Abstract
Stiffening of large elastic arteries with age increases the risk of cardiovascular diseases (CVD), but the underlying mechanisms are incompletely understood. We investigated the role of mitochondrial quality control (QC, i.e., mitophagy and biogenesis) in arterial stiffening with aging. In C57BL6 mice, aging was associated with impaired aortic expression of mitochondrial QC mediators, greater activation of the mitochondrial redox/stress sensor p66shc, elevated superoxide production and increased arterial stiffness—as indicated by ~20% higher aortic pulse wave velocity (aPWV). In old mice, supplementation with trehalose, a nutraceutical reported to enhance mitophagy, normalized mitochondrial QC markers, p66shc activation and superoxide production, and reduced aPWV and aortic collagen I (a structural protein that confers stiffness). In vitro experiments indicated that mitochondrial QC processes were enhanced in aorta from old trehalose-treated mice, and in aortic rings studied ex vivo, both aging and treatment with the mitochondrial stressor rotenone were associated with increases in p66shc activation and intrinsic mechanical stiffness, whereas co-incubation with trehalose prevented these effects. Taken together, these findings suggest that mitochondrial stress/dysfunction as a result of impaired mitochondrial QC contributes to large elastic artery stiffening with age. Enhancing mitochondrial QC with agents such as trehalose may be a novel strategy for reducing age-associated arterial stiffness and CVD.
Keywords: Collagen, mitophagy, mitochondrial biogenesis
1. Introduction
Advancing age is the primary risk factor for cardiovascular diseases (CVD) [1]. A major cause of age-associated CVD is stiffening of large elastic arteries, including the aorta [2]. Indeed, aortic pulse wave velocity (aPWV), the benchmark clinical measure of large elastic artery stiffness, is a powerful predictor of incident CVD in older adults [3]. Age-related arterial stiffening results from structural changes in the arterial wall including increases in collagen deposition and degradation of elastin [4], but the mechanisms by which these changes develop with aging are poorly understood.
A compelling but uninvestigated hypothesis is that dysregulation of mitochondrial quality control (QC) contributes to arterial stiffening with age. Mitochondrial QC requires a balance between the production of healthy mitochondria (mitochondrial biogenesis) and the degradation of damaged mitochondria (mitophagy). Impaired mitochondrial QC results in mitochondrial dysfunction that plays a central role in aging and disease [5] and could explain certain events associated with structural remodeling in the arterial wall (e.g., adverse redox/stress signaling and increased production of reactive oxygen species, such as superoxide) [6]. However, the role of mitochondrial QC in large elastic artery stiffening with aging is unknown.
We recently reported that the nutraceutical trehalose enhances autophagy, the cellular process of degrading damaged proteins and organelles, in aorta of old mice [7]. Although we did not examine specific targets of trehalose-mediated autophagy, evidence indicates that trehalose may protect against mitochondrial stress/dysfunction by inducing mitophagy [8, 9]. If so, trehalose may have the potential to improve mitochondrial QC and prevent or reverse arterial stiffening with age. Accordingly, the aims of the present study were to determine the associations between aging, markers of mitochondrial QC and arterial stiffness (aPWV), and to evaluate the efficacy of trehalose for enhancing mitochondrial QC and reducing arterial stiffness in a mouse model of arterial aging. A series of in vitro experiments was also conducted to investigate mechanisms underlying these processes.
2. Materials and methods
2.1 Animals
Young (4–5 months) and old (27–28 months; ~50% survival rate) male C57BL6 mice were obtained from the National Institute on Aging rodent colony. Controls received regular drinking water, and treated animals received 2% trehalose (Sigma-Aldrich) supplemented water for 4 weeks. Mice were kept on a 12:12 h light-dark cycle with ad libitum access to water and normal chow. All procedures conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85–23) and were approved by the University of Colorado Boulder Institutional Animal Care and Use Committee.
2.2 Aortic pulse wave velocity and arterial blood pressure
aPWV was measured as previously described [10, 11]. Mice were anesthetized with 2% isoflurane and positioned supine on a heating board with limbs secured to ECG electrodes. Pulse waves were detected at the transverse aortic arch and the abdominal aorta using Doppler probes (MouseDoppler data acquisition system; Indus Instruments). Time elapsed between the ECG R-wave and the foot of the Doppler signal was determined for each site, and aPWV was calculated as: aPWV = (distance between probes)/(Δtimeabdominal - Δtimetransverse). To examine the potential contribution of changes in arterial blood pressure to any treatment-related differences in aPWV, systolic and diastolic blood pressure were assessed using a CODA noninvasive tail-cuff system (Kent Scientific) as previously described [11]. Five acclimation cycles followed by 20 data collection cycles of blood pressure measurements were recorded on three consecutive days and averaged.
2.3 Aortic protein expression and superoxide production
Protein mediators of mitochondrial QC and arterial stiffening were analyzed in excised and cleaned thoracic aortas by standard Western blotting techniques (Criterion System; Bio-Rad) [7, 10]. Primary antibodies: Parkin (1:1000 dilution; Cell Signaling), sirtuin 3 (SIRT3, 1:1000; Abcam), BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 (BNIP3, 1:1000; Novus Biologicals), PPARγ co-activator-1α (PGC-1α, 1:1000; Novus Biologicals), Ser36 phosphorylated p66shc adaptor protein (1:500; Cell Signaling), collagen I (1:1000; Abcam), glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1000; Cell Signaling), voltage dependent anion channel (VDAC, 1:500; Cell Signaling). Proteins were detected using HRP-conjugated secondary antibodies (Jackson ImmunoResearch) and ECL chemiluminescent substrate (Pierce Biotechnology).
Aortic superoxide production was assessed by electron paramagnetic resonance (EPR) spectroscopy using the superoxide-specific spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (Enzo Life Sciences) as previously described [7, 11]. Briefly, freshly isolated 2 mm aortic segments were incubated for 60 min at 37°C in Krebs buffer containing 0.5 mM spin probe, and EPR signal amplitude was analyzed immediately on an MS300 X-band EPR spectrometer (Magnettech).
2.4 Dynamic mitochondrial QC
To estimate flux through mitochondrial QC pathways, changes in the mitochondrial marker VDAC were monitored using a modified Western blot protocol [12]. Equal length segments of thoracic aorta were snap-frozen immediately or after 8 h incubation at 37°C in either nutrient-complete Dulbecco’s Modified Eagle Medium (DMEM) or Hank’s Balanced Salt Solution (HBSS, without glucose or amino acids)—a stimulus for starvation-induced autophagy/mitophagy [13–15]—with or without the mitophagy inhibitor chloroquine (100 µM) [12, 14, 16]. Nutrient deprivation was used as a stimulus in order to avoid off-target effects associated with pharmacological mitophagy inducers. Samples were lysed and analyzed via Western blotting as described above, and the difference between VDAC levels in the presence vs. absence of chloroquine was interpreted as a rough indicator of mitochondrial QC (degradation/synthesis of mitochondria) [12–14, 16].
2.5 Intrinsic mechanical properties
To simulate age-related mitochondrial dysfunction and trehalose treatment, equal length segments of thoracic aorta were incubated in DMEM with or without the mitochondrial stressor rotenone (0.5 µM, Sigma-Aldrich) and/or trehalose (100 mM) for 48 h at 37°C. Half of each segment was used for Western blotting, and the remaining half was divided into two rings ~1 mm in length for mechanical and histological analyses as previously described [10]. Rings were loaded into a pre-heated (37°C) wire myograph chamber (DMT Inc.) in calcium-free phosphate buffered saline, pre-stretched for 3 min to 1 mm luminal diameter displacement and then returned to baseline (zero force). After 3 pre-stretch cycles, diameter was increased until 1 mN force and then increased by 10% every 3 minutes until tissue failure. Force was recorded after each interval, and stress and strain were calculated, with stress defined as: t = (λL)/(2HD) [t = one-dimensional stress; λ = strain; L = one-dimensional load; H = wall thickness, determined by histology; D = vessel length]. Strain was defined as: λ = Δd/d(i) [λ = strain, Δd = change in diameter, d(i) = initial diameter]. Slope of the stress-strain curve was used to determine elastic modulus (intrinsic mechanical stiffness).
2.6 Statistical analyses
Statistical analysis was performed using SPSS 19.0 software. Comparisons between groups/treatment conditions were made by ANOVA with Tukey post-hoc tests as appropriate, and significance was determined as P < 0.05. All results are presented as means ± SEM.
3. Results
3.1 Effects of aging and trehalose on mitochondrial QC
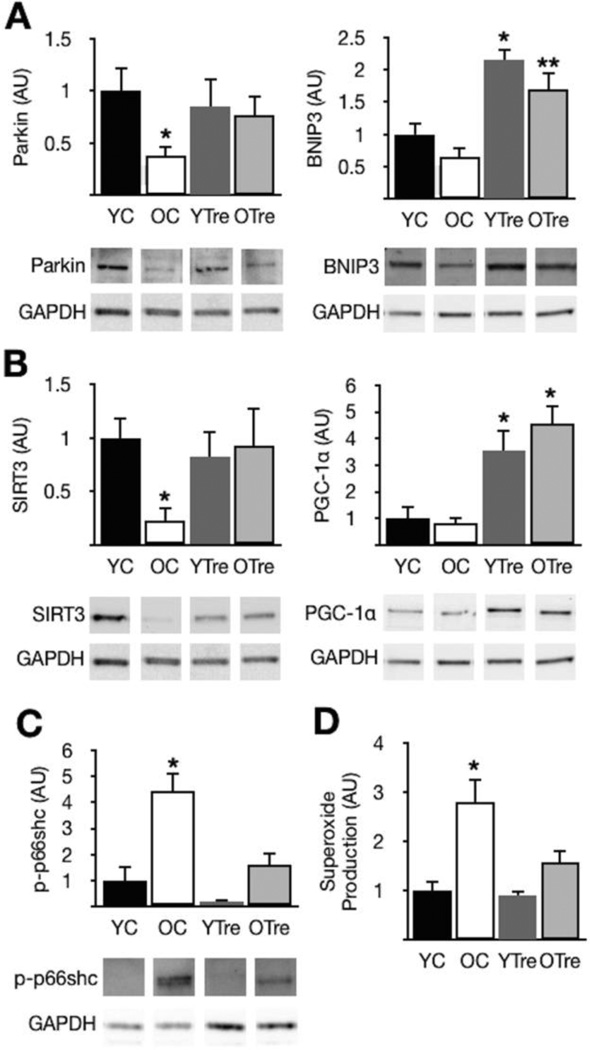
The critical mitophagy mediator Parkin [17] and the key mitochondrial fitness/biogenesis modulator SIRT3 [18] were reduced in aorta of old compared with young control mice (Fig. 1A and B). Impaired expression of these mitochondrial QC mediators was associated with significantly greater activation (phosphorylation) of the mitochondrial redox/stress sensor p66shc (Fig. 1C) [19] and elevated superoxide production (Fig. 1D). In old mice, trehalose supplementation restored Parkin and SIRT3, and also enhanced expression of the mitophagy mediator BNIP3 [20] and the mitochondrial biogenesis regulator PGC-1α [5], while markedly reducing p66shc activation and superoxide production (Fig. 1A, B, C and D).
Figure 1. Impaired expression of mitochondrial QC proteins, increased p66shc activation and elevated superoxide production in aorta of old mice; reversal by trehalose.
(A) Protein expression of the mitophagy mediators Parkin and BNIP3 in aorta of young and old control (YC and OC) and young and old trehalose treated (YTre and OTre) mice. (B) The mitochondrial biogenesis/maintenance proteins SIRT3 and PGC-1α. (C) Activation (phosphorylation at Ser36) of the mitochondrial stress/dysfunction sensor p66shc. (D) Mean EPR signal in aortic rings. Representative Western blot images below. Protein expression presented relative to GAPDH, and all data normalized to YC mean value. Means ± SEM (n = 6–8 per group). *P < 0.05 vs. YC. **P < 0.05 vs. OC.
3.2 Effects of aging and trehalose on arterial stiffness
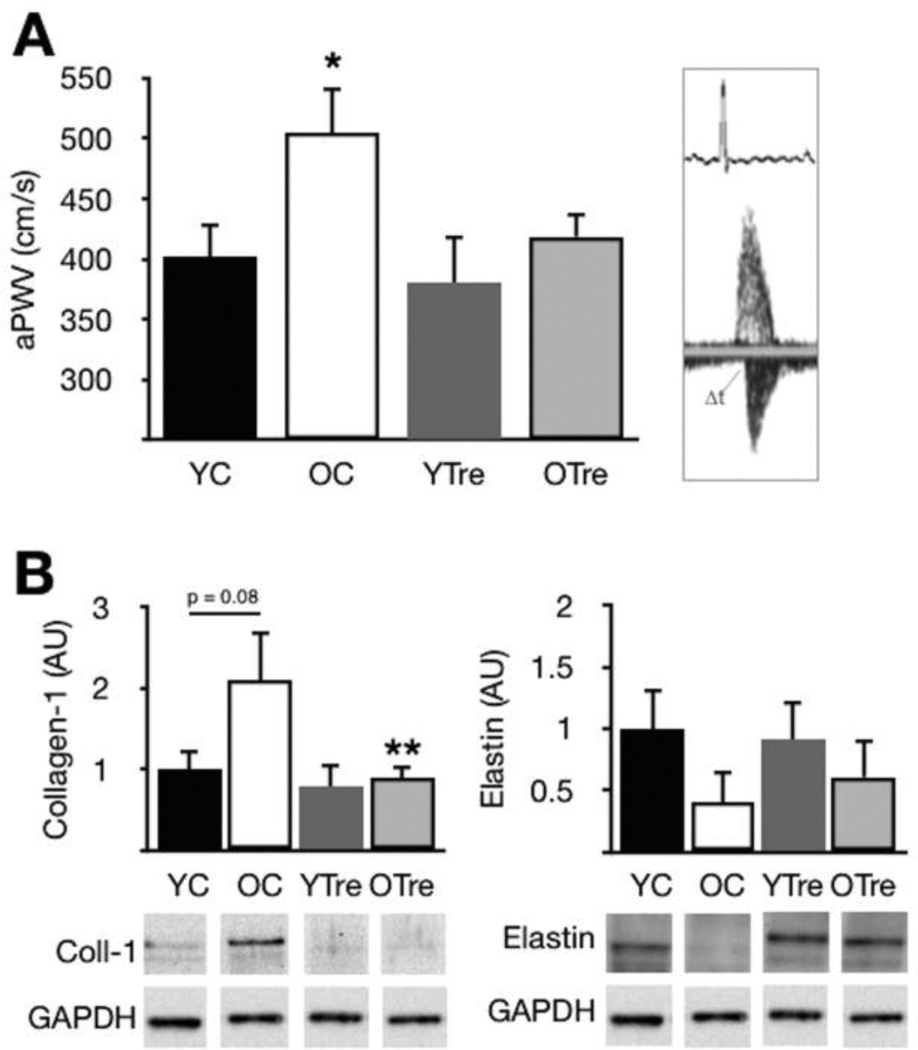
Old mice with reduced expression of mitochondrial QC mediators also had ~20% greater aPWV (Fig. 2A), increased expression of the load-bearing protein collagen I [4] and a modest reduction in elastin compared with young controls (Fig. 2B). Supplementation with trehalose normalized aPWV and aortic collagen I in old mice, but had no effect on young animals and did not influence elastin levels. In a separate cohort of old mice (n = 4), trehalose had no effect on arterial blood pressure (pre-treatment: 91 ± 2 mmHg systolic, 66 ± 2 diastolic vs. post-treatment: 90 ± 2 systolic, 64 ± 3 diastolic).
Figure 2. Increased aortic stiffness and altered structural protein expression in old mice; reversal by trehalose.
(A) Aortic pulse wave velocity (aPWV) in young and old control (YC and OC) and young and old trehalose treated (YTre and OTre) mice. Representative Doppler flows at right. (B) Collagen I and elastin protein expression. Representative Western blot images below. Protein expression data presented relative to GAPDH and normalized to YC mean value. Means ± SEM (n = 6–8 per group). *P < 0.05 vs. YC. **P < 0.05 vs. OC
3.3 Trehalose, mitochondrial QC and intrinsic mechanical properties
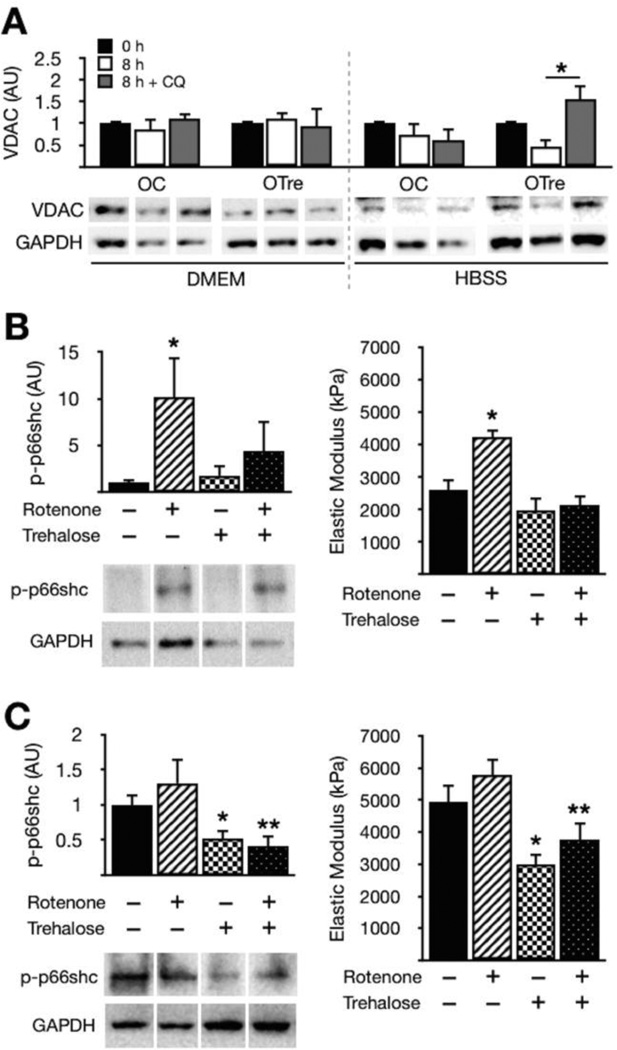
In aortas from old control and old trehalose-treated mice studied ex vivo, incubation in complete medium had no effect on markers of mitochondrial mass, whereas nutrient deprivation, a stimulus for mitophagy/autophagy [12, 15, 20], caused reductions in the mitochondrial marker VDAC (Fig. 3A). However, the mitophagy inhibitor chloroquine prevented this effect in aortas from old trehalose-treated animals, suggesting enhanced mitochondrial QC with trehalose treatment (i.e., greater degradation/synthesis of mitochondria that results in an accumulation of mitochondrial markers when mitophagy is blocked) [12, 14].
Figure 3. Enhanced mitochondrial QC in old trehalose-treated mice, simulation of age-related aortic stiffness by mitochondrial stress and prevention by trehalose.
(A) Protein expression of the mitochondrial marker VDAC in aortas from old control (OC) and old trehalose-treated mice (OTre) incubated in complete medium (DMEM) or starvation medium (HBSS) with/without the mitophagy inhibitor chloroquine (CQ, 100 µM). (B) p66shc activation (phosphorylation) and elastic modulus (intrinsic stiffness) in aortic rings from young mice incubated for 48 h with rotenone (0.5 µM) and/or trehalose (100 mM). (C) p66shc activation and elastic modulus in aortic rings from old mice incubated with rotenone and/or trehalose. Representative Western blot images below. Protein expression data presented relative to GAPDH and normalized to control/baseline condition. Means ± SEM (n = 5–6 per group/condition). *P < 0.05 vs. control; **P < 0.05 vs. rotenone condition.
In aortic rings from young control mice, incubation with the mitochondrial stressor rotenone caused an increase in p66shc activation similar in magnitude to that which we observed in arteries of old mice (Fig. 3B). Rotenone also increased the intrinsic mechanical stiffness of aortic rings (Fig. 3B), but co-incubation with trehalose prevented these effects. In contrast, rotenone had no effects on p66shc or stiffness in aortic rings from old animals (Fig. 3C), whereas trehalose reduced p66shc activation and mechanical stiffness in both untreated and rotenone-treated old aortas (Fig. 3C). These results indicate that: 1) mitochondrial stress has direct “aging-like” effects on aortic stiffness in samples from young, but not old mice; and 2) the beneficial effects of trehalose on aortic stiffness are exerted, at least in part, via protection of mitochondria.
4. Discussion
Mitochondria play a central role in aging and disease, and mitophagy and mitochondrial biogenesis are, therefore, topics of considerable research interest [5, 17, 20]. Coordinated regulation of these processes (mitochondrial QC) may be particularly important in the vasculature, as many pathways governing mitochondrial function and dynamics are also modulators of vascular physiology [6, 21]. However, until now the role of mitochondrial QC pathways in age-associated arterial stiffening was unknown. The novel findings of the present study are that impaired mitochondrial QC contributes to increased large elastic artery stiffness (increased aPWV) in old mice, and that the nutraceutical trehalose enhances mitochondrial QC and reverses age-related aortic stiffness. These findings are clinically relevant, as aPWV is a strong predictor of age-associated CVD [3]. Our results suggest that mitochondrial QC may be a promising therapeutic target for reducing the stiffness of large elastic arteries with aging and preventing age-associated CVD.
4.1 Mitochondrial QC and arterial aging
Dysfunctional mitochondria are implicated in many processes that contribute to aging and disease [5]. As such, mitochondrial QC, including mitophagy mediated by Parkin and BNIP3 and mitochondrial biogenesis/maintenance by PGC-1α and SIRT3, is necessary for survival and proper function in numerous cell types and tissues [17, 18, 20]. Exactly how mitochondrial QC influences physiological functions (e.g., arterial stiffness) remains to be determined, but the p66shc adapter protein—a sensor that couples mitochondrial dysfunction with adverse aging/disease processes such as oxidative stress and inflammation—is one important link between mitochondria and cellular/organismal health [19, 21]. Here, we report for the first time that reduced aortic expression of Parkin and SIRT3 is associated with increased p66shc activation and greater arterial stiffness (aPWV) in old mice, and that trehalose reverses these age-related changes. We also show that trehalose increases the expression of additional mitochondrial QC mediators (BNIP3, PGC-1α), an observation consistent with reports describing coordinated regulation of mitophagy and mitochondrial biogenesis [6]. Although p66shc and mitochondrial dysfunction have previously been implicated in vascular endothelial aging [21] and a recent report connects reduced mitochondrial function with increased aPWV in a transgenic mouse model [22], the present results provide the first evidence for dysregulation of mitochondrial QC per se, an event that may lie upstream of these observations, in aging arteries.
4.2 Mitochondrial QC and arterial stiffening
Alterations in arterial wall structural proteins with aging and disease have been linked with numerous adverse cellular processes including oxidative stress, inflammation, apoptosis and cellular senescence [2, 4]—all of which are also direct consequences of mitochondrial stress/dysfunction. Thus, an age-related decline in mitochondrial QC, such as observed in the present study, could exacerbate these processes and be a key early event in arterial aging. Consistent with this notion, we previously reported that oxidative stress and inflammation (inflammatory cytokine levels) are increased with aging in arteries of mice [7, 11], and that trehalose ameliorates these adverse processes [7]. The present results extend these findings and provide novel support for the idea that impaired mitochondrial QC underlies age-related aortic stiffening by demonstrating that reduced expression of mitochondrial QC mediators in aortas of old mice is associated with increased oxidative stress (superoxide production) and greater aPWV, and that restoration of mitochondrial QC proteins by trehalose reduces superoxide production and aPWV while normalizing aortic collagen I, a key contributor to age-associated arterial stiffness.
4.3 Mitochondrial QC, arterial stiffness and trehalose
Because of the multifunctional role that mitochondria play in cell physiology and signaling, definitively interpreting markers of related processes in vivo can be challenging [12, 20]. Thus, as a first step toward testing the idea that trehalose treatment enhances mitochondrial QC, we confirmed that turnover of mitochondrial proteins (VDAC) is increased in aortas from old trehalose-treated mice studied ex vivo. Then, to check for a mechanistic link between mitochondria, arterial stiffness and trehalose treatment, we simulated age-related mitochondrial dysfunction by treating aortic rings from young and old mice with the mitochondrial stressor rotenone, and we measured resulting changes in protein expression and intrinsic mechanical properties in vitro. Consistent with previous reports in cell culture [23], we found that rotenone induced activation of p66shc. Here, we extend these observations by showing that rotenone induces “aging-like” increases in mechanical stiffness in aortic rings from young mice, and that co-incubation with trehalose prevents these effects. Moreover, we show that although rotenone does not further increase p66shc activation or mechanical stiffness in samples from old mice, trehalose does reduce p66shc activation and stiffness in old aortas in both the absence and presence of rotenone. These observations suggest that aging and rotenone cause aortic stiffening via similar mechanisms (i.e., by promoting mitochondrial stress/dysfunction).
The cellular signaling pathways underlying these effects of mitochondrial stress and trehalose on stiffness remain to be determined. Evidence indicates that dysfunctional mitochondria impact cellular health/function via activation of the NLRP3 inflammasome, a pro-inflammatory signaling platform that is causally involved in stiffness-promoting processes such as collagen synthesis and fibrosis [24]. Thus, stimulation of these pathways by mitochondrial stress/dysfunction either acutely (by rotenone) or chronically (as a result of impaired mitochondrial QC) could underlie the age- and treatment-related differences observed in the present study. These possibilities require further investigation, including a more complete analysis of underlying changes in aortic mitochondrial QC mediators and flux with age (e.g., more comprehensive assays of mitophagy/biogenesis and characterization of the proteins involved). In any case, our results provide initial evidence for a causative relation between mitochondrial stress/dysfunction and arterial stiffness, proof of the ability of trehalose to prevent these events, and support for the idea that the differences in mitochondrial QC and aortic stiffness (aPWV) we observed with aging and trehalose treatment in mice are mechanistically connected.
5. Conclusions
Our findings provide the first evidence for impaired mitochondrial QC with aging in arteries and support for a link between these processes and arterial stiffness. Moreover, we show that reversal of these events by trehalose involves enhancement of mitochondrial QC, protection of mitochondria and normalization of aortic collagen, a key contributor to arterial stiffness. These findings are clinically relevant, as aortic stiffness (aPWV) is a strong predictor of CVD with age. As such, our results suggest that mitochondrial QC may be a promising therapeutic target for reducing large elastic artery stiffness and preventing age-associated CVD, and they provide an experimental basis for future studies of events downstream of mitochondrial QC (e.g., respiration, apoptosis, inflammatory signaling) that may modulate arterial stiffness with age.
Highlights.
We examined mitochondrial quality control (QC) and age-related arterial stiffening.
Reduced mitochondrial QC with age was associated with greater arterial stiffness.
The nutraceutical trehalose enhanced mitochondrial QC and reduced stiffness.
Acknowledgments
This work was supported by the National Institutes of Health: AG013038 and AG039210. The authors especially thank Jason Eng for his technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, et al. Heart disease and stroke statistics--2010 update: a report from the american heart association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a "set up" for vascular disease. Circulation. 2003;107(1):139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell GF, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zieman SJ. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(5):932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 5.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–1159. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzetti E, et al. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol. 2013;305(4):H459–H476. doi: 10.1152/ajpheart.00936.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaRocca TJ, et al. Translational evidence that impaired autophagy contributes to arterial ageing. The Journal of Physiology. 2012;590(14):3305–3316. doi: 10.1113/jphysiol.2012.229690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Navarro JA, et al. Trehalose ameliorates dopaminergic and tau pathology in parkin deleted/tau overexpressing mice through autophagy activation. Neurobiol Dis. 2010;39(3):423–438. doi: 10.1016/j.nbd.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar S, et al. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J Biol Chem. 2007;282(8):5641–5652. doi: 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- 10.Fleenor BS, et al. Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol. 2012;47(8):588–594. doi: 10.1016/j.exger.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleenor BS, et al. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell. 2012;11(2):269–276. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, Dagda RK, Chu CT. Monitoring mitophagy in neuronal cell cultures. Methods Mol Biol. 2011;793:325–339. doi: 10.1007/978-1-61779-328-8_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding WX, Yin XM. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol Chem. 2012;393(7):547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim I, Lemasters JJ. Mitochondrial degradation by autophagy (mitophagy) in GFP-LC3 transgenic hepatocytes during nutrient deprivation. Am J Physiol Cell Physiol. 2011;300(2):C308–C317. doi: 10.1152/ajpcell.00056.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster BR, et al. Restricted mitochondrial protein acetylation initiates mitochondrial autophagy. J Cell Sci. 2013;126(Pt 21):4843–4849. doi: 10.1242/jcs.131300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell EL, Guarente L. The SirT3 divining rod points to oxidative stress. Molecular Cell. 2011;42(5):561–568. doi: 10.1016/j.molcel.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gertz M, Steegborn C. The lifespan-regulator p66Shc in mitochondria: redox enzyme or redox sensor? Antioxidants and Redox Signaling. 2010;13(9):1417–1428. doi: 10.1089/ars.2010.3147. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb RA, Carreira RS. Autophagy in health and disease. 5. Mitophagy as a way of life. Am J Physiol Cell Physiol. 2010;299(2):C203–C210. doi: 10.1152/ajpcell.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai DF, Rabinovitch PS, Ungvari Z. Mitochondria and cardiovascular aging. Circulation Research. 2012;110(8):1109–1124. doi: 10.1161/CIRCRESAHA.111.246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou R-H, et al. Mitochondrial oxidative stress in aortic stiffening with age: the role of smooth muscle cell function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011 doi: 10.1161/ATVBAHA.111.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y, et al. Oxidized low-density lipoprotein activates p66Shc via lectin-like oxidized low-density lipoprotein receptor-1, protein kinase C-beta, and c-Jun Nterminal kinase kinase in human endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31(9):2090–2097. doi: 10.1161/ATVBAHA.111.229260. [DOI] [PubMed] [Google Scholar]
- 24.Artlett CM. The role of the NLRP3 inflammasome in fibrosis. Open Rheumatol J. 2012;6:80–86. doi: 10.2174/1874312901206010080. [DOI] [PMC free article] [PubMed] [Google Scholar]