Abstract
Background
Ayurveda, Indian traditional system of medicine, is practiced commonly in South East Asia and in many parts of the world. Many ayurvedic drugs contain heavy metals and may lead to metal toxicity. Of these, chronic lead poisoning is the most common. Chronic arsenic poisoning following the use of ayurvedic medication, though reported, is rare.
Case Reports
We describe three patients who presented with features of chronic arsenic poisoning following prolonged ayurvedic medication use. The diagnosis of chronic arsenic poisoning was confirmed by high arsenic levels in the blood, urine, hair, and nails in all the three patients and in ayurvedic drug in two patients. The ayurvedic medication was discontinued and treatment with d-penicillamine started. At 6 months after treatment, blood arsenic levels returned to normal with clinical recovery in all of them.
Conclusion
Arsenic poisoning following ayurvedic medication is much less common than lead poisoning, though mineral ayurvedic medicines may lead to it. We used d-penicillamine as chelator and all of them recovered. Whether withdrawal of medication alone or d-penicillamine also played a role in recovery is unclear and needs to be assessed.
Keywords: Chronic arsenic poisoning, Ayurveda, d-Penicillamine
Introduction
Ayurveda, an indigenous system of medicine, is an important source of health care in India, and its popularity is on the rise globally. The word “Ayu’s” means longevity, whereas “veda” means related to science or knowledge. Western medicine classifies it as complementary and alternative medicine. It has eight branches collectively, called as “Ashtang Ayurveda”. It asserts upon building healthy metabolic system, good digestion, and excretion. Its pharmacological section consists of medicines of herbal, mineral, and animal origin [1]. The herbal drugs are the safest, whereas mineral (rasayana) drugs can lead to toxicity and numerous cases of heavy metal toxicity, especially lead, have been reported following their use. A few cases with mercury (Hg) and arsenic (As) have also been reported [2]. We report three cases of chronic As poisoning following ayurvedic medication taken for different medical disorders.
Case Reports
Case 1
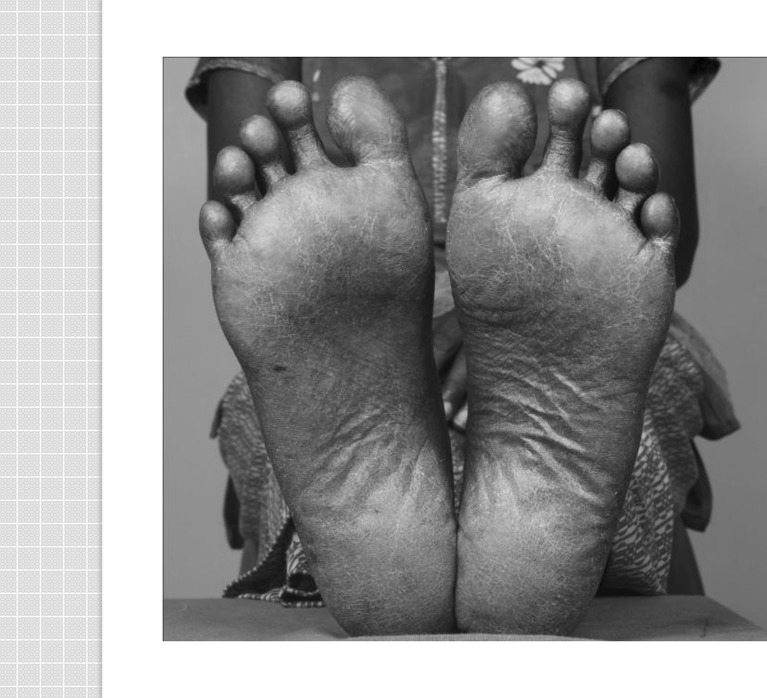
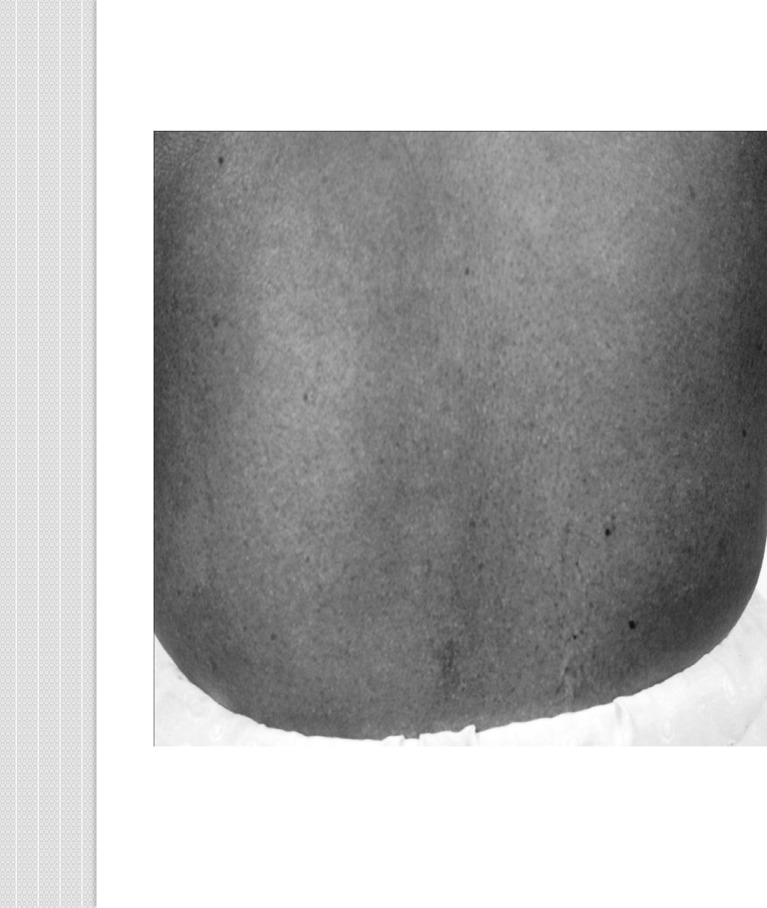
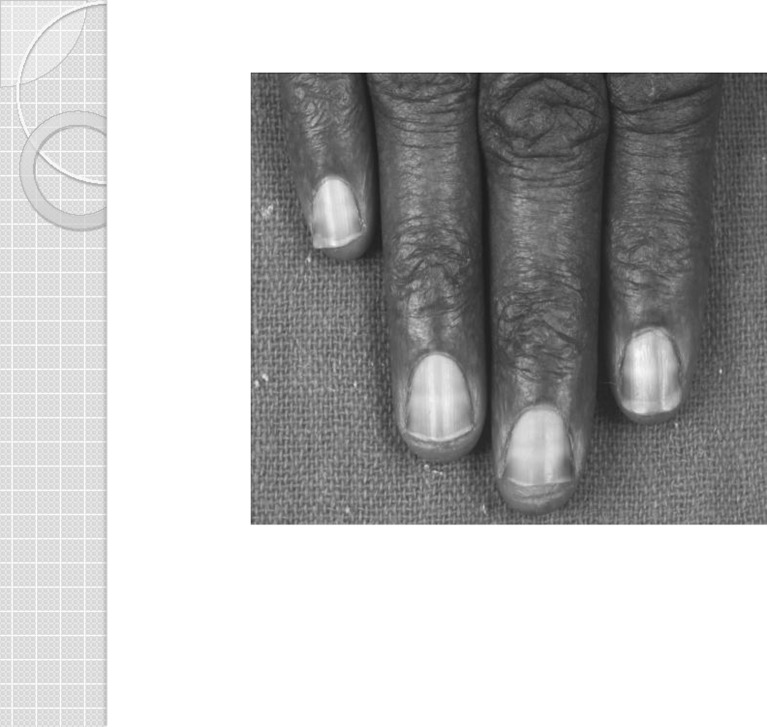
A 32-year-old lady presented with hyperpigmented skin of the palms and soles and diminished sensation and paresthesia of her feet of 6 months’ duration. She had been on oral ayurvedic medication for eczematous dermatitis for the past 5 years. In addition, she complained of recurrent episodes of abdominal pain, loose stools, and significant weight loss over the past year. She had two episodes of jaundice in the past year without cholestatic symptoms. She had also developed significant anemia unresponsive to hematinics and was requiring blood transfusions. She had no other medical problems and was not taking any other medication. She was not consuming meat or seafood as being a vegetarian, and water in the area does not have high arsenic content. On examination, she had pallor. There was hyperkeratosis of both palms and soles (Fig. 1); rain drop pigmentation over the arms, lower limbs, abdomen, and back (Fig. 2); and transverse white lines (Mee’s lines) on the nails (Fig. 3). Systemic examination revealed enlarged liver, weakness of both plantar flexors and dorsiflexors of the foot along with graded sensory loss to all sensory modalities below the knee with allodynia. Romberg’s test was positive. Bilateral ankle jerks were absent.
Fig. 1.
Hyperkeratosis of the soles
Fig. 2.
Raindrop pigmentation of the skin (back)
Fig. 3.
Mee’s lines over nails
Investigations revealed pancytopenia with macrocytosis and elevated transaminases (Table 1). Abdominal ultrasound revealed hepatomegaly with normal echotexture and cholelithiasis. There was no intrahepatic biliary dilatation, and portal vein was normal. Nerve conduction studies of the median, ulnar, common peroneal, tibial, and sural nerve revealed axonal sensorimotor polyneuropathy. The blood, urine, hair, and nails were tested for arsenic which was elevated (Table 2). As was detected in the ayurvedic medication and it was >100 μg/kg. It was immediately stopped, and she was treated with d-penicillamine (DPA) 500 mg twice daily for 6 months as DMSA was not available. Hematological parameters improved within 2 weeks with normalization of blood counts. Hyperkeratosis of the palms and soles resolved in the next 4 months. However, neuropathy only partially improved. Blood As levels normalized after 6 months. She has been now followed up for more than a year and is doing well.
Table 1.
Laboratory findings in three patients of chronic As ingestion following ayurvedic medication
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Hb (g/dL) | 7.9 | 12.2 | 7.0 |
| TLC (mm3) | 3,200 | 4,600 | 6,400 |
| DLC (%) | N54L40M4E2 | N69L29M2E0 | N66L30M3E2 |
| Platelets (mm3) | 30,000 | 300,000 | 230,000 |
| BU/creat (mg/dL) | 14/0.6 | 46/0.8 | 20/0.7 |
| TB/CB (mg/dL) | 1.4/0.4 | 0.7 | 0.7 |
| TP/albumin (g/dL) | 6/3.6 | 7.4/4.1 | 6.0/3.1 |
| AST/ALT (IU/L) | 43/19 | 10/19 | 11/7 |
| ALP | 60 | 16 | 9 |
| INR/APTT | 1.0/34 |
TLC total leukocyte count, DLC differential leukocyte count, BU/creat blood urea/creatinine, TB/CB total and conjugated bilirubin, TP/albumin total serum proteins/serum albumin
Table 2.
The pretreatment and 6 months posttreatment As levels in the blood and urine in three patients and in ayurvedic drug in two patients
| Case 1 | Case 2 | Case 3 | ||
|---|---|---|---|---|
| Pretreatment | Blood (<23.0 μg/L) | 93.4 | 330 | 133 |
| 24-h urine (<50 μg/L) | 8.9 | NA | 187 | |
| Hair (15–30 μg/kg) | 18.2 | 1,330 | 333 | |
| Nails (30–320 μg/kg) | 32.4 | NA | 30 | |
| Posttreatment | Blood (μg/L) | 18.9 | ||
| Urine (μg/L) | 0.9 | NA | NA | |
| Drug | >100 μg/kg | NA | >100 μg/kg |
Case 2
A 20-year-old male presented to us with weakness of both lower limbs followed by that of upper limbs for 2 months. He was detected with type I diabetes 2 years ago and was controlled with insulin. On the advice of an ayurvedic practitioner, he discontinued the use of insulin and began treatment with ayurvedic medication since 4 months. On examination, he had bilateral foot drop and wrist drop with graded sensory loss. Blood counts, liver, and renal functions were within normal limits (Table 1). Nerve conduction revealed bilateral severe axonal polyneuropathy with absent sensory nerve action potentials in the median, ulnar, peroneal, and sural nerves. As levels in the blood and hair were elevated (Table 2). We were unable to procure the ayurvedic medication he had been taking for estimation of As content. There was no other apparent source of exposure to it such as water or seafood. In the hospital, we stopped his ayurvedic medication, controlled his diabetes with insulin, and chelation therapy was started with DPA 500 mg twice a day and was administered for 6 months. Blood As levels became normal at 6 months and were normal till 1 year when last estimated, and he improved clinically, though had residual neuropathy.
Case 3
A 19-year-old lady was diagnosed with vitiligo 9 years back and was on oral ayurvedic treatment since then. She presented to us with fever, cough, and shortness of breath of 2 weeks’ duration. Examination revealed pallor, pedal edema, raindrop pigmentation of the skin, hyperkeratosis of the palms and soles, coarse crepitations in both lung fields and splenomegaly. She had anemia with normal leukocyte and platelet counts. Liver function tests revealed mild hypoalbuminemia (Table 1). Computerized tomography of the chest revealed bilateral bronchiectasis, and ultrasound of the abdomen showed normal liver echotexture, splenomegaly, and dilated portal vein suggestive of non-cirrhotic portal hypertension (NCPF). Liver biopsy was done which was inconclusive. She was suspected with chronic As toxicity following ayurvedic medication as no other apparent source of it was there. As levels in the blood, urine, nails, and hair were elevated (Table 2). Ayurvedic medication was stopped, and she was started on DPA (250 mg twice daily) which was administered for 6 months. The As content in ayurvedic medicine was more than 100 μg/kg. Her cutaneous manifestations improved, and blood As levels became normal after 6 months. She lived well for 2 year, after which, she succumbed to severe bronchopneumonia and sepsis.
Discussion
Arsenic is a metalloid used since ancient times both as a poison and for medicinal purposes. Although its use has declined with time, arsenic trioxide is being used for treatment of acute promyelocytic leukemia [3]. Mineral drugs called as “bhasmas” are widely used in ayurveda. Bhasmas are prepared after extensive purification (shodhana) of metals which supposedly renders them non-toxic [4]. In an experimental study with Tamra bhasma (incinerated copper), Jagtap et al. [5] found that only Tamra bhasma which did not undergo proper shodhana process was pathological and pure (shoditha) Tamra was safe. However, not all ayurvedic medications available undergo stringent quality control. A recent study by Saper et al. [6] measured lead, Hg, and As in ayurvedic medications and found one fifth had levels of at least one of these in excess of permissible limits.
Most of chronic arsenic toxicity has been described from regions where ground water is contaminated with arsenic. It manifests with characteristic cutaneous raindrop pigmentation, hyperkeratosis of the palms and soles, and transverse white bands on nails called Mee’s lines which are due to deposition of As in keratin-rich areas. In addition, there is increased predisposition to premalignant and malignant lesions like Bowen’s disease, squamous cell carcinoma, and basal cell carcinoma of the skin. Arsenic neuropathy is a distal axonopathy with predominant sensory involvement [3, 7]. Hepatotoxicity of arsenic is well studied, and one study showed that over 90 % of patients with chronic arsenic toxicity had evidence of NCPF on liver biopsy [8]. One of our patients (case 3) had bronchiectasis. Chronic exposure to arsenic is associated with respiratory diseases such as chronic bronchitis, interstitial lung disease, and bronchiectasis [9]. Several other manifestations have been reported and include peripheral vascular disease (blackfoot disease), gastrointestinal disturbances, renal involvement, and increased risk of visceral malignancies [3]. Bad obstetrical outcome such as increased incidence of spontaneous abortion, stillbirth, and higher preterm birth have been observed in women exposed to contaminated drinking water [10]. Epidemiological evidence also suggests an increased incidence of diabetes mellitus in them.
Treatment of chronic arsenic toxicity includes avoiding further exposure and symptomatic and chelation therapy. Dimercaprol or British anti-Lewisite (BAL) was introduced during the Second World War and was the first chelator used for its toxicity. However, due to its adverse effects, it was soon superseded by dimercaptosuccinic acid (DMSA) and 2,3-dimercapto-1-propane sulfonate (DMPS) [8, 11]. However, limited availability and cost complicates treatment with these agents. d-Penicillamine (DPA) is another chelating agent that is used in Wilson’s disease and lead and Hg poisoning. It is a monothiol and its thiol group forms complexes with certain metals [11]. DPA has been used in arsenic toxicity prior to introduction of DMSA and DMPS in a number of reports [12–14]. Murphy et al. reported two cases of As neuropathy treated with DPA. In both these patients, urinary excretion of As increased and they improved though residual neuropathy persisted [15]. However, the dose and duration of treatment with DPA is not clear. We used DPA for 6 months.
Since DMSA and DMPS have become available, DPA has fallen out of favor because of adverse effects mainly hypersensitivity reactions like fever, skin eruptions, lymphadenopathy, neutropenia, thrombocytopenia, and proteinuria. Despite favorable outcome observed in anecdotal reports, most of the experimental studies have not found DPA to be effective in As toxicity. Kreppel et al. [16] in an experimental model of As poisoning, observed that DPA did not prevent the lethal effects or reduce the tissue concentration of As. Similar observations were made by Hilmy et al. [17] and Aposhian et al. [18]. It is difficult to assess in our patients the individual contribution of stoppage of culprit drug and chelation therapy. Our patients had rapid improvement after cessation of the offending drug and institution of DPA. Though improvement occurred, it will be difficult to asses if the role of DPA as simple removal of ayurvedic drug could have led to improvement, and the role of DPA in arsenic toxicity remains unclear.
Conclusion
Clinicians should be aware that chronic As toxicity can develop following ingestion of mineral ayurvedic drugs as stopping the drug in time can prevent a fatal outcome. All our patients improved with cessation of the offending drug and institution of DPA. It is difficult to assess the contribution of DPA to clinical improvement as cessation of the culprit medication could also have led to it. DPA was well tolerated in our patients. Although other chelators have demonstrated greater effectiveness in the treatment of arsenic poisoning, when they are unavailable, DPA may be considered; though further investigations are needed to establish its effectiveness and its dose, duration of treatment needs to be determined.
References
- 1.The departments of AYUSH. Ministry of Health and family welfare . Ayurveda. The science of life. New Delhi: Government of India; 2012. Basic principles; pp. 24–31. [Google Scholar]
- 2.Lynch E, Braithwaite R. A review of the clinical and toxicological aspects of ‘traditional’ (herbal) medicines adulterated with heavy metals. Expert Opin Drug Saf. 2005;4:769. doi: 10.1517/14740338.4.4.769. [DOI] [PubMed] [Google Scholar]
- 3.Sengupta SR, Das NK, Datta PK. Pathogenesis, clinical features and pathology of chronic arsenicosis. Indian J Dermatol Venereol Leprol. 2008;74:559. doi: 10.4103/0378-6323.45097. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Nair AG, Reddy AV, Garg AN. Bhasmas: unique ayurvedic metallic-herbal preparations, chemical characterization. Biol Trace Elem Res. 2006;109:231. doi: 10.1385/BTER:109:3:231. [DOI] [PubMed] [Google Scholar]
- 5.Jagtap CY, Ashok BK, Patgiri BJ, Prajapati PK, Ravishankar B. Acute and subchronic toxicity study of Tamra bhasma (incinerated copper) prepared from Ashodhita (unpurified) to Shodhita (purified Tamra) in rats. Indian J Pharmaceut Sci. 2013;75(3):346. doi: 10.4103/0250-474X.117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, Thuppil V, Kales SN. Lead, mercury, and arsenic in US- and Indian-manufactured ayurvedic medicines sold via the internet. JAMA. 2008;300:915. doi: 10.1001/jama.300.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das NK, Sengupta SR. Arsenicosis: diagnosis and treatment. Indian J Dermatol Venereol Leprol. 2008;74:571. doi: 10.4103/0378-6323.45098. [DOI] [PubMed] [Google Scholar]
- 8.Santra A, Das Gupta J, De BK, Roy B, Guha Mazumder DN. Hepatic manifestations in chronic arsenic toxicity. Indian J Gastroenterol. 1999;18:152. [PubMed] [Google Scholar]
- 9.Mazumder DN, Steinmaus C, Bhattacharya P, von Ehrenstein OS, Ghosh N, Gotway M. Bronchiectasis in persons with skin lesions resulting from arsenic in drinking water. Epidemiology. 2005;16:760. doi: 10.1097/01.ede.0000181637.10978.e6. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, Hadi SA, Talukder HK. Arsenic in drinking water and pregnancy outcomes. Environ Health Perspect. 2001;109(6):629. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flora SJ, Pachauri V. Chelation in metal intoxication. Int J Environ Res Public Health. 2010;7:2745. doi: 10.3390/ijerph7072745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuruvilla A, Bergeson PS, Done AK. Arsenic poisoning in childhood. An unusual case report with special notes on therapy with penicillamine. Clin Toxicol. 1975;8:535. doi: 10.3109/15563657508988097. [DOI] [PubMed] [Google Scholar]
- 13.Peterson RG, Rumack BH. D-penicillamine therapy of acute arsenic poisoning. J Pediatr. 1977;91:661. doi: 10.1016/S0022-3476(77)80528-3. [DOI] [PubMed] [Google Scholar]
- 14.Cullen NM, Wolf LR, St Clair D. Pediatric arsenic ingestion. Am J Emerg Med. 1995;13(4):432. doi: 10.1016/0735-6757(95)90133-7. [DOI] [PubMed] [Google Scholar]
- 15.Murphy MJ, Lyon WL, Taylor JW. Subacute arsenic neuropathy: clinical and electrophysiological observations. J Neurol Neurosurg Psychiatry. 1981;44:899. doi: 10.1136/jnnp.44.10.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreppel H, Reichl FX, Forth W, Fichtl B. Lack of effectiveness of D-penicillamine in experimental arsenic poisoning. Vet Hum Toxicol. 1989;31(1):1. [PubMed] [Google Scholar]
- 17.Hilmy AM, El-Domiaty NA, Kamal MA, Mohamed MA, Abou Samra WE. Effect of some arsenic antagonists on the toxicity, distribution and excretion of arsenite and arsenate in rats. Comp Biochem Physiol. 1991;99:357. doi: 10.1016/0300-9629(91)90014-4. [DOI] [PubMed] [Google Scholar]
- 18.Aposhian HV, Tadlock CH, Moon TE. Protection of mice against lethal effects of sodium arsenite—a quantitative comparison of a number of chelating agents. Toxicol Appl Pharmacol. 1981;61(3):385. doi: 10.1016/0041-008X(81)90360-4. [DOI] [PubMed] [Google Scholar]