Abstract
Introduction
We report the case of an adolescent with anticholinergic toxidrome from diphenhydramine overdose, whose symptoms were treated with a novel application of dexmedetomidine.
Case Report
A 13-year-old female developed an anticholinergic toxidrome after intentionally ingesting 9.5 mg/kg of diphenhydramine. Despite routine supportive therapies, to include appropriate doses of lorazepam, she continued to have significant agitation, psychosis, and hallucinations. A dexmedetomidine infusion was started to aid in the treatment of her agitation and psychosis with marked improvement of her symptoms.
Discussion
Using dexmedetomidine for the treatment of anticholinergic toxidrome has not been previously described in the literature, but there are multiple reports of its use in alcohol withdrawal syndrome. We suggest that adding dexmedetomidine as an adjunctive agent in the therapy of anticholinergic toxidrome may relieve the symptoms of agitation, psychosis, tachycardia, and hypertension, without the attendant risk of respiratory depression associated with high doses of benzodiazepines.
Keywords: Diphenhydramine toxicity, Anticholinergic toxidrome, Dexmedetomidine
Introduction
In this article, we present the case of intentional diphenhydramine overdose with subsequent anticholinergic toxidrome. After appropriate doses of benzodiazepines were given, with little therapeutic response, the patient was started on an infusion of dexmedetomidine with improvement in her agitation, psychosis, tachycardia, and hypertension. We suggest the use of dexmedetomidine as a novel treatment for the symptoms of an anticholinergic toxidrome.
Case
The patient was a healthy 13-year-old Caucasian female, who was brought to the emergency department (ED) by ambulance for altered mental status after intentional ingestion of diphenhydramine. By report, the patient’s younger sibling found the patient outside their home, speaking incoherently, and unaware of her surroundings. Emergency medical services were contacted and transported the patient to a local ED.
In the initial history and later conversations, it was determined that the patient and her friend were intentionally trying to “get high” and that the patient took approximately 24 pills of diphenhydramine (25 mg each, total dose 600 mg, 9.5 mg/kg). The patient could not recall the exact timing of the ingestion, but based on the time that she and her friend were unattended, the ingestion occurred sometime in the early evening. She later denied taking any other medications or drugs of abuse. The patient’s past medical history was significant only for attention deficit hyperactivity disorder, treated with dextroamphetamine and amphetamine (Adderall XR®) 20 mg by mouth daily, with no reported surgeries, prior hospitalizations, or known drug allergies. Her parents reported two known previous attempts of intentional ingestions of pseudoephedrine, with the goal of intoxication, as well as recreational use of marijuana.
On arrival to the ED at 2154, her exam was notable for reactive but dilated pupils (6–7 mm), dry mucus membranes, and she was speaking incoherently. Her vital signs on check in were heart rate (HR) 180 bpm, blood pressure (BP) 131/60 mmHg, respiration rate (RR) 20/min, and pulse-oximetry 100 % on room air (RA). The remainder of her exam was normal. The emergency department consulted Poison Control, and anticholinergic toxidrome from diphenhydramine ingestion was felt to be the most likely explanation of her symptoms. Her labs were notable for urine drug screen positive for amphetamines (likely her prescribed Adderall XR®) but negative for other drugs of abuse, ethanol, or salicylates. Her urine pregnancy screen was negative. A comprehensive metabolic panel was normal except for a potassium of 2.9 mmol/L (3.3–4.6 mmol/L). An electrocardiogram (ECG) revealed a QTc of 390 ms (HR 155, QT 240 ms). She received 5 mg of lorazepam for agitation. A Foley catheter was placed to prevent urinary retention (as recommended by Poison Control), and she was then transported to the pediatric intensive care unit (PICU) for continued care. At the time of transfer (0012, approximately 2 h after her initial presentation), her vital signs were HR 138 bpm, BP 116/81 mmHg, RR 28/min, and pulse-oximetry 98 % on RA.
On arrival to the PICU, she was agitated, attempting to climb out of bed despite soft limb restraints, speaking incoherently, and appeared to be having visual hallucinations. Initial vital signs were temperature 99.3 °F, HR 134 bpm, RR 21/min, BP 111/61 mmHg, and pulse-oximetry 98 % on RA. Physical exam revealed a girl who was disheveled, agitated, flushed, combative, with dilated (6–7 mm) but reactive pupils, and tachycardic with a grade 1/6 systolic flow murmur at the lower sternal border. Her Richmond Agitation Sedation Scale (RASS) score was 4+ [1, 2]. The remainder of her physical exam was unremarkable. A repeat basic metabolic panel at the time of admission was normal, with a potassium of 3.7 mmol/L (3.3–4.6 mmol/L).
In addition to standard care and monitoring, she was given lorazepam for agitation and treatment of her anticholinergic toxidrome. Despite 12 mg of lorazepam IV over the first 5 h of admission, she continued to be agitated, combative, confused, and a risk of injury to herself and the medical staff. A dexmedetomidine loading dose of 1 mcg/kg IV was given followed by an infusion of 0.5 mcg/kg/h IV. After the loading dose and start of the infusion, she rested comfortably with no further agitation or combative behavior, had a RASS score of 3−, and her tachycardia improved significantly.
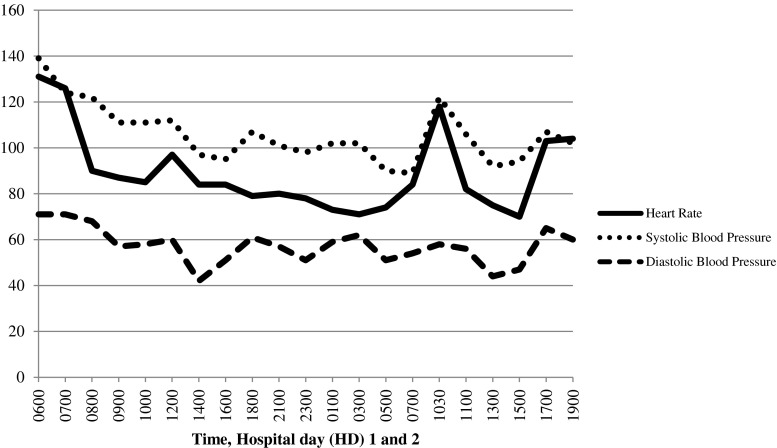
The dexmedetomidine infusion was continued for approximately 25 h. At that time, the patient was sleeping but easily arousable, able to follow commands, exhibited no anxiety or agitation, and had a RASS score of 1− to 3−. Given the improvement in her mental status, the infusion was held and she woke easily, was lucid, calm, and followed commands. Approximately 90 min after stopping the infusion, the patient reported feelings of the “bed moving” along with tactile and visual hallucinations and increasing anxiety. She was given 2 mg of lorazepam with little improvement. The dexmedetomidine infusion was restarted with subsequent resolution of her symptoms. The infusion was continued for another 8 h and then held, with no return of her previous symptoms. Figure 1 shows her HR and BP over time, starting prior to the infusion and until it was finally discontinued. Once her symptoms had resolved, she was interviewed by child psychiatry and found to be safe for transfer to the pediatric ward for further observation and evaluation. She reported very mild symptoms of anxiety the evening after stopping the dexmedetomidine infusion, which were controlled with oral lorazepam. Ultimately, she was discharged home with close follow-up in the pediatric and psychiatry clinics.
Fig. 1.
Vital signs over time. Dexmedetomidine infusion started at 0600 HD1, held at 0700 HD2, restarted at 1030 HD2, and finally held at 1500 HD2
Discussion
In this report, we suggest the use of dexmedetomidine as an adjunctive therapy for the treatment of the anticholinergic toxidrome due to diphenhydramine overdose. This is both a novel use of dexmedetomidine and treatment for anticholinergic toxidrome. There is a growing body of literature describing the use of dexmedetomidine for the treatment of alcohol withdrawal syndrome, which has some similarities with anticholinergic toxidrome [3–8].
Diphenhydramine is a common over-the-counter drug used for mild nighttime sedation, prevention of motion sickness, as an antitussive, and for the symptomatic relief of allergic symptoms [9]. Diphenhydramine competes with histamine for the H1-receptor sites on effector cells in the gastrointestinal (GI) tract, blood vessels, and respiratory tract [9]. In higher doses, it can cause an anticholinergic toxidrome, which has been well characterized in the past [10]. Ingestions continue to be a major cause of morbidity and mortality in the USA, and antihistamines are the eighth most common ingestion listed in the most recent Annual Report of the American Association of Poison Controls [11]. Further, the frequency of antihistamine abuse has been increasing in recent years [11]. Diphenhydramine intoxication commonly occurs from either accidental overdose or intentional overdose, with the goal of intoxication or suicide [12–14].
The symptoms of anticholinergic toxidrome include peripheral and central effects. Central effects include delusions, psychosis, agitation, and seizures, whereas peripheral effects manifest as mydriasis, cutaneous vasodilation, hyperthermia, tachycardia, anhidrosis, gastrointestinal dysmotility, and urinary retention [13, 15]. The effects of diphenhydramine overdose are dose dependent and range in severity. Mild intoxications can occur at doses less than 300 mg and, if there are no mental status changes, can usually be managed as an outpatient [16]. Severe effects, including seizures, delirium, psychosis, rhabdomyolysis, coma, and death, can occur in doses that exceed 1 g [17, 18]. In massive overdose, death can result from wide-complex tachycardia leading to tachydysrhythmia and widening of the QT interval [12, 17].
The currently recommended therapies for anticholinergic toxidrome are largely supportive. Benzodiazepines have been the mainstay therapy for moderate to severe agitation and seizures. Physostigmine is a potent, short-acting anticholinesterase that can treat the central and peripheral effects of anticholinergic overdose, but its use is somewhat limited by its short half-life (approximately 15 min). Physostigmine can be re-dosed or given as an infusion and has been shown to reduce the need for benzodiazepines in the setting of anticholinergic toxidromes [15, 18–21]. With our patient, we chose not to give a trial dose of physostigmine due in part to length of time of getting it from the pharmacy, relative unfamiliarity with the side effect profile, and for concern of the disparity of the relative half-lives of diphenhydramine and physostigmine.
Dexmedetomidine is a selective α2-adrenergic agonist first approved by the Food and Drug Administration in 1999 for sedation of adult patients in the intensive care unit (ICU) setting [22]. Since then, multiple studies have shown that its sedative, anxiolytic, and analgesic properties are safe for a broad range of uses, including sedation in the operating room and ICU settings, procedural sedation, and opioid and alcohol withdrawal [23, 24]. Dexmedetomidine is chemically similar to clonidine but has a much higher affinity for α2 receptors versus α1 (approximately 1,620:1) with a much shorter half-life than clonidine (2–3 versus 12–24 h) [25, 26]. Its primary action is stimulation of the receptors in the medulla, which decreases sympathetic outflow in the locus coeruleus, leading to increased action of the inhibitory GABA neurons, with resultant sedation, analgesia, and REM sleep [26]. Dexmedetomidine is generally given as a continuous intravenous infusion, with or without a preceding bolus dose, but can be given intranasally or orally as well [23, 26]. The most commonly encountered adverse effects of dexmedetomidine infusion are hypotension and bradycardia [22–24, 26]. It has been shown to have very little effect on the respiratory drive and airway patency, even in the setting of accidental overdoses [23]. If used for greater than 24 h, tolerance and tachyphylaxis may occur, and in rare cases, withdrawal symptoms have been reported but do not appear to be a significant issue in pediatric patients [22, 23]. The onset of action for an IV bolus is 5–10 min with a peak effect seen in 15–30 min and a duration of 60–120 min. It is approximately 94 % protein bound, metabolized in the liver, and 95 % of the metabolites are excreted in the urine [22–26].
In our review of the literature, we found no reports of the use of dexmedetomidine to treat anticholinergic toxidrome. There is a growing body of case reports and retrospective single-center analyses for use of dexmedetomidine as an adjunctive therapy in alcohol withdrawal syndrome (AWS) [4, 8]. AWS is an abstinence syndrome with similarities to anticholinergic toxidrome. AWS includes autonomic hyperactivity with symptoms of anxiety, agitation, elevated blood pressure, tachycardia, and tremor, as well as hallucinations and seizures which are caused by disrupted GABA signaling and subsequent increase in glutamate, leading to central nervous system hyperexcitation [6, 8].
As with anticholinergic toxidrome, the suggested standard treatment for AWS is supportive therapy and benzodiazepines for agitation, anxiety, psychosis, and seizures. However, those with significant alcohol tolerance often have cross-tolerance to the GABA-mediated actions of benzodiazepines. The tolerance to benzodiazepines can lead to the administration of large doses, which increases the risk of respiratory insufficiency, aspiration, and the need for intubation. Benzodiazepine tolerance also increases complication rates, length of hospital stay, and potential cost of hospitalization [6]. Alpha-2 agonists, such as dexmedetomidine, may have an adjunctive role in management of the symptoms of AWS by their amelioration of the sympathetic overdrive which is part of AWS [5].
Dexmedetomidine’s sedative, analgesic, anxiolytic, and sympatholytic effects, along with its favorable side effect profile, make it potentially very useful in the treatment of AWS [7]. Most importantly, dexmedetomidine’s effects are mediated by agonism of central, presynaptic α2-autoreceptors with no effect on GABA or opioid receptors, which allows for sedation with very little effect on the respiratory drive [3, 4, 23, 24, 26]. Additionally, it does not significantly depress a patient’s neurologic status and allows for cooperative sedation. The growing body of literature supporting use of dexmedetomidine in AWS suggests that there are significant reductions in the need for high doses of benzodiazepines, alcohol withdrawal score, restraint use, tachycardia, and systolic hypertension [4–6, 8]. Additionally, studies have shown a decrease in the rate of intubation due to high-dose benzodiazepine administration [5, 6, 8]. The major limitation of this case report is it represents the experience with a single patient and her response and cannot, without more trials, be generalized to all patients with anticholinergic toxidrome. However, as described above, dexmedetomidine has a growing support in the treatment of AWS, which is similar to anticholinergic intoxication. As with AWS, we suggest that considering dexmedetomidine as an adjunctive agent in the therapy of anticholinergic toxidrome may relieve the symptoms of agitation, psychosis, tachycardia, and hypertension, without the attendant risk of respiratory depression associated with high doses of benzodiazepines. Further research is required to assess the safety and generalized efficacy of dexmedetomidine in drug-induced delirium (particularly in children).
Acknowledgments
Disclaimer
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, nor the US Government.
This work was prepared as part of the authors’ official duties. Title 17 U.S.C. 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C. 101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
Conflict of Interest
The authors have no conflicts of interest to report.
Funding
No funding was given for this work.
Previous Presentations
This work has been submitted and selected for the Naval Medical Center Portsmouth, Portsmouth, VA 2014 Academic Research Competition.
References
- 1.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 2.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA J Am Med Assoc. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 3.Darrouj J, Puri N, Prince E, Lomonaco A, Spevetz A, Gerber DR. Dexmedetomidine infusion as adjunctive therapy to benzodiazepines for acute alcohol withdrawal. Ann Pharmacother. 2008;42(11):1703–1705. doi: 10.1345/aph.1K678. [DOI] [PubMed] [Google Scholar]
- 4.Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of alpha2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother. 2011;45(5):649–657. doi: 10.1345/aph.1P575. [DOI] [PubMed] [Google Scholar]
- 5.Muzyk AJ, Revollo JY, Rivelli SK. The use of dexmedetomidine in alcohol withdrawal. J Neuropsychiatry Clin Neurosci. 2012;24(3):E45–E46. doi: 10.1176/appi.neuropsych.11080194. [DOI] [PubMed] [Google Scholar]
- 6.Rayner SG, Weinert CR, Peng H, Jepsen S, Broccard AF. Dexmedetomidine as adjunct treatment for severe alcohol withdrawal in the ICU. Ann Intensiv Care. 2012;2(1):12. doi: 10.1186/2110-5820-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rovasalo A, Tohmo H, Aantaa R, Kettunen E, Palojoki R. Dexmedetomidine as an adjuvant in the treatment of alcohol withdrawal delirium: a case report. Gen Hosp Psychiatry. 2006;28(4):362–363. doi: 10.1016/j.genhosppsych.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Tolonen J, Rossinen J, Alho H, Harjola VP. Dexmedetomidine in addition to benzodiazepine-based sedation in patients with alcohol withdrawal delirium. Eur J Emerg Med. 2013;20(6):425–427. doi: 10.1097/MEJ.0b013e32835c53b3. [DOI] [PubMed] [Google Scholar]
- 9.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Giffin SL. 2008 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 26th Annual Report. Clin Toxicol (Phila) 2009;47(10):911–1084. doi: 10.3109/15563650903438566. [DOI] [PubMed] [Google Scholar]
- 10.Jones J, Dougherty J, Cannon L. Diphenhydramine-induced toxic psychosis. Am J Emerg Med. 1986;4(4):369–371. doi: 10.1016/0735-6757(86)90312-8. [DOI] [PubMed] [Google Scholar]
- 11.Mowry JB, Spyker DA, Cantilena LR, Jr, Bailey JE, Ford M. 2012 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol (Phila) 2013;51(10):949–1229. doi: 10.3109/15563650.2013.863906. [DOI] [PubMed] [Google Scholar]
- 12.Ramachandran K, Sirop P. Rare complications of diphenhydramine toxicity. Conn Med. 2008;72(2):79–82. [PubMed] [Google Scholar]
- 13.Rinder CS, D’Amato SL, Rinder HM, Cox PM. Survival in complicated diphenhydramine overdose. Crit Care Med. 1988;16(11):1161–1162. doi: 10.1097/00003246-198811000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Williams JF, Kokotailo PK. Abuse of proprietary (over-the-counter) drugs. Adolesc Med Clin. 2006;17(3):733–750. doi: 10.1016/j.admecli.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Erickson T, Ahrens W, Aks S, Baum C, Ling L (2005) Pediatric toxicology. 1st ed. New York, NY: McGraw-Hill http://www.r2library.com/Resource/Title/0071417362. Accessed 3 Nov 2014
- 16.Scharman EJ, Erdman AR, Wax PM, et al. Diphenhydramine and dimenhydrinate poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2006;44(3):205–223. doi: 10.1080/15563650600585920. [DOI] [PubMed] [Google Scholar]
- 17.Clark RF, Vance MV. Massive diphenhydramine poisoning resulting in a wide-complex tachycardia: successful treatment with sodium bicarbonate. Ann Emerg Med. 1992;21(3):318–321. doi: 10.1016/S0196-0644(05)80897-2. [DOI] [PubMed] [Google Scholar]
- 18.Quesada H, Stuckas H, Skibinski DO. Heteroplasmy suggests paternal co-transmission of multiple genomes and pervasive reversion of maternally into paternally transmitted genomes of mussel (Mytilus) mitochondrial DNA. J Mol Evol. 2003;57(Suppl 1):S138–S147. doi: 10.1007/s00239-003-0019-y. [DOI] [PubMed] [Google Scholar]
- 19.Koppel C, Ibe K, Tenczer J. Clinical symptomatology of diphenhydramine overdose: an evaluation of 136 cases in 1982 to 1985. J Toxicol Clin Toxicol. 1987;25(1–2):53–70. doi: 10.3109/15563658708992613. [DOI] [PubMed] [Google Scholar]
- 20.Sharma AN, Hexdall AH, Chang EK, Nelson LS, Hoffman RS. Diphenhydramine-induced wide complex dysrhythmia responds to treatment with sodium bicarbonate. Am J Emerg Med. 2003;21(3):212–215. doi: 10.1016/S0735-6757(02)42248-6. [DOI] [PubMed] [Google Scholar]
- 21.Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374–381. doi: 10.1016/S0196-0644(00)70057-6. [DOI] [PubMed] [Google Scholar]
- 22.Precedex (dexmedetomidine hydrochloride) Lake Forest, IL: Hospira, Inc.; 2013
- 23.Mason KP, Lerman J. Review article: dexmedetomidine in children: current knowledge and future applications. Anesth Analg. 2011;113(5):1129–1142. doi: 10.1213/ANE.0b013e31822b8629. [DOI] [PubMed] [Google Scholar]
- 24.Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28(1):3–6. doi: 10.1097/EJA.0b013e32833e266d. [DOI] [PubMed] [Google Scholar]
- 25.Dexmedetomidine. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6715. Accessed Oct 10 2013
- 26.McMorrow SP, Abramo TJ. Dexmedetomidine sedation: uses in pediatric procedural sedation outside the operating room. Pediatr Emerg Care. 2012;28(3):292–296. doi: 10.1097/PEC.0b013e3182495e1b. [DOI] [PubMed] [Google Scholar]