the setting of AIDS. Among our patients with AIDS, immunologic and virologic factors were not clearly associated with altered risk of cataract, although a modest protective effect of HAART could not be excluded. Cataract risk was affected by age and ocular disease (retinal detachment and inflammation). Given the greatly increased survival of persons with AIDS in an era of frequently effective antiretroviral therapy, the burden of cataract among these individuals likely will increase substantially over time. Additional research, especially with a longer period of surveillance, would strengthen our understanding regarding whether modest but potentially important increases in the risk of cataract exist among patients with AIDS free of ocular opportunistic complications.
Supplementary Material
Acknowledgments
Grant Support: Supported by cooperative agreements from the National Eye Institute to the Icahn School of Medicine at Mount Sinai (U10 EY 08052), The Johns Hopkins University Bloomberg School of Public Health (U10 EY 08057), and the University of Wisconsin, Madison School of Medicine (U10 EY 08067). Additional support provided by National Center for Research Resources through General Clinical Research Center grants to (5M01 RR 00350) Baylor College of Medicine, (5M01 RR 00052) Johns Hopkins University School of Medicine, (5M01 RR 05096) LSU/Tulane/Charity Hospital, (5M01 RR 00865) University of California, Los Angeles, (5M01 RR00046) University of North Carolina, (5M01 RR00043) University of Southern California, (5M01 RR00047) Weill Medical College of Cornell University. Support also provided through cooperative agreements (U01 AI 27674) Louisiana State University/Tulane, (U01 AI 27660) University of California, Los Angeles, (U01 AI 27670) University of California, San Diego, (U01 AI 27663 University of California, San Francisco, (U01 AI25868) University of North Carolina, (U01 AI25903) Washington University at St. Louis, (U01 AI32783) University of Pennsylvania.
Additional support was provided by the Paul and Evanina Mackall Foundation, Research to Prevent Blindness (Drs. Kempen, Jabs and Lewis), and NEI grant EY004505 (Dr. Jabs).
The Los Angeles Latino Eye Study is supported by a cooperative agreement between the National Eye Institute and the University of Southern California (U10-EY-11753). Dr. Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
Longitudinal Study of Ocular Complications of AIDS Clinical Centers - Credit Roster (as of November 21, 2013)
Key Personnel (LSOCA certified) 1997 – 2013
Baylor College of Medicine, Cullen Eye Institute, Houston, TX: Richard Alan Lewis, MD, MS (Director); Robert E. Coffee III, MD; Valerie Gudell, DMA; Joseph F. Morales, CRA; Silvia Orengo-Nania, MD; Steven S. Spencer, BA, COMT, CCRP; Mitchell P. Weikert, MD. Former Members: Richard C. Allen, MD; John Michael Bourg; Victor Fainstein, MD; Pamela Frady, COMT; Ronald Gross, MD; Zbigniew Krason, CRA; Tobias C. Samo, MD; Allison Schmidt, CRA; Laura Shawver, COT/CCRP; James Shigley, CRA (deceased); Benita Slight, COT; Rachel Sotuyo, COT; Kay R. Stephenson, COT, BA, CCRP; Stephen Travers, CRA.
Emory University Eye Center, Atlanta, GA: Steven Yeh, MD (Director); Deborah Gibbs, COMT, CCRC, CCRP; Debora Jordan, CRA; Janna Rutter, CRA. Former Members: Antonio Capone, Jr. MD; David Furukuwa, PA; Allison Gibbs, BS; Baker Hubbard, MD; Steven Kim, MD; Daniel F. Martin, MD; Bob Myles, CRA; Bryan Schwent, MD; Sunil K. Srivastava, MD.
Johns Hopkins University School of Medicine, Baltimore, MD: J.P. Dunn, MD (Director); Kristen Brotherson, BS; Bryn Burkholder, MD; Nicholas Butler, MD; Dennis Cain; David Emmert; Theresa Gan Leung, MD; Charles Mark Herring; Ahmadreza Moradi, MD; Antonia Nwankwo-Marshall; Jennifer E. Thorne, MD, PhD. Former Members: Ellen Arnold, BS; Patricia Barditch-Crovo, MD; Patricia Barnabie, BS; Marie-Lyne Bélair, MD; Stephen G. Bolton, CRNP; Joseph B. Brodine; Diane M. Brown, RN; Lisa M. Brune, RN, BSN; Anat Galor, MD; Douglas A. Jabs, MD, MBA; Adam Jacobowitz, MD; Meera Kapoor; Sanjay R. Kedhar, MD; John H. Kempen, MD, PhD; Stephen J. Kim, MD; Henry A. Leder, MD; Alison G. Livingston, RN, BSN; Yavette Morton; Kisten D. Nolan, RN, BSN, MPH; Armando L. Oliver, MD; George B. Peters, III, MD; Richard D. Semba, MD, MPH; Priscilla Soto; Ricardo Stevenson, MD; Michelle Tarver-Carr, MD, PhD; Lynnet Tirabassi, RN, BSN; Susan Wittenberg, MD; Michelle Yue Wang, MD.
Louisiana State University Health Sciences Center, New Orleans, LA: Donald Bergsma, MD (Director); Rebecca Clark, MD; Robin Cooper, COMT; Christine Jarrott, RN, ACRN; P. Sean O’Sullivan, MD; Maria Reinoso, MD Christine Romero, COT, ROUB. Former Members: Bruce Barron, MD; Robin Bye, RN; Mandi Conway, MD; Larry Dillon, COT/CRA; Jasmine Elison, MD; Butler Fuller, MD; Audrey Lombard, RN; Lynn Otillio, COT; Gholman Peyman, MD.
Memorial Sloan Kettering Center, New York, NY: Murk-Hein Heinemann, MD (Director); Susana Coleman; Sara Daniel; Roberta Janis, RN, BSN; Andrzej Kozbial; Kent Sepkowitz, MD. Former Members: Kenneth Boyd; Robinson V.P. Chan, MD; Cynthia Chiu, MD; Minhee Cho, MD; Charles Cole, MD; Charles Doering, MD; Jasmine Elison, MD; Aziz Khanifar, MD; Fang Lu; Joseph Murphy; Sophia Pachydaki, MD; Christiana Peroni, MD; Firas M. Rahhal, MD; Ashok Reddy, MD.
New York University Medical Center, New York, NY: Dorothy N. Friedberg, MD, PhD (Director); Adrienne Addessi, MA, RN; Douglas Dieterich, MD; Monica Lorenzo-Latkany, MD; Maria Pei, COA. Former Member: Alex McMeeking, MD.
Northwestern University, Chicago, IL: Alice T. Lyon, MD (Director); Lori Ackatz, RN, MPH; Manjot Gill, MD; Lori Kaminski, RN, MS; Rukshana Mirza, MD; Robert Murphy, MD; Frank Palella, MD; Carmen Ramirez; Zuzanna Rozenbajgier, MA; Dawn Ryan, CRA; Evica Simjanoski, BFA, CRA; Former Members: Alexander Habib; Jill Koecher; Jeevan Mathura, MD; Annmarie Muñana, RN; Jonathan Shankle; David V. Weinberg, MD; James Yuhr.
University of California, Los Angeles, CA: Gary N. Holland, MD (Director); Robert D. Almanzor, COA; Margrit E. Carlson, MD; Jose T. Castellanos, COT; Serina Gonzales; Ann K. Johiro, MN, RN,BC, FNP-C, AACRN, AAHIVS; Susan S. Ransome, MD. Former Members: Suzette A. Chafey, RN, NP; Alexander C. Charonis, MD; Jeffrey A. Craddock, COT; Partho S. Kalyani, MD; Michael A. Kapamajian, MD; Peter J. Kappel, MD; David L. LeBeck (deceased); Kristin M. Lipka; Ardis A. Moe, MD; Germán Piñón; Angela Sanderson; Kayur H. Shah, MD; Robert Stalling, COA; Dennis Thayer, CRA; Jean D. Vaudaux, MD.
University of California, San Diego, CA: Cheryl A. Arcinue, MD (Director); Payam Amini, MD; Janne Chuang; Isaac Ezon, MD; William R. Freeman, MD; Leonard Holmes; Azadeh Khatibi, MD; Veronica Mendoza. Former Members: Sunan Chaidhawanqual, MD; Lingyun Cheng, MD; Tom Clark; Mark Cleveland; Denise Cochran; Randall L. Gannon; Claudio Garcia, MD; Daniel Goldberg, MD; Joshua Hedaya, MD; Marietta Karavellas, MD; Tiara Kemper; Brian Kosobucki; Igor Kozak, MD (Director); Megan Loughran; Luzandra Magana; Alona Mask; Victoria Morrison, MD; Vivian Nguyen; Stephen Oster, MD; Nicole Reagan MD; Mi-Kyoung Song, MD; Francesca Torriani, MD; Dorothy Wong; Karen Yesensky; Tekeena Young.
University of California, San Francisco, CA: Jacque Duncan, MD (Director); Robert Bhisitkul, MD, PhD; David Clay; Michael Deiner; Donald Eubank; Mark Jacobson, MD; Mary Lew, COT; Todd Margolis, MD, PhD; Arshia Mian. Former Members: Judith Aberg, MD; Fermin Ballesteros, Jr.; Debra Brown; Jacqueline Hoffman; Alexander Irvine, MD; James Larson; Jody Lawrence, MD; Michael Narahara; Monique Trinidad.
University of North Carolina, Chapel Hill, NC: Travis A. Meredith, MD (Director); Sandy Barnhart, MPH; Debra Cantrell; Seema Garg, MD, PhD; Odette Houghton, MD; Megha Karmalkar; Maurice B. Landers, MD; Sarah Moyer; David Wohl, MD. Former Members: Cynthia Aurrichio, OD; Stephanie Betran; Kelly DeBoer; Elizabeth DuBose, MPH; David Eifrig, MD; John Foley, MD; Elizabeth Hartnett, MD; Angela Jeffries; Harpreet Kaur; Jan Kylstra, MD; Barbara Longmire; Sharon Myers; Fatima N’Dure, COA; Kean T. Oh, MD; Jeremy Pantell; Susan Pedersen, RN; Cadmus Rich, MD; Cecilia A. Sotelo, RN; Charles van der Horst, MD; Samir Wadhvania.
University of Pennsylvania Medical Center, Philadelphia, PA: Charles W. Nichols, MD (Director); Mark Bardsley, BSN; John Beaver, RN; Cheryl C. Devine; Jay Kostman, MD; Albert Maguire, MD; William Nyberg. Former Members: Chris Helker, RN; RobRoy MacGregor, MD; Karen McGibney, RN; Keith Mickelberg, RN; Leslie Smith, RN.
University of South Florida, Tampa, FL Peter Reed Pavan, MD (Director); Ken Albritton; Linda Clark; JoAnn Leto, COT; Brian Madow, MD; Lori, Mayor; Richard Oehler, MD; Wyatt Saxon. Former Members: Linda Bergen-Losee; Andrew Burrows, MD; Steve Carlton; Burton Goldstein, MD; Sandra Gompf, MD; Bonnie Hernandez, COT; Mohan Iyer, MD; Patrick Kelty, MD; Amy Kramer, COT; Julie Larkin, MD; Sharon Millard, RN, COT; Jeffrey Nadler, MD; Robert Nelson, MD; Nandesh Patel, MD; Scott E. Paulter, MD; James Powers, MD; Susan Sherouse, COT; Jennifer Tordilla-Wadia, MD; Nancy Walker, COA.
Chairman’s Office, Mount Sinai School of Medicine, New York, NY: Douglas A. Jabs, MD, MBA (Study Chairman); Amanda Allen; Yasmin Hilal, MHS; Karen Pascual, MBA; Jill Slutsky-Sanon, MPA. Former member: Amy Cooperstein, MPH; Melissa Nieves, BA; Judith C. Southall; Maria Stevens, CM.
Coordinating Center, The Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD: Curtis L. Meinert, PhD (Director); Alka Ahuja, MS; Debra A. Amend-Libercci; Karen L. Collins; Betty J. Collison; John Dodge; Michele Donithan, MHS; Lea T. Drye, MS, PhD; Cathleen Ewing; Janet T. Holbrook, MS, MPH, PhD; Milana R. Isaacson, BS; Rosetta M. Jackson; Patrick May, MS; Girlie Reyes; Lee Sensinger, MA; Efe Sezgin, PhD; Jacki Smith, AA; Michael Smith, BS; Elizabeth Sugar, PhD; Jennifer E. Thorne, MD, PhD; James A. Tonascia, PhD; Vijay Vaidya, MS, MPH; Mark L. Van Natta, MHS; Annette Wagoner. Former members: Carley Benham; Laura Coleson-Schreur, RN; Ryan Colvin, MPH; Kathryn Connor, BA; Gregory Foster, MS; Kevin Frick, PhD; Judith Harle; Adele M. Kaplan Gilpin, JD, PhD; John H. Kempen, MD, PhD; Hope Livingston; Barbara K. Martin, PhD; Nancy Min, MPH, PhD; Laurel Murrow, MS; Maria J. Oziemkowska, MS, MPH; Bonnie Piantadosi, MS; Milo Puhan, MD, PhD; Wai Ping Ng, BS; Shoshana Reshef, PhD, MPH; Pamela E. Scott, MA; Erica Smothers; Emily West, PhD; Claudine Woo, MPH; Albert Wu, MD, MPH; Alice Zong.
Fundus Photograph Reading Center, University of Wisconsin, Madison, WI: Ronald Danis, MD (Director); Charles Chandler; Gregory Guilfoil; Jeffrey Joyce; Nancy Robinson; Dennis Thayer; Jeong Won Pak, PhD; Grace Zhang. Former members: Michael Altaweel, MD; Jane Armstrong; Matthew D. Davis, MD; Sapna Gangaputra, MD, MPH; Sheri Glaeser; Larry Hubbard, MAT; Katrina Hughes; Dolores Hurlburt; Linda Kastorff; Michael Neider, BA; Thomas Pauli; Therese Traut; Marilyn Vanderhoof-Young; Hugh Wabers.
National Eye Institute, Bethesda, MD: Natalie Kurinij, PhD; Steven Oversby, PsyD. Former Project Officer: Richard Mowery, PhD.
Officers of the Study: Douglas A. Jabs, MD, MBA (Chair); Ronald Danis, MD; Natalie Kurinij, PhD; Curtis L. Meinert, PhD; Steven Oversby, PsyD; Jennifer E. Thorne, MD, PhD. Former Members: Matthew D. Davis, MD; Janet T. Holbrook, MS, MPH, PhD.
Steering Committee: Douglas A. Jabs, MD, MBA (Chair); Lori Ackatz, RN, MPH; Ronald Danis, MD; Dorothy Friedberg, MD; Gary N. Holland, MD; Milana R. Isaccson, BS; Mark Jacobson, MD; Ann Johiro, MN, RN, BC, FNP-C, AACRN; Natalie Kurinij, PhD; Alice Lyon, MD; Curtis L. Meinert, PhD; Christine Romero, COT; Steven Oversby, PsyD; Jennifer E. Thorne, MD, PhD. Former Members: Adrienne Addessi, MA, RN; Lisa Brune, RN, BSN; Rebecca Clark, MD; Tom Clark, CRA; Janet Davis, MD; Matthew D. Davis, MD; James P. Dunn, MD; William R. Freeman, MD; Dorothy Friedberg, MD; James Gilman; Janet T. Holbrook, MS, MPH, PhD; John Horna; Larry Hubbard, MAT; Mark Jacobson, MD; Richard Lewis, MD, MS; Daniel F. Martin, MD; Travis A. Meredith, MD; Annmarie Muñana, RN; Robert Murphy, MD; Kisten D. Nolan, RN, BSN, MPH; William Nyberg; Frank Palella, MD; P. Reed Pavan, MD; Steven Spencer, BA, COMT; Tim Steffens, CRA; Dennis Thayer; Charles van der Horst, MD; Fran Wallach.
Policy and Data Monitoring Board: John P. Phair, MD (Chair); Brian P. Conway, MD; Ronald Danis, MD; Barry R. Davis, MD, PhD; Douglas A. Jabs, MD, MBA; Natalie Kurinij, PhD; Curtis L. Meinert, PhD; David Musch, PhD; Robert B. Nussenblatt, MD; Steven Oversby, PsyD; Jennifer E. Thorne, MD, PhD; Richard Whitley, MD; Leslie Wolf, JD, MPH. Former Members: Beverly Alston, MD; B. William Brown, Jr., PhD; Matthew D. Davis, MD; James Grizzle, PhD; Argye Hillis, PhD; Janet T. Holbrook, MS, MPH, PhD; Harmon Smith, PhD; James A. Tonascia, PhD.
Visual Function Quality Assurance Committee: Steven Spencer, BA, COMT, CCRP (Chair); Robert D. Almanzor, COT; Deborah Gibbs, COMT; Milana Isaacson, BS; Mary Lew, COT ;Richard Alan Lewis, MD, MS (Advisor);. Former Members: Ferman Ballesteros; Jeff Grijalva, COT; Karen Lopez; Laura G. Neisser, COT; Rosa Paez-Boham, COST.
The Los Angeles Latino Eye Study Group (LALES II):
University of Southern California, Los Angeles, CA: Rohit Varma, MD, MPH (Principal Investigator); Stanley P. Azen, PhD (Co–Principal Investigator); Mina Torres, MS (Project Director); Jaime Barrera; Farzana Choudhury, MBBS, MPH; Lupe Cisneros, COA; Jessica Chung, MPH, PhD (2008); Elizabeth Corona; Carolina Cuestas, OD; Anne DiLauro, MPH (2005–2007); Jeanne Dzekov (2005–2010); Ana Evans (2004–2007); Athena W.P. Foong(2007–2008); Carlos Lastra, MD; Mei-Ying Lai, MS (2004–2006); George Martinez; Roberta McKean-Cowdin, PhD; Carlos Moya; Sylvia H. Paz, MS (2004–2005); Fernando Pena, MD (2004–2005); Corina Shtir, MS (2008–2009); Ronald E. Smith, MD; LaVina Tetrow (2004–2005); Heather Volk, PhD (2008); Ying Wang, MS (2006–2007); Joanne Wu, MPH (2004–2006).
Battelle Survey Research Center, St. Louis, MO: Lisa John, MSW; Karen Tucker, MA; Natasha Van Leeuwen.
Former clinics
Indiana University, Indianapolis, IN [Active from October 1997 to July 2008]: Former Members: Mitchell Goldman, MD (Director); Janice Brown; Thomas Ciulla, MD; Jean Craft, RN, CS; Ronald Danis, MD; Paul Fry; Hua Gao,MD; Samir Gupta, MD; Janet Hernandez, RN; Debra Poe; Linda Pratt, RN; James D. Richardson, MD; Tim Steffens, CRA; L. Joseph Wheat, MD; Beth Zwickl, RN, CS, MSN.
New Jersey Medical School, Newark, NJ [Active from April 1995 to January 2009]: Former Members : Ronald Rescigno, MD (Director); Neelakshi Bhagat, MD; Rosa Paez-Boham, COMT; Marta Paez-Quinde.
Rush University, Chicago, IL [Active from June 2001 to January 2009]: Former Members: Mathew W. MacCumber, MD, PhD (Director); Bruce Gaynes, OD, PharmD; Christina Giannoulis; Pamela Hulvey; Harold Kessler, MD; Heena S. Khan; Andrea Kopp; Pauline Merrill, MD; Frank Morini; Nada Smith; Allen Tenorio, MD; Denise Voskuil-Marre; Kisung Woo.
University of California, Irvine [Active from April 1998 to January 2009]: Former Members: Baruch D. Kuppermann, MD, PhD (Director); Bogdan Alexandiescu, MD; Donald N. Forthal, MD; Jeff Grijalva, COT; Faisal Jehan, MD; Karen Lopez; Rosie Magallon, BA; Nader Moinfar, MD; Bret Trump; Melody Vega, COA; Randy Williams.
University of Southern California, Los Angeles, CA [Active from April 1998 to July 2008]: Former Members: Jennifer I. Lim, MD (Director); Rizwan Bhatti, MD; John Canzano, MD; Thomas S. Chang, MD; Alexander Charonis, MD; Lawrence Chong, MD; Robert Equi, MD; Amani Fawzi, MD; Christina Flaxel, MD; Jesus Garcia; Todd Klesert, MD; Francoise Kramer, MD; Lori Levin, MPH; Tracy Nichols, COA, CRA; Christopher Pelzek, MD; Margaret Podilla, BS; Len Richine; Danny Romo, COA; Srinivas Sadda, MD; Richard Scartozzi, MD; Robert See, MD; Kevin Shiramizu, MD; Mark Thomas; A. Frances Walonker, CO, MPH; Alexander Walsh, MD; Ziquiang Wu, MD.
University of Texas Medical Branch, Galveston, TX [Active from July 1997 to January 2009]: Former Members: Garvin Davis, MD (Director); Robert Blem, MD; J. Mike Bourg, BA; Gibran Khurshid, MD; John Horna, BS; Craig Kelso; Vivian Keys; Zbigniew Krason, BS; Helen K. Li, MD; Lan-Chi Nguyen, COMT; Rhonda Nolen, BS, CRC; Michelle Onarato, MD; David Paar, MD; Steven Rivas; Vicky Seitz, COT; Happy Spillar; Sami Uwaydat, MD.
LSOCA Grant Support:
Supported by cooperative agreements from the National Eye Institute to Mount Sinai School of Medicine (U10 EY 08052), The Johns Hopkins University Bloomberg School of Public Health (U10 EY 08057), and the University of Wisconsin, Madison School of Medicine (U10 EY 08067). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Eye Institute or the National Institutes of Health.
Additional support provided by National Center for Research Resources through General Clinical Research Center grants:
5M01 RR 00350 (Baylor College of Medicine)
5M01 RR00039 (Emory University)
5M01 RR 05096 (LSU/Tulane/Charity Hospital)
5M01 RR00096 (New York University Medical Center, New York)
5M01 RR 00865 (University of California, Los Angeles)
5M01 RR00046 (University of North Carolina)
5M01 RR00043 (University of Southern California)
ULI RR024996 (Weill Medical College of Cornell University)
Support also provided through cooperative agreements:
U01 AI 27674 (Louisiana State University/Tulane)
U01 AI 27660 (University of California, Los Angeles)
U01 AI 27670 (University of California, San Diego)
U01 AI 27663 (University of California, San Francisco)
U01 AI25868 (University of North Carolina)
U01 AI32783 (University of Pennsylvania)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Proprietary Interest: No authors have either proprietary or financial interests in the subject matter or materials discussed in the manuscript.
Prior Presentation: A subset of these data reported here was presented at the Fourth Symposium on Ophthalmic Epidemiology, Sarasota, Florida, February, 2007.
References
- 1.Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya'ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 3.Jabs DA. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc. 1995;93:623–683. [PMC free article] [PubMed] [Google Scholar]
- 4.Eye Diseases Prevalence Research Group. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122:487–494. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2004. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. [Accessed June 3, 2004]. pp. 1–46. Available at: http://www.cdc.gov/hiv/pdf/statistics_2004_HIV_Surveillance_Report_vol_16.pdf. [Google Scholar]
- 6.Joint United Nations Programme on HIV/AIDS, World Health Organization. AIDS Epidemic Update: Special Report on HIV Prevention. December, 2005. Geneva, Switzerland: UNAIDS; 2005. [Accessed June 3, 2014]. pp. 1–98. Available at: http://data.unaids.org/publications/irc-pub06/epi_update2005_en.pdf. [Google Scholar]
- 7.Detels R, Tarwater P, Phair JP, et al. Effectiveness of potent antiretroviral therapies on the incidence of opportunistic infections before and after AIDS diagnosis. AIDS. 2001;15:347–355. doi: 10.1097/00002030-200102160-00008. [DOI] [PubMed] [Google Scholar]
- 8.Palella FJ, Jr, Delaney KM, Moorman AC, et al. HIV Outpatient Study Investigators. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 9.Baril L, Jouan M, Agher R, et al. Impact of highly active antiretroviral therapy on onset of Mycobacterium avium complex infection and cytomegalovirus disease in patients with AIDS. AIDS. 2000;14:2593–2596. doi: 10.1097/00002030-200011100-00023. [DOI] [PubMed] [Google Scholar]
- 10.Deayton JR, Wilson P, Sabin CA, et al. Changes in the natural history of cytomegalovirus retinitis following the introduction of highly active antiretroviral therapy. AIDS. 2000;14:1163–1170. doi: 10.1097/00002030-200006160-00013. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson MA, Stanley H, Holtzer C, et al. Natural history and outcome of new AIDS-related cytomegalovirus retinitis diagnosed in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30:231–233. doi: 10.1086/313612. [DOI] [PubMed] [Google Scholar]
- 12.Varani S, Spezzacatena P, Manfredi R, et al. The incidence of cytomegalovirus (CMV) antigenemia and CMV disease is reduced by highly active antiretroviral therapy. Eur J Epidemiol. 2000;16:433–437. doi: 10.1023/a:1007619323939. [DOI] [PubMed] [Google Scholar]
- 13.Yust I, Fox Z, Burke M, et al. EuroSIDA. Retinal and extraocular cytomegalovirus end-organ disease in HIV-infected patients in Europe: a EuroSIDA study, 1994–2001. Eur J Clin Microbiol Infect Dis. 2004;23:550–559. doi: 10.1007/s10096-004-1160-2. [DOI] [PubMed] [Google Scholar]
- 14.Aberg JA. Aging, inflammation, and HIV infection. Top Antivir Med. 2012;20:101–105. [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia R, Ryscavage P, Taiwo B. Accelerated aging and human immunodeficiency virus infection: emerging challenges of growing older in the era of successful antiretroviral therapy. J Neurovirol. 2012;18:247–255. doi: 10.1007/s13365-011-0073-y. [DOI] [PubMed] [Google Scholar]
- 16.Pathai S, Lawn SD, Weiss HA, et al. Increased ocular lens density in HIV-infected individuals with low nadir CD4 counts in South Africa: evidence of accelerated aging. J Acquir Immune Defic Syndr. 2013;63:307–314. doi: 10.1097/QAI.0b013e31828ad759. [DOI] [PubMed] [Google Scholar]
- 17.Kempen JH, Sugar EA, Lyon AT, et al. Studies of Ocular Complications of AIDS Research Group. Risk of cataract in persons with cytomegalovirus retinitis and the acquired immune deficiency syndrome. Ophthalmology. 2012;119:2343–2350. doi: 10.1016/j.ophtha.2012.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorne JE, Jabs DA, Kempen JH, et al. Studies of Ocular Complications of AIDS Research Group. Causes of visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology. 2006;113:1441–1445. doi: 10.1016/j.ophtha.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Jabs DA, Van Natta ML, Kempen JH, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2002;133:48–61. doi: 10.1016/s0002-9394(01)01322-8. [DOI] [PubMed] [Google Scholar]
- 20.Jabs DA, Van Natta ML, Thorne JE, et al. Studies of Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 2 Second eye involvement and retinal detachment. Ophthalmology. 2004;111:2232–2239. doi: 10.1016/j.ophtha.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Jabs DA, Van Natta ML, Thorne JE, et al. Studies of Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 1 Retinitis progression. Ophthalmology. 2004;111:2224–2231. doi: 10.1016/j.ophtha.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Jabs DA, Holbrook JT, Van Natta ML, et al. Studies of Ocular Complications of AIDS Research Group. Risk factors for mortality in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology. 2005;112:771–779. doi: 10.1016/j.ophtha.2004.10.049. [DOI] [PubMed] [Google Scholar]
- 23.Kempen JH, Martin BK, Wu AW, et al. Studies of Ocular Complications of AIDS Research Group. The effect of cytomegalovirus retinitis on the quality of life of patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology. 2003;110:987–995. doi: 10.1016/S0161-6420(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 24.Kempen JH, Min YI, Freeman WR, et al. Studies of Ocular Complications of AIDS Research Group. Risk of immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis. Ophthalmology. 2006;113:684–694. doi: 10.1016/j.ophtha.2005.10.067. [DOI] [PubMed] [Google Scholar]
- 25.Thorne JE, Jabs DA, Kempen JH, et al. Studies of Ocular Complications of AIDS Research Group. Incidence of and risk factors for visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology. 2006;113:1432–1440. doi: 10.1016/j.ophtha.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Semba RD, Martin BK, Kempen JH, et al. The impact of anemia on energy and physical functioning in individuals with AIDS. Arch Intern Med. 2005;165:2229–2236. doi: 10.1001/archinte.165.19.2229. [DOI] [PubMed] [Google Scholar]
- 27.Brown DM, Thorne JE, Foster GL, et al. Factors affecting attrition in a longitudinal study of patients with AIDS. AIDS Care. 2006;18:821–829. doi: 10.1080/09540120500466747. [DOI] [PubMed] [Google Scholar]
- 28.Ferris FL, III, Bailey I. Standardizing the measurement of visual acuity for clinical research studies: guidelines from the Eye Care Technology Forum. Ophthalmology. 1996;103:181–182. doi: 10.1016/s0161-6420(96)30742-2. [DOI] [PubMed] [Google Scholar]
- 29.Klein BE, Klein R, Linton KL. Prevalence of age-related lens opacities in a population The Beaver Dam Eye Study. Ophthalmology. 1992;99:546–552. doi: 10.1016/s0161-6420(92)31934-7. [DOI] [PubMed] [Google Scholar]
- 30.McCarty CA, Nanjan MB, Taylor HR. Operated and unoperated cataract in Australia. Clin Experiment Ophthalmol. 2000;28:77–82. doi: 10.1046/j.1442-9071.2000.00276.x. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell P, Cumming RG, Attebo K, Panchapakesan J. Prevalence of cataract in Australia: the Blue Mountains Eye Study. Ophthalmology. 1997;104:581–588. doi: 10.1016/s0161-6420(97)30266-8. [DOI] [PubMed] [Google Scholar]
- 32.West SK, Munoz B, Schein OD, et al. Racial differences in lens opacities: the Salisbury Eye Evaluation (SEE) Project. Am J Epidemiol. 1998;148:1033–1039. doi: 10.1093/oxfordjournals.aje.a009579. [DOI] [PubMed] [Google Scholar]
- 33.Broman AT, Hafiz G, Munoz B, et al. Cataract and barriers to cataract surgery in a US Hispanic population: Proyecto VER. Arch Ophthalmol. 2005;123:1231–1236. doi: 10.1001/archopht.123.9.1231. [DOI] [PubMed] [Google Scholar]
- 34.Richter GM, Chung J, Azen SP, Varma R Los Angeles Latino Eye Study Group. Prevalence of visually significant cataract and factors associated with unmet need for cataract surgery: Los Angeles Latino Eye Study. Ophthalmology. 2009;116:2327–2335. doi: 10.1016/j.ophtha.2009.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varma R, Paz SH, Azen SP, et al. Los Angeles Latino Eye Study Group. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111:1121–1231. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 36.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 37.Karnofsky DA, Burchenal BJ. The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents: Symposium. New York: Columbia Univ. Press; 1949. pp. 191–205. [Google Scholar]
- 38.Klein BE, Klein R, Moss SE. Incident cataract surgery: the Beaver Dam Eye Study. Ophthalmology. 1997;104:573–580. doi: 10.1016/s0161-6420(97)30267-x. [DOI] [PubMed] [Google Scholar]
- 39.Rasmussen LD, Kessel L, Molander LD, et al. Risk of cataract surgery in HIV-infected individuals: a Danish nationwide population-based cohort study. Clin Infect Dis. 2011;53:1156–1163. doi: 10.1093/cid/cir675. [DOI] [PubMed] [Google Scholar]
- 40.Tanna AP, Kempen JH, Dunn JP, et al. Incidence and management of cataract after retinal detachment repair with silicone oil in immune compromised patients with cytomegalovirus retinitis. Am J Ophthalmol. 2003;136:1009–1015. doi: 10.1016/s0002-9394(03)00724-4. [DOI] [PubMed] [Google Scholar]
- 41.Harper SL, Chorich LJ, III, Foster CS. Diagnosis of uveitis. In: Foster CS, Vitale AT, editors. Diagnosis and Treatment of Uveitis. Philadelphia, PA: Saunders; 2002. p. 92. [Google Scholar]
- 42.Urban RC, Jr, Cotlier E. Corticosteroid-induced cataracts. Surv Ophthalmol. 1986;31:102–110. doi: 10.1016/0039-6257(86)90077-9. [DOI] [PubMed] [Google Scholar]
- 43.Cunningham ET., Jr Uveitis in HIV positive patients. Br J Ophthalmol. 2000;84:233–235. doi: 10.1136/bjo.84.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holland GN. AIDS and ophthalmology: the first quarter century. Am J Ophthalmol. 2008;145:397–408. doi: 10.1016/j.ajo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 45.West SK, Valmadrid CT. Epidemiology of risk factors for age-related cataract. Surv Ophthalmol. 1995;39:323–334. doi: 10.1016/s0039-6257(05)80110-9. [DOI] [PubMed] [Google Scholar]
- 46.Chang JR, Koo E, Agron E, et al. Age-Related Eye Disease Study Group. Risk factors associated with incident cataracts and cataract surgery in the Age-related Eye Disease Study (AREDS): AREDS report number 32. Ophthalmology. 2011;118:2113–2119. doi: 10.1016/j.ophtha.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tebas P. Insulin resistance and diabetes mellitus associated with antiretroviral use in HIV-infected patients: pathogenesis, prevention, and treatment options. J Acquir Immune Defic Syndr. 2008;49(suppl):S86–S92. doi: 10.1097/QAI.0b013e31818651e6. [DOI] [PubMed] [Google Scholar]
- 48.Varma R, Richter GM, Torres M, et al. Los Angeles Latino Eye Study Group. Four-year incidence and progression of lens opacities: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2010;149:728–734. doi: 10.1016/j.ajo.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.