Abstract
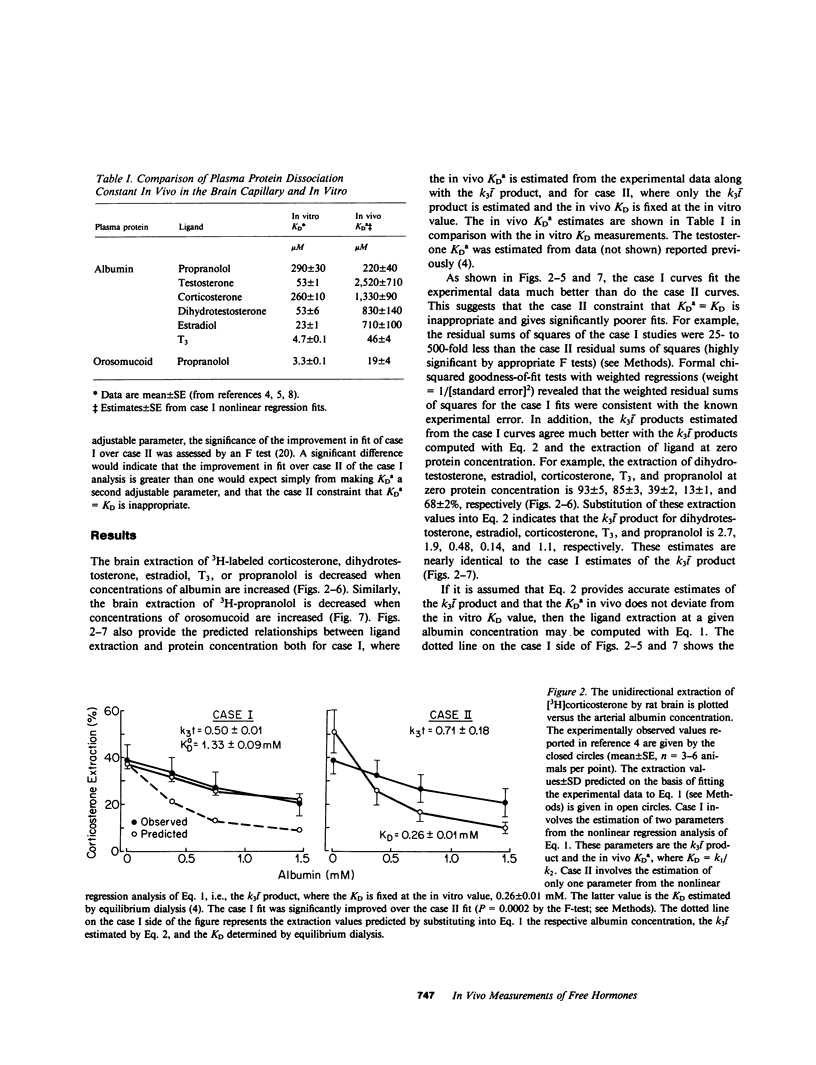
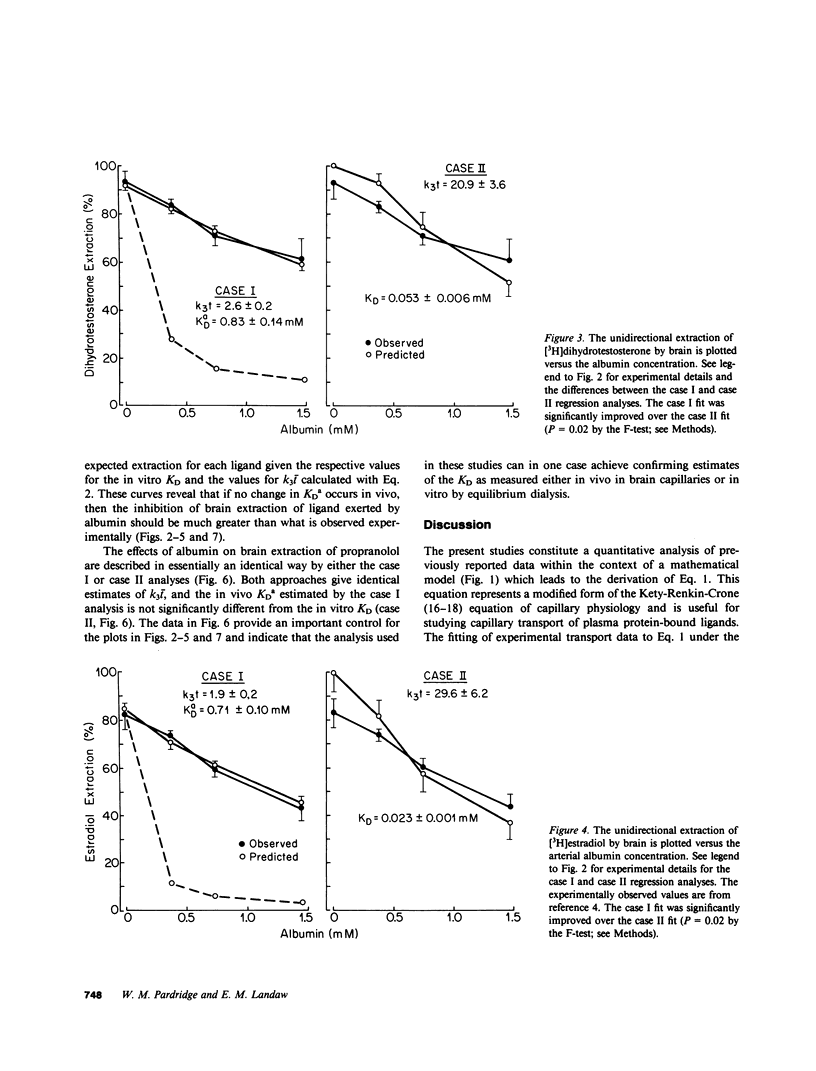
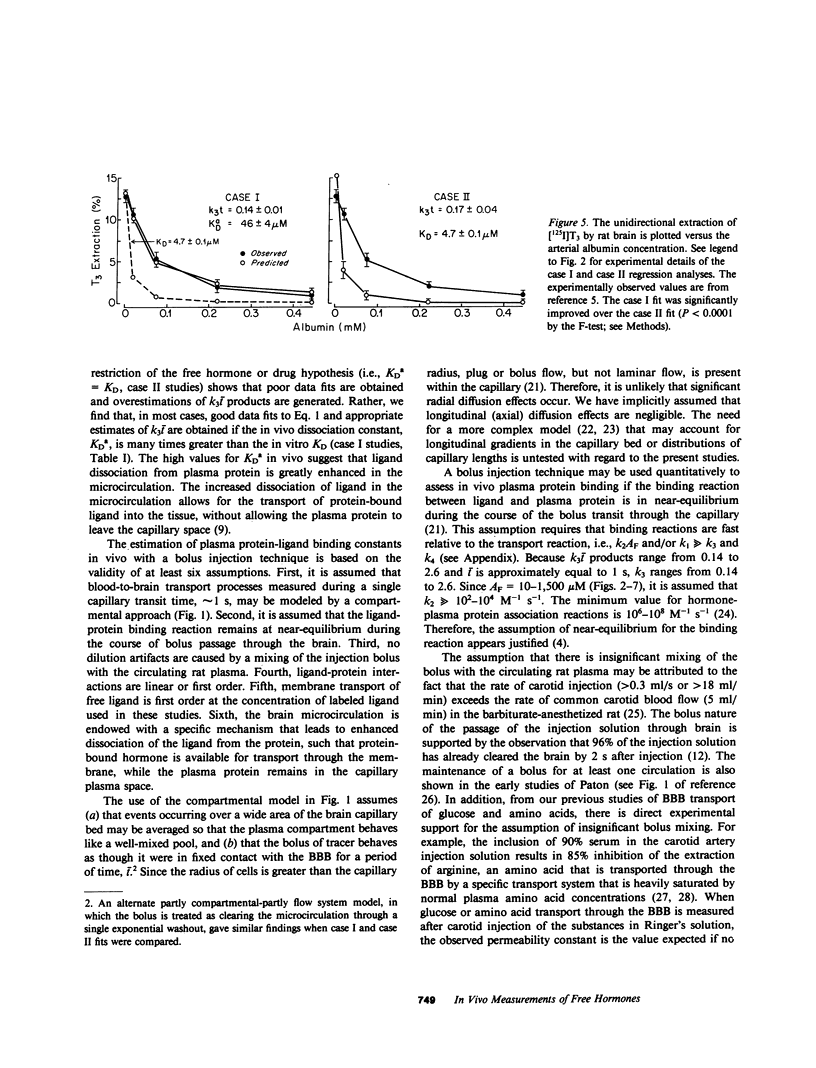
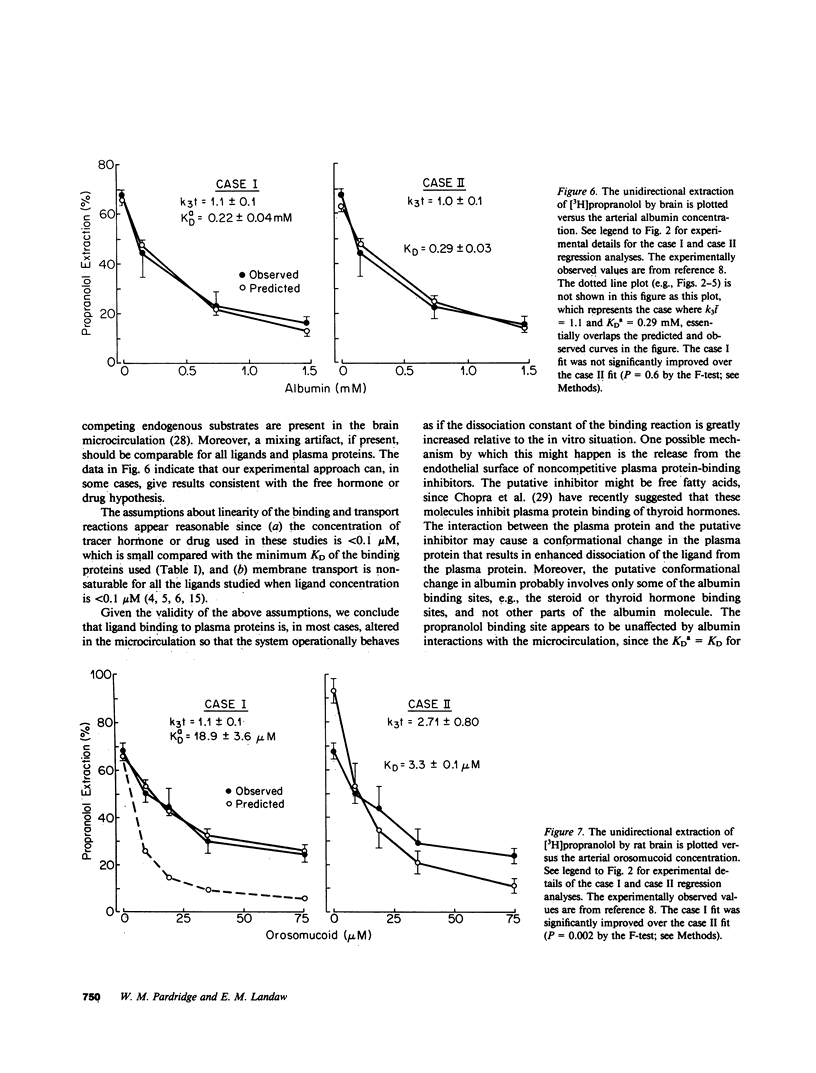
Previous studies have shown that the fraction of hormone or drug that is plasma protein bound is readily available for transport through the brain endothelial wall, i.e., the blood-brain barrier (BBB). To test whether these observations are reconcilable with the free-hormone hypothesis, a tracer-kinetic model is used in the present investigations to analyze in vivo initial extraction data on BBB transport of protein-bound steroid hormones (dihydrotestosterone, testosterone, estradiol, and corticosterone), thyroid hormones (triiodothyronine), and lipophilic amine drugs (propranolol). The plasma proteins used are bovine albumin and human orosomucoid. Transport data was fit to a modification of the Kety-Renkin-Crone equation of capillary physiology; the modified equation incorporates the principles of both capillary physiology and plasma protein-ligand mass action binding relationships. In most cases, the experimental data is best fit to the model equation when the apparent in vivo dissociation constant, KDa, of the ligand protein binding reaction increases to values that are 5- to 50-fold greater than the in vitro dissociation constant, KD. This result indicates that the rate of ligand dissociation from the plasma protein is accelerated in the capillary bed relative to the in vitro situation. It is hypothesized that the major factor leading to the rapid transport in vivo of protein-bound ligands into tissues such as brain is an endothelial-induced decrease in the affinity of the plasma protein for the ligand. Under these conditions, the amount of plasma ligand available for tissue clearance in vivo parallels the protein-bound fraction, not the free hormone.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassingthwaighte J. B. A concurrent flow model for extraction during transcapillary passage. Circ Res. 1974 Sep;35(3):483–503. doi: 10.1161/01.res.35.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasberg R. G., Fenstermacher J. D., Patlak C. S. Transport of alpha-aminoisobutyric acid across brain capillary and cellular membranes. J Cereb Blood Flow Metab. 1983 Mar;3(1):8–32. doi: 10.1038/jcbfm.1983.2. [DOI] [PubMed] [Google Scholar]
- CRONE C. THE PERMEABILITY OF CAPILLARIES IN VARIOUS ORGANS AS DETERMINED BY USE OF THE 'INDICATOR DIFFUSION' METHOD. Acta Physiol Scand. 1963 Aug;58:292–305. doi: 10.1111/j.1748-1716.1963.tb02652.x. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Huang T. S., Hurd R. E., Beredo A., Solomon D. H. A competitive ligand binding assay for measurement of thyroid hormone-binding inhibitor in serum and tissues. J Clin Endocrinol Metab. 1984 Apr;58(4):619–628. doi: 10.1210/jcem-58-4-619. [DOI] [PubMed] [Google Scholar]
- Cornford E. M., Pardridge W. M., Braun L. D., Oldendorf W. H. Increased blood--brain barrier transport of protein-bound anticonvulsant drugs in the newborn. J Cereb Blood Flow Metab. 1983 Sep;3(3):280–286. doi: 10.1038/jcbfm.1983.42. [DOI] [PubMed] [Google Scholar]
- Crane P. D., Braun L. D., Cornford E. M., Cremer J. E., Glass J. M., Oldendorf W. H. Dose dependent reduction of glucose utilization by pentobarbital in rat brain. Stroke. 1978 Jan-Feb;9(1):12–18. doi: 10.1161/01.str.9.1.12. [DOI] [PubMed] [Google Scholar]
- Forker E. L., Luxon B. A. Albumin-mediated transport of rose bengal by perfused rat liver. Kinetics of the reaction at the cell surface. J Clin Invest. 1983 Nov;72(5):1764–1771. doi: 10.1172/JCI111136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goresky C. A. The processes of cellular uptake and exchange in the liver. Fed Proc. 1982 Dec;41(14):3033–3039. [PubMed] [Google Scholar]
- KETY S. S. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol Rev. 1951 Mar;3(1):1–41. [PubMed] [Google Scholar]
- Koch-Weser J., Sellers E. M. Binding of drugs to serum albumin (first of two parts). N Engl J Med. 1976 Feb 5;294(6):311–316. doi: 10.1056/NEJM197602052940605. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am J Physiol. 1971 Dec;221(6):1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H., Braun L. D. [H] Tryptamine and 3H-water as diffusible internal standards for measuring brain extraction of radio-labeled substances following carotid injection. Brain Res. 1976 Aug 20;113(1):219–224. doi: 10.1016/0006-8993(76)90024-x. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H., Pardridge W. M., Braun L. D., Crane P. D. Measurement of cerebral glucose utilization using washout after carotid injection in the rat. J Neurochem. 1982 May;38(5):1413–1418. doi: 10.1111/j.1471-4159.1982.tb07920.x. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Brain metabolism: a perspective from the blood-brain barrier. Physiol Rev. 1983 Oct;63(4):1481–1535. doi: 10.1152/physrev.1983.63.4.1481. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M. Carrier-mediated transport of thyroid hormones through the rat blood-brain barrier: primary role of albumin-bound hormone. Endocrinology. 1979 Sep;105(3):605–612. doi: 10.1210/endo-105-3-605. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Mietus L. J. Palmitate and cholesterol transport through the blood-brain barrier. J Neurochem. 1980 Feb;34(2):463–466. doi: 10.1111/j.1471-4159.1980.tb06621.x. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Mietus L. J. Transport of steroid hormones through the rat blood-brain barrier. Primary role of albumin-bound hormone. J Clin Invest. 1979 Jul;64(1):145–154. doi: 10.1172/JCI109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Mietus L. J. Transport of thyroid and steroid hormones through the blood-brain barrier of the newborn rabbit: primary role of protein-bound hormone. Endocrinology. 1980 Dec;107(6):1705–1710. doi: 10.1210/endo-107-6-1705. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Moeller T. L., Mietus L. J., Oldendorf W. H. Blood-brain barrier transport and brain sequestration of steroid hormones. Am J Physiol. 1980 Jul;239(1):E96–102. doi: 10.1152/ajpendo.1980.239.1.E96. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Sakiyama R., Fierer G. Blood-brain barrier transport and brain sequestration of propranolol and lidocaine. Am J Physiol. 1984 Sep;247(3 Pt 2):R582–R588. doi: 10.1152/ajpregu.1984.247.3.R582. [DOI] [PubMed] [Google Scholar]
- Pardridge W. M., Sakiyama R., Fierer G. Transport of propranolol and lidocaine through the rat blood-brain barrier. Primary role of globulin-bound drug. J Clin Invest. 1983 Apr;71(4):900–908. doi: 10.1172/JCI110844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M. Transport of protein-bound hormones into tissues in vivo. Endocr Rev. 1981 Winter;2(1):103–123. doi: 10.1210/edrv-2-1-103. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. Transport of potassium-42 from blood to tissue in isolated mammalian skeletal muscles. Am J Physiol. 1959 Dec;197:1205–1210. doi: 10.1152/ajplegacy.1959.197.6.1205. [DOI] [PubMed] [Google Scholar]
- Weisiger R., Gollan J., Ockner R. Receptor for albumin on the liver cell surface may mediate uptake of fatty acids and other albumin-bound substances. Science. 1981 Mar 6;211(4486):1048–1051. doi: 10.1126/science.6258226. [DOI] [PubMed] [Google Scholar]



