Abstract
The Toxicology Investigators Consortium (ToxIC) Case Registry was established in 2010 by the American College of Medical Toxicology. The Registry includes all medical toxicology consultations performed at participating sites. This report summarizes the Registry data for 2013. A query of the ToxIC Registry was carried out for the dates of January 1 through December 31, 2013. Specific data reviewed for analysis included demographics (age, gender), source of consultation, reasons for consultation, agents involved in toxicological exposures, signs, symptoms and clinical findings, and treatment. A total of 8,598 cases were entered into the Registry in 2013. Females accounted for 49.2 % of cases, males for 47.7 %, and gender was not reported in 3.1 %. The majority of patients (63.4 %) were adults between the ages of 19 and 65 years. There were 93 fatalities (1.1 %). Most referrals for medical toxicology consultation originated from the emergency department (59.7 %) or inpatient services (16.7 %). Exposures to pharmaceutical products (intentional and unintentional) made up 50.0 % of cases. Illicit drug abuse (8.0 %) and adverse drug reactions (ADRs) (4.8 %) were the next most frequent reasons for consultation. Similar to past years, nonopioid analgesics, sedative-hypnotics, and opioids were the most commonly encountered agents. Symptoms or clinical findings were documented in 71.1 % of patients. Of all cases, 54.6 % required some form of medical treatment (antidotes, antivenom, chelation, specific types of supportive care). This report serves as a comprehensive survey of medical toxicology practice within participating institutions. Prior trends continued to apply this year and indicate analgesic (opioid and nonopioid), sedative-hypnotic/muscle relaxant agents, illicit drug use, and ADRs continue to be major toxicological problems. Cases requiring medical toxicology consultation in 2013 predominantly involved pharmaceuticals and illicit drugs. Reasons for these drug exposures were diverse and included intentional overdose, unintentional exposure, withdrawal syndromes, and ADRs. Nonopioid analgesics, sedative-hypnotic agents, and opioids remained the most frequently encountered agent classes. While over half of cases required some form of medical treatment, fatalities were uncommon.
Keywords: Poisoning, Overdose, Epidemiology
Introduction
In 2010, the Toxicology Investigators Consortium (ToxIC) of the American College of Medical Toxicology (ACMT) created a registry intended to provide a tool for clinical toxicology research and toxico-surveillance [1]. Unlike other poisoning databases, the ToxIC Registry prospectively collects information on patients seen in consultation by medical toxicologists as hospital inpatients or in outpatient clinics. These toxicologists enter the patient data directly into the Registry. Since its inception in 2010 with four initial participating institutions, the ToxIC Registry has expanded to include investigators at 38 sites who see patients at 69 separate facilities. The object of this report is to summarize the Registry’s 2013 data.
Patients entered from January 1, 2013 through December 31, 2013 are described in this report. This is the fourth annual report for the Registry [2–4].
Several sub-registries were developed after the inception of ToxIC. Three were added in 2013 focusing on North American snake bites, prescription drug misuse, and a clinical poisoning severity score. These, in addition to the existing ones on caustic ingestions and lipid resuscitation therapy, bring the total number of sub-registries to five. During 2013, six abstracts based on Registry data were presented at three national and international meetings.
Methods
Participating investigators agree to enter data on all of their medical toxicology consultations into the Registry. Cases are entered on a password-protected, online data collection form. The site is maintained by the ACMT and is overseen by the ToxIC Registry Steering Committee. The Registry is Health Insurance Portability and Accountability Act compliant and does not collect any identifying patient information. Participation in the Registry is compliant with local Institutional Review Board policies and procedures as well as the Western Institutional Review Board.
Collected data include presenting signs, symptoms, clinical course, treatments, patient demographics, location, and the reason for toxicological exposure. The term consultation is used in this report to describe any encounter with the medical toxicologist, including admission to a toxicology inpatient service and consultation on other inpatient units, emergency department, and outpatient clinic visits. The online collection form is formatted to ensure that the data remain organized and easily searchable. There are also areas for free-text entry where providers may describe the case in more detail or offer supplementary information that is not available as a preexisting check box. As part of the Registry’s mission of providing a real-time toxico-surveillance tool, a component of the standard data form is a sentinel detection field that signals novel or unusual cases.
For this report, a search of the database was performed to identify encounters recorded from January 1, 2013 through December 31, 2013. Additional details collected in the sub-registries will be published separately.
In tables describing agent classes, unless otherwise indicated, substances with fewer than five occurrences were not listed as separate items. Exceptions to this practice include the agent comprising a significant fraction of the class or if there was only one agent in the table. Percentages noted in tables of specific agents represent the proportion of those agents within its agent class.
Results
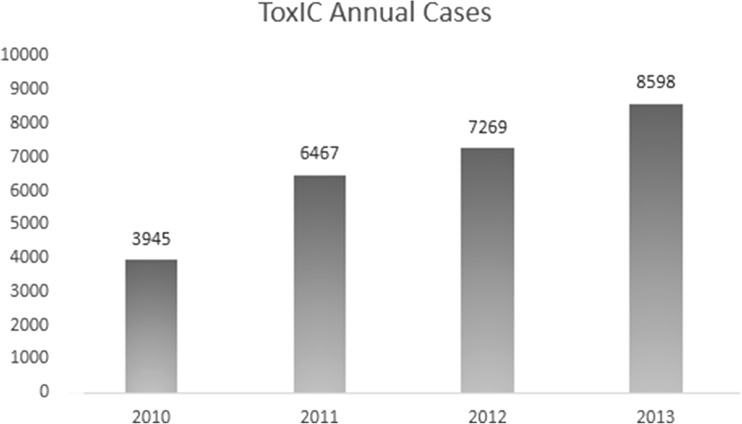
The individual institutions participating in the ToxIC Registry are listed in Table 1. In 2013, a total of 8,598 cases, representing an increase of 18.3 % over 2012 (Fig. 1), were entered.
Table 1.
Participating institutions
| Arizona | Minnesota | Pennsylvania |
| Phoenix | St. Paul | Harrisburg |
| Banner Good Samaritan Medical Center | Regions Hospital | Harrisburg Hospital |
| Phoenix Children’s Hospital | Philadelphia | |
| Missouri | Einstein Medical Center | |
| California | Kansas City | Hahnemann University Hospital |
| Fresno | Children’s Mercy Hospital & Clinics | St. Christopher’s Hospital for Children |
| UCSF Fresno Medical Center | St. Louis | Mercy Fitzgerald Hospital |
| Los Angeles | Barnes Jewish Hospital | Mercy Philadelphia Hospital |
| USC Verdugo Hills Hospital | Einstein Medical Center Montgomery | |
| San Francisco | Nebraska | Einstein Medical Center Elkins Park |
| San Francisco General Hospital | Omaha | Pittsburgh |
| University of Nebraska Medical Center | UPMC Presbyterian/Shadyside | |
| Colorado | UPMC Children’s Hospital of Pittsburgh | |
| Denver | New Jersey | UPMC Magee Women’s Hospital |
| Denver Health Medical Center | Morristown | |
| University of Colorado Hospital | Morristown Medical Center | Texas |
| Porter and Littleton Adventist Hospital | New Brunswick | Dallas |
| Children’s Hospital Colorado | Robert Wood Johnson University Hospital | Parkland Memorial Hospital |
| Swedish Medical Center | Newark | Children’s Medical Center of Dallas |
| University Hospital | UT Southwestern Medical Center | |
| Connecticut | Newark Beth Israel Medical Center | Houston |
| Hartford | Ben Taub General Hospital | |
| Hartford Hospital | New York | Texas Children’s Hospital |
| CT Children’s Medical Center | Manhasset | San Antonio |
| North Shore University Hospital | San Antonio Military Medical Center | |
| Indiana | New Hyde Park | |
| Indianapolis | Long Island Jewish Medical Center | Utah |
| IU Health Methodist Hospital | New York | Salt Lake City |
| Wishard Memorial Hospital | Mount Sinai Hospital | Primary Children’s Hospital |
| Riley Hospital for Children | Staten Island University Hospital | University Hospital |
| IU Health University Hospital | Bellevue Hospital Center | |
| NYU Langone Medical Center | Virginia | |
| Illinois | Elmhurst Medical Center | Charlottesville |
| Chicago | Rochester | University of Virginia Health Systems |
| John H. Stroger, Jr. Hospital of Cook County | Strong Memorial Hospital | Richmond |
| Evanston | Huther Doyle | VCU Medical Center |
| North Shore University Health System | Highland Hospital | |
| Wisconsin | ||
| Massachusetts | North Carolina | Milwaukee |
| Boston | Charlotte | Froedtert Hospital |
| Beth Israel Deaconess Medical Center | Carolinas Medical Center | Children’s Hospital of Wisconsin |
| Boston Children’s Hospital | ||
| Worcester | Ohio | Australia |
| UMass Memorial Medical Center | Cincinnati | Blacktown, New South Wales |
| Cincinnati Children’s Hospital Medical Center | Blacktown and Mt. Druitt Hospital | |
| Michigan | ||
| Grand Rapids | Oregon | Israel |
| Spectrum Health Hospitals | Portland | Haifa |
| Oregon Health & Science University Hospital | Rambam Health Care Campus | |
| Doernbecher Children’s Hospital |
Fig. 1.
Annual ToxIC cases
Demographics
Females comprised 4,234 (49.2 %) cases, compared to 4,100 (47.7 %) males. Gender was not reported in 3.1 % of cases. Fifty-six (1.3 %) were pregnant, 0.6 % of all cases. Adults between the ages of 19 and 65 accounted for a majority (63.4 %) of reported cases. Adolescents (13 to 18 years) were the next most frequent, making up 14.9 %. Additional demographic data are summarized in Table 2.
Table 2.
Demographics of Registry cases
| N (%) | |
|---|---|
| Male | 4,100 (47.7) |
| Female | 4,234 (49.2) |
| Pregnant | 56 (0.6) |
| Gender unspecified | 264 (3.1) |
| Age (years) | |
| <2 | 324 (3.8) |
| 2–6 | 435 (5.1) |
| 7–12 | 183 (2.1) |
| 13–18 | 1,283 (14.9) |
| 19–65 | 5,455 (63.4) |
| 66–89 | 453 (5.3) |
| >89 | 33 (0.4) |
| Unspecified | 432 (5.0) |
Source of Referrals and Primary Reason for Encounter
Hospital emergency departments were the most frequent source of referral, accounting for 5,133 (59.7 %) of Registry cases (Table 3). Inpatient services and patients transferred directly from other facilities were the next most common source of consultation. There were 384 outpatient referrals, accounting for 4.5 %. As seen in Table 4, exposures to pharmaceuticals, both intentional and unintentional, were the reason for referral in half of all cases. Drug abuse, encompassing illicit, prescription, and over-the-counter agents, was the next most frequent. Collectively, drug abuse was responsible for 11.7 % of cases. Adverse drug reactions (ADRs), defined in the Registry as undesirable effects of therapeutic drug use, were the reason for 4.8 %.
Table 3.
Referral sources
| N (%) | |
|---|---|
| Emergency department (ED) | 5,133 (59.7) |
| Admitting service (inpatient) | 1,433 (16.7) |
| Outside hospital transfer | 629 (7.3) |
| Request from other hospital service (non-ED) | 413 (4.8) |
| Primary care or outpatient physician | 384 (4.5) |
| Unspecified | 264 (3.1) |
| Self-referral | 241 (2.8) |
| Employer, workers’ compensation, etc. | 55 (0.6) |
| Poison control center | 46 (0.5) |
Table 4.
Reasons for medical toxicology consultation
| N (%) | |
|---|---|
| Intentional exposure—pharmaceutical | 3,445 (40.1) |
| Unintentional exposure—pharmaceutical | 851 (9.9) |
| Drug abuse—illicit drug | 692 (8.0) |
| Adverse drug reaction (ADR, undesirable effect of therapeutic drug use) | 412 (4.8) |
| Unintentional exposure—nonpharmaceutical | 410 (4.8) |
| Not documented | 361 (4.2) |
| Intentional exposure—nonpharmaceutical | 351 (4.1) |
| Unidentified agent | 272 (3.2) |
| Drug abuse—prescription drug | 266 (3.1) |
| Organ system dysfunction | 256 (3.0) |
| Withdrawal—ethanol | 186 (2.2) |
| Interpretation of toxicology lab data | 154 (1.8) |
| Alcohol (ethanol) abuse | 148 (1.7) |
| Envenomation—snake | 147 (1.7) |
| Environmental evaluation | 143 (1.7) |
| Withdrawal—opioid | 117 (1.4) |
| Adverse drug event (ADE, medication error resulting in harm) | 107 (1.2) |
| Occupational evaluation | 91 (1.1) |
| Withdrawal—sedative/hypnotic | 52 (0.6) |
| Drug abuse—OTC drug | 50 (0.6) |
| Envenomation—spider | 43 (0.5) |
| Envenomation—scorpion | 16 (0.2) |
| Withdrawal—other | 13 (0.2) |
| Envenomation—other | 6 (0.1) |
| Marine/fish poisoning | 6 (0.1) |
| Withdrawal—cocaine/amphetamine | 3 (0.1) |
Agent Classes
A total of 11,279 individual agents were listed in case entries. The distribution of these agents among the 40 different substance classes predefined in the Registry is shown in Table 5. Exposure to more than one agent was reported in 27.9 % of cases. Nonopioid analgesics, sedative-hypnotic agents including muscle relaxants, and opioids were the most common categories comprising over a third of all reported agents. Overall, exposures to pharmaceutical products including prescription and nonprescription drugs were reported in 80.9 % of cases.
Table 5.
Agent classes involved in consultation
| N (%) | |
|---|---|
| Analgesic (nonopioid) | 1,490 (13.2) |
| Sedative-hypnotic/muscle relaxant | 1,383 (12.26) |
| Opioid | 1,250 (11.1) |
| Antidepressant | 1,056 (9.4) |
| Ethanol | 737 (6.5) |
| Sympathomimetic | 702 (6.2) |
| Cardiovascular | 687 (6.1) |
| Antipsychotic | 626 (5.6) |
| Anticholinergic/antihistamine | 617 (5.5) |
| Anticonvulsant | 408 (3.6) |
| Psychoactive | 302 (2.7) |
| Envenomation | 188 (1.7) |
| Diabetic medications | 181 (1.6) |
| Lithium | 166 (1.5) |
| Metals | 154 (1.4) |
| Cough and cold products | 134 (1.2) |
| Gases/irritants/vapors/dusts | 126 (1.1) |
| Herbal products/dietary supplements | 119 (1.1) |
| Antimicrobial | 113 (1.0) |
| Household product | 113 (1.0) |
| Toxic alcohol | 95 (0.8) |
| Caustic | 88 (0.8) |
| Unknown agent | 88 (0.8) |
| Hydrocarbon | 84 (0.8) |
| Plants and fungi | 71 (0.6) |
| Anticoagulant | 58 (0.5) |
| Gastrointestinal agents | 34 (0.3) |
| Endocrine | 34 (0.3) |
| Other pharmaceutical product | 30 (0.3) |
| Insecticide | 27 (0.2) |
| Chemotherapeutic/immunological | 23 (0.2) |
| Parkinson’s disease agents | 19 (0.2) |
| Rodenticide | 15 (0.1) |
| Other nonpharmaceutical product | 14 (0.1) |
| Herbicides | 11 (0.1) |
| Anesthetics | 11 (0.1) |
| Pulmonary | 7 (0.1) |
| WMD/riot agent/radiological | 3 (0.0) |
| Ingested foreign object | 3 (0.0) |
| Fungicide | 1 (0.0) |
Percentages are out of the total number of reported agents (11,279). Of registry cases, 27.9 % reported exposure to multiple agents
WMD weapons of mass destruction
Signs and Symptoms
At least one clinical sign or symptom was reported in 6,116 (71.1 %) cases. These are summarized and organized by clinical syndrome or organ system in Table 6. Neurological signs or symptoms were described most frequently, with coma or CNS depression found in 26 % of all cases. This finding is consistent with the sedative-hypnotic toxidrome being most common among the syndromes. Agitation and delirium were also common, with each being reported in 9.2 % of cases. Among vital sign abnormalities, significant tachycardia (defined in the Registry as a heart rate of greater than 140 beats per minute) was the most frequent, described in 9.9 % of cases. All other categories of symptoms or findings were documented in less than 7 % of cases.
Table 6.
Clinical signs and symptoms
| N (%) | |
|---|---|
| Toxidrome | |
| Sedative-hypnotic | 589 (6.9) |
| Anticholinergic | 401 (4.7) |
| Opioid | 259 (3.0) |
| Sympathomimetic | 167 (1.9) |
| Serotonin syndrome | 151 (1.8) |
| Sympatholytic | 26 (0.3) |
| Overlap syndromes (chronic fatigue, multiple chemical sensitivity, etc.) | 13 (0.2) |
| Neuroleptic malignant syndrome | 11 (0.1) |
| Cholinergic | 6 (0.1) |
| Major vital sign abnormalities | |
| Tachycardia (HR > 140) | 848 (9.9) |
| Hypotension (systolic BP < 80 mmHg) | 508 (5.9) |
| Bradycardia (HR < 50) | 286 (3.3) |
| Hypertension (systolic BP > 200 mmHg or diastolic BP > 120 mmHg) | 180 (2.1) |
| Bradypnea (RR < 10) | 151 (1.8) |
| Hyperthermia (temp > 105 °F) | 28 (0.3) |
| Cardiovascular | |
| Prolonged QTc (≥500 ms) | 289 (3.3) |
| Prolonged QRS (≥120 ms) | 165 (1.9) |
| Ventricular dysrhythmia | 71 (0.8) |
| Atrioventricular block (>1st degree) | 44 (0.5) |
| Pulmonary | |
| Respiratory depression | 483 (5.6) |
| Aspiration pneumonia | 151 (1.8) |
| Acute lung injury/ARDS | 63 (0.7) |
| Asthma/reactive airway disease | 39 (0.5) |
| Neurological | |
| Coma/CNS depression | 2,233 (26.0) |
| Delirium | 795 (9.2) |
| Agitation | 795 (9.2) |
| Hyperreflexia/myoclonus/tremor | 533 (6.2) |
| Seizures | 337 (3.9) |
| Hallucinations | 218 (2.5) |
| Dystonia/rigidity/extrapyramidal symptoms | 109 (1.3) |
| Weakness/paralysis | 78 (0.9) |
| Numbness/paresthesia | 62 (0.7) |
| Peripheral neuropathy (objective) | 15 (0.2) |
| Metabolic | |
| Metabolic acidosis (pH < 7.2) | 350 (4.1) |
| Elevated anion gap (>20) | 254 (3.0) |
| Hypoglycemia (glucose < 50 mg/dL) | 146 (1.7) |
| Elevated osmole gap (>20) | 42 (0.5) |
| Gastrointestinal/hepatic | |
| Hepatotoxicity (AST ≥ 1,000 IU/L) | 312 (3.6) |
| Gastrointestinal bleeding | 58 (0.7) |
| Pancreatitis | 35 (0.4) |
| Corrosive injury | 25 (0.3) |
| Hematological | |
| Coagulopathy (PT > 15 s) | 205 (2.4) |
| Thrombocytopenia (platelets < 100 K/μL) | 74 (0.9) |
| Leukocytosis (WBC > 20 K/μL) | 74 (0.9) |
| Hemolysis (Hgb < 10 g/dL) | 20 (0.2) |
| Pancytopenia | 12 (0.1) |
| Methemoglobinemia (MetHgb ≥ 2 %) | 9 (0.1) |
| Renal/musculoskeletal | |
| Acute kidney injury (creatinine > 2.0 mg/dL) | 312 (3.6) |
| Rhabdomyolysis (CPK > 1,000 IU/L) | 245 (2.8) |
| Dermatological | |
| Rash | 101 (1.2) |
| Blisters/bullae | 32 (0.7) |
| Necrosis | 21 (0.2) |
| Angioedema | 12 (0.1) |
CPK creatine phosphokinase, Hgb hemoglobin, WBC white blood cells, PT prothrombin time, AST aspartate aminotransferase, CNS central nervous system, ARDS acute respiratory distress syndrome, RR respiratory rate, BP blood pressure, HR heart rate
Fatalities
There were 93 (1.1 %) fatalities (Table 7) with 47.3 % being female. Their average age was 46.5 years. Nonopioid analgesics were the agent class most frequently associated with fatalities. At least one analgesic drug was implicated in 22.6 % of the fatalities. Opioids were the second most frequently implicated, comprising 17.2 % of the deaths. Cardiovascular drugs comprised the third most common class in fatal cases (15.1 %).
Table 7.
Fatalities
| Case | Age/gender | Agents involved | Symptoms and clinical findings | Treatment |
|---|---|---|---|---|
| 1 | 43 M | Acetaminophen, hydrocodone, carisoprodol | HT, CNS, HPT, CPT, AKI | NAC, NaHCO3 |
| 2 | 61 M | Metformin | HT, ALI, RD, CNS, HGY, MA, PNC, CPT | Continuous renal replacement |
| 3 | 66 M | Metformin | MA, AG | Carnitine, hemodialysis |
| 4 | 65 F | None listed | AG, CPT | |
| 5 | 86 M | Citalopram, quetiapine, dextromethorphan | TC, AP, DLM, EPS, RFX | Antipsychotics, benzodiazepines |
| 6 | 74 F | Acetaminophen | MA, AKI | None listed |
| 7 | 85 M | Acetaminophen | HT, TC, RAD, HPT, PLT | NAC, albuterol, intubation |
| 8 | 36 M | Kratom, lamotrigine, paroxetine, quetiapine | HTN, VD, QRS, RD, CNS, SZ, RBM | Atropine, lipid resuscitation, naloxone, NaHCO3, neuromuscular blocker, opioids, vasopressors, CPR, intubation, IV fluids |
| 9 | 23 M | None listed | HT, TC, BP, VD, RD, MA, AG, HPT, AKI, RBD | Atropine, naloxone, NaHCO3, vasopressors, gastric lavage, charcoal, continuous renal replacement, CPR, intubation, IV fluids |
| 10 | 19 F | Sympathomimetic unspecified | HT, TC, HYT, VD, AGT, SZ, MA | Calcium, lipid resuscitation, NaHCO3, antiarrhythmics, benzodiazepines, neuromuscular blockers, vasopressors, CPR, intubation, IV fluids |
| 11 | 29 M | Methamphetamine | HT, TC, VD, AGT, CNS, DLM, HYS, CPT, RBD | Factor replacement, lipid resuscitation, methylene blue, vasopressors, continuous renal replacement, CPR, intubation, transfusion |
| 12 | 47 M | Cocaine, heroin, methadone | TC, HYT, AGT, HCN, AG, PNC, AKI | Benzodiazepines, neuromuscular blockers, opioids, intubation, IV fluids |
| 13 | 49 F | Methamphetamine | HT, RD, CNS, MA, HPT, CPT, AKI, RBD | None listed |
| 14 | 38 M | Heroin | BP, ALI, CNS, MA, AG, HPT, CPT, PLT, WBC, AKI, RBD | NAC, naloxone, NaHCO3, neuromuscular blockers, vasopressors, CPR, intubations, IV fluids |
| 15 | 65 M | Diphenhydramine | RD, AGT, CNS, DLM, EPS, RFX | Flumazenil, benzodiazepines, opioids, intubation |
| 16 | 26 M | Oxycodone | HT, TC, CNS, MA, HPT | NAC, intubation, IV fluids |
| 17 | 40 M | Methadone | HT, BC, MA | Vasopressors, CPR, intubation, IV fluids |
| 18 | 86 M | Hydrochloric acid | MA, AG, CRV, NEC | None listed |
| 19 | 43 F | Heroin, carisoprodol | HT, TC, CNS, MA, AG | Lipid resuscitation, naloxone, vasopressors, CPR, intubation, IV fluids |
| 20 | 17 F | Methanol | HT, TC, RD, CNS, SZ, MA, AG | Fomepizole, continuous renal replacement, ECMO |
| 21 | 17 F | Cannabinoid-synthetic | None listed | Vasopressors, CPR, intubation, IV fluids |
| 22 | 56 M | Alkyl nitrite | HT, CNS, MA | Methylene blue, vasopressors, cardioversion, intubation, IV fluids |
| 23 | 61 M | Cyanide | BC, BP, VD, RD, CNS | CPR, intubation |
| 24 | 19 M | Cyanide | HT, TC, QRD, RD, CNS, MA, AG | Hydroxocobalamin, NaHCO3, vasopressors, intubation, IV fluids |
| 25 | 15 M | LSD | HT, BC, VD, RD, CNS, SZ, MA | NaHCO3, vasopressors, CPR, intubation |
| 26 | 21 M | Metformin | HT, TC, CNS, MA, AG | Fomepizole, NaHCO3, hemodialysis, intubation |
| 27 | (>89) F | Atenolol | BC, AVB, AKI | Atropine, glucagon, |
| 28 | 31 F | Aripiprazole, acetaminophen, prazosin, cyclobenzaprine | HT, CNS, MA, AG, HPT | NAC |
| 29 | 69 M | Amlodipine, acetaminophen, oxycodone | HT, TC, AP, RD, CNS, HGY, MA, AG, HPT, PNC, CPT, PLT, WBC, AKI, RBD | NAC, anticonvulsants, benzodiazepines, glucose, vasopressors, hemodialysis, intubation, IV fluids |
| 30 | 17 M | Aspirin | TC, VD, RD, AGT, CNS, MA, CPT | NaHCO3, antiarrhythmics, anticonvulsants, benzodiazepines, neuromuscular blockers, vasopressors, urinary alkalinization, cardioversion, CPR, intubation, IV fluids |
| 31 | 55 M | Verapamil, diltiazem | HT, BC, VD, QRS, QTc, AVB, AP, CNS, MA, HYS, CPT, PLT, WBC, AKI | Calcium, insulin-euglycemic therapy, methylene blue, NaHCO3, benzodiazepines, neuromuscular blockers, vasopressors, charcoal, continuous renal replacement, CPR, intubation, IV fluids, pacemaker, transfusion |
| 32 | 22 M | Methamphetamine | TC, QTc, AGT, DLM | None listed |
| 33 | (Unknown) F | Acetaminophen | ALI, MA, AG, HPT, AKI | NAC |
| 34 | (Unknown) F* | Acetaminophen | HT, HGY, MA, AG, HPT, GIB, PNC, CPT, AKI | NAC, glucose, steroids, vasopressors, hemodialysis, continuous renal replacement, CPR, intubation, IV fluids |
| 35 | (Unknown) F | Oxycodone | HT, BP, QRS, AP, RD, CNS, MA, AG, HPT, CPT, AKI | NAC, naloxone, benzodiazepines, neuromuscular blockers, vasopressors, CPR, intubation, IV fluids |
| 36 | 45 M | Lisinopril, amlodipine | HT, ALI, HGY | Insulin-euglycemic therapy, lipid resuscitation, glucose, vasopressors, intubation, IV fluids |
| 37 | 27 F | Acetaminophen | HPT | NAC |
| 38 | 52 F | Acetaminophen, lorazepam | HT, CNS, MA, AG, HPT, PLT, PCT, AKI, | NAC, vasopressors, hemodialysis, intubation, IV fluids |
| 39 | 57 F | Acetaminophen | HT, TC, AP, CNS, HGY, RSH, BB | NAC, albuterol, glucose, vasopressors, intubation, IV fluids |
| 40 | 61 F | Unknown agent | HT, BC, VD, CNS | Vasopressors, CPR, intubation |
| 41 | 70 M | Hydrocodone | HT, HGY, MA, AKI | NAC, vasopressors, CPR, intubation |
| 42 | 58 F | Acetaminophen, hydrocodone, morphine | BP, CNS, HPT, CPT | NAC, naloxone, vasopressors |
| 43 | 65 F | Amlodipine, losartan, lorazepam | HT, CNS | Calcium, glucagon, insulin-euglycemic therapy, lipid resuscitation, vasopressors, intubation, IV fluids |
| 44 | 46 M | Ethylene glycol | CNS, MA, AG, OG, WBC, AKI, RBM | Fomepizole, vasopressors |
| 45 | 48 F | Diltiazem | HT, BC, AP, ALI, CNS, MA, AKI | Atropine, calcium, flumazenil, insulin-euglycemic therapy, lipid resuscitation, albuterol, benzodiazepines, neuromuscular blockers, vasopressors, glucose, CPR, intubation, IV fluids, pacemaker |
| 46 | 29 F | Quetiapine, desvenlafaxine, escitlopram, bupropion | HT, QRS, CNS, MA, AG, AKI | Lipid resuscitation, vasopressors, CPR, intubation, IV fluids |
| 47 | 25 F | Pseudoephedrine | TC, CNS | Benzodiazepines |
| 48 | 70 M | Amlodipine, metformin | HT, CNS, MA, AKI | Calcium, insulin-euglycemic therapy, vasopressors, IV fluids |
| 49 | 33 F | Acetaminophen, ibuprofen, diphenhydramine | RD, AG, HPT, CPT, AKI | Flumazenil, NAC, physostigmine, benzodiazepines, hemodialysis, intubation, IV fluids |
| 50 | 59 F | Acetaminophen | HT, TC, VD, CNS, RFX, HGY, MA, AG, HPT, GIB, CPT, PLT, AKI | Factor replacement, NAC, naloxone, NaHCO3, benzodiazepines, glucose, vasopressors, continuous renal replacement, intubation, IV fluids |
| 51 | 52 M | Lorazepam | AP, ALI, RD, CNS, RFX | None listed |
| 52 | 37 F | Hydrocodone, acetaminophen | VD, CNS, HPT, GIB, AKI, RBM | NAC, benzodiazepines, neuromuscular blockers, vasopressors |
| 53 | 22 F | Diphenhydramine, hydroxyzine, quetiapine, citalopram, sertraline | HT, BC, QRS, RD, CNS, MA, RBM | None listed |
| 54 | 49 M | Acetaminophen, ethanol | CNS, MA, AG, HPT, CPT, AKI | NaHCO3, vasopressors, continuous renal replacement, intubation, IV fluids |
| 55 | 50 F | Verapamil, metoprolol | HT, BC, VD, AVB, RD, CNS, MA, CPT, AKI | Calcium, insulin-euglycemic therapy, lipid resuscitation, methylene blue, NaHCO3, benzodiazepines, neuromuscular blockers, vasopressors, CPR, intubation, IV fluids, pacemaker |
| 56 | 56 F | None listed | None listed | None listed |
| 57 | 69 F | Cyanide | HT, TC, RD, CNS, MA, BB | Hydroxocobalamin, vasopressors, intubation, IV fluids |
| 58 | 46 M | None listed | HT, TC, BC, ALI, RD, CNS, HGY, MA, AG, HPT, GIB, HYS, CPT, PLT | NAC, vasopressors, hemodialysis, continuous renal replacement, cardioversion, CPR, intubation, IV fluids |
| 59 | 57 F | None listed | VD, CN, SZ, MA, AG, HPT, PCT, AKI, RBM | None listed |
| 60 | 45 F | None listed | HT, CNS DLM, RFX, WKN, HGY, MA, HPT | None listed |
| 61 | 61 M | Metformin | HT, CNS, DLM, HGY, MA, AG, CPT, WBC, AKI | NaHCO3, steroids, vasopressors, continuous renal replacement, intubation, transfusion |
| 62 | 56 M | None listed | MA, HPT | NAC |
| 63 | 54 M | Opioid unspecified | HT, AP, CNS, MA, HPT, CPT, AKI, RBM, BB | Insulin-euglycemic therapy, NAC, naloxone, benzodiazepines, opioids, vasopressors, intubation, IV fluids |
| 64 | 74 M | Rivaroxaban | HT, HPT, AKI | NAC, vasopressors, intubation, IV fluids |
| 65 | 52 F | Ethanol | HT, CNS | CPR, intubation, IV fluids |
| 66 | 47 F | Carisoprodol | CNS, RFX | Benzodiazepines |
| 67 | 66 M | Fentanyl | CNS | Naloxone |
| 68 | 35 F | None listed | HTN, RD, CNS, MA, CPT, AKI | Hemodialysis, CPR, intubation, IV fluids, transfusion |
| 69 | 43 M | Gabapentin, ethanol | SZ | Vasopressors, CPR, intubation, IV fluids |
| 70 | 33 M | NaHCO3 | HT, RD, SZ, AKI | Vasodilators, vasopressors, intubation, IV fluids |
| 71 | 28 M | Heroin | BC, BP, VD, QTc, AP, ALI, CNS, MA, AG, CPT, AKI, RBM | Atropine, naloxone, NaHCO3, vasopressors, CPR, intubation, |
| 72 | 50 M | Methylene chloride | HT, BC, BP, RD, CNS, MA, AG, RBM | Vasopressors, intubation, IV fluids |
| 73 | 65 F | Acetaminophen, hydrocodone | CNS, AG, HPT, CPT | NAC, intubation, IV fluids |
| 74 | 17 F | Ethanol | RD, CNS | IV fluids |
| 75 | 46 M | Aspirin | TC, AGT, MA, AG, GIB, AKI | NaHCO3 |
| 76 | 83 F | Digoxin, amlodipine | WKN | Digoxin Fab |
| 77 | 76 M | Digoxin | VD, AVB | Digoxin Fab |
| 78 | (>89) M | Digoxin | BC, AVB | Digoxin Fab |
| 79 | (Unknown) M | None listed | HGY | Glucose |
| 80 | 16 F | None listed | HT, TC, BC, RD, CNS, SZ, MA, AG, HPT, CPT, WBC, AKI | NaHCO3, vasopressors, continuous renal replacement, CPR, ECMO, intubation, IV fluids |
| 81 | 6 M | None listed | TC, ALI, CNS, HPT | None listed |
| 82 | (Unknown) M | None listed | BC | None listed |
| 83 | 39 F | Unknown agent | HT, VD, QTc, AP, MA, AKI | NaHCO3, vasopressors |
| 84 | 49 F | Metoprolol | HT, NC, BP, ALI, RD, CNS, MA, GIB, CPT | Calcium, insulin-euglycemic therapy, lipid resuscitation, NaHCO3, neuromuscular blockers, vasopressors, CPR, intubation, IV fluids, pacemaker, transfusion |
| 85 | 58 F | Acetaminophen | HT, TC, QRS, AGT, CNS, HGY, MA, AG, HPT, PNC, CPT, PLT | NAC, glucose, vasopressors, continuous renal replacement, intubation, IV fluids, transfusion |
| 86 | 39 F | None listed | AGT, CNS, HGY, MA, AG, OG, PLT, WBC, AKI | Fomepizole, NAC |
| 87 | 18 M | None listed | None listed | None listed |
| 88 | 69 F | Acetaminophen | HT, AP, CNS, MA, AG, RBM | Lipid resuscitation |
| 89 | 59 M | Ethanol | HT, TC, RD, MA, AG, HPT, GIB, PNC, HYS, AKI | Antiarrhythmics, benzodiazepines, hemodialysis, intubation |
| 90 | 26 F | None listed | HT, BC, BP, CNS | Naloxone, vasopressors, cardioversion, CPR, intubation |
| 91 | 55 M | Acetaminophen, ethanol | HT, CNS, HGY, MA, AG, OG, HPT, CPT, AKI | Fomepizole, NAC, thiamine, vasopressors, continuous renal replacement, CPR, intubation, IV fluids |
| 92 | 34 F | None listed | None listed | None listed |
| 93 | 61 M | None listed | RD | None listed |
TC tachycardia, HT hypotension, BC bradycardia, HTN hypertension, BP bradypnea, HYT hyperthermia, QTc QTc prolongation, QRS QRS prolongation, VD ventricular dysrhythmia, AVB AV block, RD respiratory depression, AP aspiration pneumonia, ALI acute lung injury/ARDS, RAD asthma/reactive airway disease CNS coma/CNS depression, DLM delirium, AGT agitation, RFX hyperreflexia/tremor, SZ seizures, HCN hallucinations, EPS dystonia, WKN weakness/paralysis, PST paresthesia, NP neuropathy, MA metabolic acidosis, AG anion gap, HGY hypoglycemia, OG osmole gap, HPT hepatoxicity, GIB GI bleeding, PNC pancreatitis, CRV corrosive injury, CPT coagulopathy, PLT thrombocytopenia, WBC leukocytosis, HYS hemolysis, PCT pancytopenia, MET methemoglobinemia, AKI acute kidney injury, RBM rhabdomyolysis, RSH rash, BB blisters/bullae, NEC dermal necrosis, AE angioedema
Individual Agent Classes
Tables 8 through 40 summarize the frequency of specific drugs enumerated by class.
Table 9.
Sedative-hypnotics/muscle relaxants
| N (%) | |
|---|---|
| Benzodiazepines | 802 (58.0) |
| Clonazepam | 254 (18.3) |
| Alprazolam | 242 (17.5) |
| Lorazepam | 146 (10.6) |
| Diazepam | 76 (5.5) |
| Benzodiazepine unspecified | 47 (3.4) |
| Temazepam | 17 (1.2) |
| Midazolam | 5 (0.4) |
| Miscellaneousa | 15 (1.1) |
| Muscle relaxants | 271 (19.6) |
| Cyclobenzaprine | 97 (7.0) |
| Carisoprodol | 82 (5.9) |
| Baclofen | 63 (4.6) |
| Tizanidine | 20 (1.4) |
| Methocarbamol | 6 (0.4) |
| Miscellaneousb | 3 (0.2) |
| Barbiturates | 51 (3.7) |
| Butalbital | 33 (2.4) |
| Phenobarbital | 12 (0.9) |
| Miscellaneousc | 6 (0.4) |
| Nonbenzodiazepine GABA agonists (“Z-drugs”) | 121 (8.7) |
| Zolpidem | 112 (8.1) |
| Eszopiclone | 7 (0.5) |
| Miscellaneousd | 2 (0.1) |
| Other sedatives | 138 (10.0) |
| Gabapentin | 88 (6.3) |
| Buspirone | 21 (1.5) |
| Pregabalin | 15 (1.1) |
| Miscellaneouse | 8 (0.5) |
| Sedative-hypnotic unspecified | 6 (0.4) |
aIncludes chlordiazepoxide, oxazepam, flurazepam, nitrazepam, chlorazepate, triazolam, and brotizolam
bIncludes chlorzoxazone, and metaxalone
cIncludes butabarbital, pentobarbital, and barbiturate unspecified
dIncludes zaleplon, and zopiclone
eIncludes phenibut, ramelteon, meprobamate, propofol, and chloral hydrate
Table 10.
Opioids
| N (%) | |
|---|---|
| Heroin | 322 (25.7) |
| Oxycodone | 269 (21.5) |
| Methadone | 169 (13.5) |
| Hydrocodone | 132 (10.6) |
| Tramadol | 85 (6.8) |
| Buprenorphine | 70 (5.6) |
| Morphine | 67 (5.4) |
| Fentanyl | 39 (3.1) |
| Hydromorphone | 35 (2.8) |
| Codeine | 30 (2.4) |
| Opioid unspecified | 8 (0.6) |
| Oxymorphone | 5 (0.4) |
| Tapentadol | 5 (0.4) |
| Miscellaneousa | 14 (1.1) |
aIncludes loperamide, meperidine, naloxone, naltrexone, propoxyphene, diphenoxylate, and opium
Table 11.
Antidepressants
| N (%) | |
|---|---|
| Selective serotonin reuptake inhibitors (SSRIs) | 387 (36.6) |
| Citalopram | 143 (13.5) |
| Sertraline | 101 (9.6) |
| Fluoxetine | 71 (6.7) |
| Escitalopram | 48 (4.5) |
| Paroxetine | 24 (2.3) |
| Tricyclic antidepressants (TCAs) | 161 (15.2) |
| Amitriptyline | 115 (10.9) |
| Doxepin | 24 (2.3) |
| Nortriptyline | 13 (1.2) |
| Miscellaneous† | 9 (0.9) |
| Serotonin-norepinephrine reuptake inhibitors (SNRIs) | 107 (10.1) |
| Venlafaxine | 67 (6.3) |
| Duloxetine | 24 (2.3) |
| Desvenlafaxine | 9 (0.9) |
| Fluvoxamine | 7 (0.7) |
| Other antidepressants | 401 (38.0) |
| Bupropion | 198 (18.6) |
| Trazodone | 142 (13.4) |
| Mirtazapine | 41 (3.9) |
| Vilazodone | 6 (0.6) |
| Antidepressant unspecified | 5 (0.5) |
| Miscellaneousb | 9 (0.9) |
aIncludes imipramine, desipramine, and clomipramine
bIncludes agomelatine, dosulepin, sibutramine, tranylcypromine, and phenelzine
Table 12.
Sympathomimetics
| N (%) | |
|---|---|
| Cocaine | 313 (44.6) |
| Methamphetamine | 115 (16.4) |
| Amphetamine | 61 (8.7) |
| Methylphenidate | 47 (6.7) |
| Methylenedioxy-N-methamphetamine | 32 (4.6) |
| Dextroamphetamine | 30 (4.3) |
| Sympathomimetic unspecified | 23 (3.3) |
| Mephedrone | 16 (2.3) |
| Dexmethylphenidate | 12 (1.7) |
| Atomoxetine | 10 (1.4) |
| Pseudoephedrine | 8 (1.1) |
| Phenylephrine | 7 (1.0) |
| 25I-NBOMe | 5 (0.7) |
| Miscellaneousa | 23 (3.3) |
aIncludes lisdexamfetamine, cathinone, phentermine, tetrahydrozoline, α-pyrrolidinopentiophenone (α-PVP), epinephrine, 2C-T-7, ephedrine, phendimetrazine, phenylpropanolamine, propylhexedrine, and methylone
Table 13.
Cardiovascular agents
| N (%) | |
|---|---|
| Beta blockers | 185 (26.9) |
| Metoprolol | 69 (10.0) |
| Propranolol | 41 (6.0) |
| Atenolol | 37 (5.4) |
| Carvedilol | 13 (1.9) |
| Labetalol | 13 (1.9) |
| Nebivolol | 6 (0.9) |
| Miscellaneousa | 6 (0.9) |
| Calcium channel antagonists | 116 (16.9) |
| Amlodipine | 61 (8.9) |
| Diltiazem | 24 (3.5) |
| Verapamil | 19 (2.8) |
| Nifedipine | 10 (1.5) |
| Miscellaneousb | 2 (0.3) |
| ACE inhibitors | 64 (9.3) |
| Lisinopril | 53 (7.7) |
| Enalapril | 6 (0.9) |
| Miscellaneousc | 6 (0.9) |
| Angiotensin receptor blockers | 10 (1.5) |
| Losartan | 6 (0.9) |
| Miscellaneousd | 4 (0.6) |
| Cardiac glycosides | 53 (7.7) |
| Digoxin | 52 (7.6) |
| Digitoxin | 1 (0.1) |
| Sympatholytics | 154 (22.4) |
| Clonidine | 129 (18.8) |
| Guanfacine | 21 (3.1) |
| Xylazine | 4 (0.6) |
| Diuretics | 32 (4.7) |
| Hydrochlorothiazide | 10 (1.5) |
| Furosemide | 9 (1.3) |
| Miscellaneouse | 13 (1.9) |
| Other antihypertensives and vasodilators | 44 (6.4) |
| Prazosin | 13 (1.9) |
| Cilostazol | 11 (1.6) |
| Miscellaneousf | 20 (2.9) |
| Antidysrhythmics | 11 (1.6) |
| Amiodarone | 5 (0.7) |
| Miscellaneousg | 6 (0.9) |
| Other cardiovascular agents | 12 (1.7) |
| Atorvastatin | 5 (0.7) |
| Miscellaneoush | 7 (1.0) |
aIncludes nadolol, bisoprolol, and pindolol
bIncludes aranidipine and felodipine
cIncludes benazepril, perindopril, quinapril
dIncludes valsartan, and candesartan
eIncludes acetazolamide, spironolactone, pamabrom, chlorthalidone, and torsemide
fIncludes alkyl nitrite, isosorbide, antihypertensive unspecified, doxazosin, nitroglycerin, terazosin, amyl nitrite, hydralazine, and tamsulosin
gIncludes propafenone, flecainide, and mexiletine
hIncludes cardiovascular agent unspecified, lovastatin, pravastatin, and ranolazine
Table 14.
Antipsychotics
| N (%) | |
|---|---|
| Quetiapine | 304 (48.5) |
| Risperidone | 94 (15.0) |
| Olanzapine | 60 (9.6) |
| Aripiprazole | 54 (8.6) |
| Haloperidol | 42 (6.7) |
| Ziprasidone | 18 (2.9) |
| Clozapine | 13 (2.1) |
| Chlorpromazine | 11 (1.8) |
| Miscellaneousa | 30 (4.8) |
aIncludes lurasidone, paliperidone, perphenazine, asenapine, fluphenazine, loxapine, droperidol, iloperidone, prochlorperazine, zuclopenthixol, and antipsychotic unspecified
Table 15.
Anticholinergics and antihistamines
| N (%) | |
|---|---|
| Diphenhydramine | 334 (54.1) |
| Hydroxyzine | 72 (11.7) |
| Promethazine | 36 (5.8) |
| Benztropine | 34 (5.5) |
| Chlorpheniramine | 32 (5.2) |
| Doxylamine | 20 (3.2) |
| Antihistamine unspecified | 16 (2.6) |
| Loratidine | 13 (2.1) |
| Dicyclomine | 11 (1.8) |
| Meclizine | 10 (1.6) |
| Cyproheptadine | 7 (1.1) |
| Hyoscyamine | 6 (1.0) |
| Pyrilamine | 6 (1.0) |
| Cetirizine | 5 (0.8) |
| Scopolamine | 5 (0.8) |
| Miscellaneous† | 11 (1.8) |
aIncludes anticholinergic unspecified, dimenhydrinate, oxybutynin, atropine, brompheniramine, chlorcyclizine, and trihexyphenidyl
Table 16.
Anticonvulsants and mood stabilizers
| N (%) | |
|---|---|
| Valproic acid | 132 (32.4) |
| Lamotrigine | 82 (20.1) |
| Phenytoin | 62 (15.2) |
| Carbamazepine | 59 (14.5) |
| Topiramate | 26 (6.4) |
| Oxcarbazepine | 22 (5.4) |
| Levetiracetam | 12 (2.9) |
| Lacosamide | 5 (1.2) |
| Miscellaneousa | 8 (2.0) |
| Lithiumb | 166 (100.0) |
aIncludes primidone, zonisamide, clobazam, and ethosuximide
bLithium is considered a separate category
Table 17.
Psychoactives
| N (%) | |
|---|---|
| Marijuana | 123 (40.7) |
| Phencyclidine | 62 (20.5) |
| Cannabinoid—synthetic | 52 (17.2) |
| Gamma hydroxybutyrate (GHB) | 15 (5.0) |
| Lysergic acid diethylamide (LSD) | 15 (5.0) |
| Cannabinoid—nonsynthetic | 11 (3.6) |
| Nicotine | 7 (2.3) |
| Ketamine | 5 (1.7) |
| Miscellaneousa | 12 (4.0) |
aIncludes donepezil, disulfiram, hallucinogen unspecified, dimethyltryptamine (DMT), gamma butyrolactone, ibogaine, mescaline, and rotundine
Table 18.
Envenomations and marine poisonings
| N (%) | |
|---|---|
| Crotalus spp. | 66 (35.1) |
| Agkistrodon spp. | 39 (20.7) |
| Loxosceles spp. | 24 (12.8) |
| Unspecified snake | 12 (6.4) |
| Dendroaspis spp. | 9 (4.8) |
| Centuroides spp. | 8 (4.3) |
| Latrodectus spp. | 8 (4.3) |
| Unspecified envenomation | 5 (2.7) |
| Miscellaneousa | 17 (9.0) |
aIncludes unspecified scorpion, unspecified spider, Vipera palestinae, jellyfish, ciguatera poisoning, unspecified insect, hymenoptera, scombroid poisoning, Sistrurus spp., and stingray envenomation
Table 19.
Diabetic medications
| N (%) | |
|---|---|
| Metformin | 55 (30.4) |
| Insulin | 44 (24.3) |
| Glipizide | 31 (17.1) |
| Glyburide | 28 (15.5) |
| Glimepiride | 11 (6.1) |
| Miscellaneousa | 12 (6.6) |
aIncludes sitagliptin, sulfonylurea unspecified, pioglitazone, and linagliptin
Table 20.
Metals
| N (%) | |
|---|---|
| Lead | 35 (22.7) |
| Cobalt | 25 (16.2) |
| Mercury | 25 (16.2) |
| Chromium | 21 (13.6) |
| Iron | 19 (12.3) |
| Arsenic | 9 (5.8) |
| Copper | 6 (3.9) |
| Miscellaneousa | 13 (8.4) |
aIncludes manganese, aluminum, cadmium, cesium, molybdenum, nickel, selenium, tin, and titanium
Table 21.
Cough and cold products
| N (%) | |
|---|---|
| Dextromethorphan | 109 (81.3) |
| Cough and cold product unspecified | 20 (14.9) |
| Miscellaneousa | 5 (3.7) |
aIncludes guaifenesin and camphor
Table 22.
Gases, irritants, vapors, and dusts
| N (%) | |
|---|---|
| Carbon monoxide | 57 (45.2) |
| Cyanide | 12 (9.5) |
| Smoke | 7 (5.6) |
| Nitrogen oxides | 6 (4.8) |
| Hydrogen sulfide | 5 (4.0) |
| Petroleum vapors | 5 (4.0) |
| Unspecified gas | 5 (4.0) |
| Miscellaneousa | 29 (23.0) |
aIncludes dust, spray duster (canned air), asbestos, tungsten hexafluoride, boron hydride, acetonitrile, chlorine, radon, carbon dioxide, chloramine, fiberglass, liquefied petroleum gas, silica, and sulfur dioxide
Table 23.
Herbal products and dietary supplements
| N (%) | |
|---|---|
| Caffeine | 56 (47.1) |
| Melatonin | 15 (12.6) |
| Herbal product unspecified | 10 (8.4) |
| Menthol | 6 (5.0) |
| Multiple vitamin | 6 (5.0) |
| Miscellaneousa | 26 (21.8) |
aIncludes 1,3-dimethylamine, thymol, eucalyptus oil, Garcinia cambogia, calcium, prenatal vitamins, zinc, arginine, St. John’s Wort, tea tree oil, vitamin D, niacin, and garlic
Table 24.
Antimicrobials
| N (%) | |
|---|---|
| Antibiotics | 65 (57.5) |
| Trimethoprim/sulfamethoxazole | 14 (12.4) |
| Amoxicillin | 9 (8.0) |
| Metronidazole | 6 (5.3) |
| Penicillin | 5 (4.4) |
| Miscellaneousa | 31 (27.4) |
| Antivirals | 27 (23.9) |
| Emtricitabine | 6 (5.3) |
| Tenofovir | 6 (5.3) |
| Miscellaneousb | 15 (13.3) |
| Antifungalsc | 6 (5.3) |
| Other antimicrobials | 15 (13.2) |
| Levamisole | 5 (4.4) |
| Miscellaneousd | 10 (8.8) |
aIncludes cephalexin, dapsone, daptomycin, doxycycline, levofloxacin, linezolid, azithromycin, cefidinir, ceftriaxone, cefuroxime, isoniazid, meropenem, nitrofurantoin, vancomycin, cefepime, cefpodoxime, ciprofloxacin, and minocycline
bIncludes amantadine, efavirenz, lamivudine, oseltamivir, zidovudine, ribavirin, ritonavir, and valacyclovir
cIncludes fluconazole, terbinafine, griseofulvin, and itraconazole
dIncludes antimicrobial unspecified, chloroquine, benzoic acid, chlorhexidine, primaquine, an silver sulfadiazine
Table 25.
Household products
| N (%) | |
|---|---|
| Detergent pods | 33 (29.2) |
| Cleaning solutions and disinfectants | 25 (22.1) |
| Sodium hypochlorite (concentration < 6 %) | 21 (18.6) |
| Detergents | 17 (15.0) |
| Household product unspecified | 11 (9.7) |
| Miscellaneousa | 6 (5.3) |
aIncludes ammonia (concentration <10 %), paints, and talc
Table 26.
Alcohols and glycols
| N (%) | |
|---|---|
| Ethanola | 737 (100.0) |
| Nonethanol alcohols and glycols | |
| Ethylene glycol | 39 (41.0) |
| Isopropanol | 34 (35.8) |
| Methanol | 12 (12.6) |
| Miscellaneousb | 10 (10.5) |
aEthanol is considered a separate category
bIncludes glycol ethers, acetone, butanol, methyl ethyl ketone, and propylene glycol
Table 27.
Caustics
| N (%) | |
|---|---|
| Caustic Unspecified | 16 (18.0) |
| Sodium hypochlorite (unknown concentration) | 16 (18.0) |
| Sodium hydroxide | 12 (13.5) |
| Hydrochloric acid | 7 (7.9) |
| Potassium hydroxide | 7 (13.5) |
| Sulfuric acid | 5 (5.6) |
| Miscellaneousa | 27 (30.7) |
aIncludes acetic acid, boric acid, hydrogen peroxide (>10 %), sodium hypochlorite (>6 %), ammonium bifluoride, ammonium chloride, ammonium nitrate, formaldehyde, nitric acid, peroxyacetic acid, potassium permanganate, and zinc chloride
Table 28.
Hydrocarbons
| N (%) | |
|---|---|
| Hydrocarbon unspecified | 40 (47.6) |
| Carbon tetrachloride | 10 (11.9) |
| Toluene | 5 (6.0) |
| Gasoline | 4 (4.8) |
| Methylene chloride | 3 (3.6) |
| Miscellaneous (<3 cases)a | 22 (26.2) |
aIncludes methylene chloride, chlorofluorocarbons, difluoroethane, kerosene, methane, mineral oil, paraffin oil, tetrachloroethylene, vinyl chloride, mineral spirits (Stoddard solvent), naphthaline, pine oil, polychlorinated biphenyls (PCB), xylene, and trichloroethylene
Table 29.
Plants and fungi
| N (%) | |
|---|---|
| Mold | 28 (39.4) |
| Mushroom unspecified | 16 (22.5) |
| Nerium oleander | 5 (7.0) |
| Mushroom (Psilocybe spp.) | 4 (5.6) |
| Atropa belladonna (deadly nightshade) | 3 (4.2) |
| Miscellaneous (<3 cases)a | 15 (21.1) |
aIncludes Piper methysticum (kava), Dieffenbachia spp., Digitalis spp., Illicium spp. (star anise), Mentha pulegium (pennyroyal), Rhododendron spp., Ricinus communis (castor bean), Solanum dulcamara (bitter nightshade), Sophora spp. (kowhai), strychnine, valerian root, Phytolacca americana (pokeweed), Taxus spp. (yew), and Toxicodendron spp. (poison ivy)
Table 30.
Anticoagulants
| N (%) | |
|---|---|
| Warfarin | 46 (79.3) |
| Rivoroxaban | 4 (6.9) |
| Enoxaparin | 3 (5.2) |
| Miscellaneous (<3 cases)a | 5 (8.6) |
aIncludes unspecified anticoagulant, clopidogrel, and dabigatran
Table 31.
Gastrointestinal agents
| N (%) | |
|---|---|
| Ondansetron | 8 (22.9) |
| Ranitidine | 6 (17.1) |
| Metoclopramide | 5 (14.3) |
| Miscellaneousa | 15 (44.1) |
aIncludes bismuth subsalicylate, sodium bicarbonate, omeprazole, bisacodyl, docusate, pancrelipase, sennosides, sulfasalazine, famotidine, dexlansoprazole, esomeprazole, and cimetidine
Table 32.
Endocrine
| N (%) | |
|---|---|
| Levothyroxine | 17 (50.0) |
| Dexamethasone | 3 (8.8) |
| Prednisone | 3 (8.8) |
| Miscellaneous (<3 cases)a | 11 (32.4) |
aIncludes methimazole, anabolic steroid unspecified, hydrocortisone, medroxyprogesterone, mestranol, methylprednisolone, progesterone, triiodothyronine, andosterone, and ethinyl estradiol
Table 33.
Other pharmaceutical products
| N (%) | |
|---|---|
| Hydrogen peroxide (concentration < 10 %) | 6 (20.0) |
| Tadalafil | 3 (10.0) |
| Miscellaneous (<3 cases)a | 21 (70.0) |
aIncludes modafinil, pyridostigmine, radiological contrast dye, succinylcholine, sumatriptan, memantine, sildenafil, ursodiol, filgrastim, hyaluronic acid, methylergometrine, N-acetylcysteine, and rivastigmine
Table 34.
Pesticides
| N (%) | |
|---|---|
| Insecticides | |
| Pyrethroid unspecified | 9 (33.3) |
| Aldicarb | 2 (7.4) |
| Insecticide unspecified | 2 (7.4) |
| Pentachlorophenol | 2 (7.4) |
| Terbufos | 2 (7.4) |
| Cyhalothrin | 2 (7.4) |
| Miscellaneous (<2 cases)a | 8 (29.6) |
| Herbicides | |
| Dicamba | 3 (27.3) |
| Glyphosate | 3 (27.3) |
| Diquat | 2 (18.2) |
| Miscellaneous (<2 cases)b | 3 (27.3) |
| Rodenticides | |
| Brodifacoum | 10 (66.7) |
| Unspecified rodenticide | 5 (33.3) |
| Fungicidesc | 1 (100.0) |
aIncludes abamectin, chlorfenapyr, dimethoate, esfenvalerate, permethrin, phenothrin, bifenthrin, and hydropene
bIncludes triclopyr, iprodione, and 2,4,5-trichlorophenoxyacetic acid
cIncludes daconil
Table 35.
Chemotherapeutic and immunological agents
| N (%) | |
|---|---|
| Hydroxychloroquine | 6 (26.1) |
| Methotrexate | 5 (21.4) |
| Colchicine | 3 (13.0) |
| Miscellaneous (<3 cases)a | 9 (39.1) |
aIncludes psoralen, bicalutamine, bosutinib, diphtheria/tetanus/pertussis vaccine, isotretinoin, tacrolimus, dimethylfumarate, and pazopanib
Table 36.
Antiparkinsonism drugs
| N (%) | |
|---|---|
| Levodopa/carbidopa | 9 (47.4) |
| Ropinirole | 5 (26.3) |
| Miscellaneousa | 5 (26.3) |
aIncludes entacapone, rasagiline, selegiline, and pramipexole
Table 37.
Other nonpharmaceutical products
| N (%) | |
|---|---|
| Nitrates | 5 (35.7) |
| Miscellaneousa | 9 (64.3) |
aIncludes citric acid, iodine monochloride, isocyanates, methacrylates, silicone, tetrabenazine, tetrodotoxin, water, and sodium carbonate
Table 38.
Anesthetics
| N (%) | |
|---|---|
| Benzonatate | 6 (54.5) |
| Lidocaine | 2 (18.2) |
| Miscellaneous (<2 cases)a | 3 (27.3) |
aIncludes bupivacaine, mepivacaine, and sevoflurane
Table 39.
Pulmonary agents
| N (%) | |
|---|---|
| Albuterol | 5 (55.6) |
| Clenbuterol | 2 (22.2) |
| Miscellaneous (<2 cases)a | 2 (22.2) |
aIncludes theophylline and treprostinil
Table 8.
Analgesics (nonopioid)
| N (%) | |
|---|---|
| Acetaminophen | 995 (66.8) |
| Aspirin | 222 (14.9) |
| Ibuprofen | 198 (13.3) |
| Naproxen | 29 (1.9) |
| Salicylamide | 14 (0.9) |
| Analgesic unspecified | 8 (0.5) |
| Miscellaneousa | 24 (1.6) |
aIncludes diclofenac, meloxicam, phenazopyridine, methylsalicylate, metamizole, indomethacin, ketorolac, phenylbutazone, etodolac, nabumetone, and piroxicam
Table 40.
Miscellaneous nonpharmaceuticals
| N (%) | |
|---|---|
| Ingested foreign object | |
| Batteries | 3 (100.0) |
| WMD/riot agents/radiological | |
| Mace | 2 (66.7) |
| Botulinum toxin | 1 (33.3) |
WMD weapons of mass destruction
Treatment
Specific treatments were provided to 4,695 (54.6 %) patients. At least one antidote was given to 2,334 (27.1 %) patients and 356 (4.1 %) received more than one, resulting in 2,825 instances of separate antidote administration (Table 41). N-acetylcysteine accounted for nearly a third (30.5 %) of all antidote administrations. Opioid antagonists (naloxone or nalmefene) and sodium bicarbonate were the next most frequent collectively comprising another 32.2 % of total antidote use. Antivenoms and chelators were used in 1.4 and 0.3 % of cases, respectively (Tables 42 and 43). Almost all antivenom treatments (91.8 %) involved polyvalent anti-Crotalidae Fab fragments. Over half of chelation treatments (53.6 %) utilized DMSA, with an additional 21.4 % of cases involving deferoxamine.
Table 41.
Antidotal therapy
| N (%)a | |
|---|---|
| N-acetylcysteine | 861 (30.5) |
| Naloxone/nalmefene | 573 (20.3) |
| Sodium bicarbonate | 337 (11.9) |
| Physostigmine | 186 (6.6) |
| Flumazenil | 154 (5.5) |
| Glucagon | 89 (3.2) |
| Thiamine | 82 (2.9) |
| Fomepizole | 79 (2.8) |
| Calcium | 76 (2.7) |
| Folate | 46 (1.6) |
| Atropine | 43 (1.5) |
| l-carnitine | 42 (1.5) |
| Octreotide | 42 (1.5) |
| Vitamin K | 42 (1.5) |
| Fab for digoxin | 36 (1.3) |
| Lipid resuscitation | 34 (1.2) |
| Insulin-euglycemic therapy | 33 (1.2) |
| Cyproheptadine | 24 (0.8) |
| Methylene Blue | 13 (0.5) |
| Hydroxocobalamin | 11 (0.4) |
| Pyridoxine | 5 (0.2) |
| Bromocriptine | 4 (0.1) |
| Coagulation factor replacement | 3 (0.1) |
| 2-PAM | 2 (0.1) |
| Botulinum antitoxin | 2 (0.1) |
| Dantrolene | 2 (0.1) |
| Ethanol | 2 (0.1) |
| Anticoagulation reversal | 1 (0.0) |
| Nitrites | 1 (0.0) |
aPercentages are out of the total number antidotes administered (2,825); 4.1 % of registry cases received more than one antidote
Table 42.
Antivenom therapy
| N (%) | |
|---|---|
| Polyvalent anti-Crotalidae Fab fragments | 112 (91.8) |
| Scorpion antivenom | 4 (3.3) |
| Spider antivenom | 4 (3.3) |
| Other snake antivenom | 2 (1.6) |
Table 43.
Chelation therapy
| N (%) | |
|---|---|
| DMSA | 15 (53.6) |
| Deferoxamine | 6 (21.4) |
| Dimercaprol (BAL) | 3 (10.7) |
| EDTA | 3 (10.7) |
| Penicillamine | 1 (3.6) |
DMSA dimercaptosuccinic acid, EDTA ethylenediaminetetraacetic acid
Supportive care with pharmacologic agents was provided in 1,681 (19.6 %) cases (Tables 44 and 45). A total of 2,089 treatments were documented, and 336 (3.9 %) patients received more than one type of treatment. Benzodiazepine administration accounted for over half (56.0 %) of pharmacologic supportive measures. Nonpharmacologic forms of supportive care were given in 1,527 (17.8 %) cases, with 350 (4.1 %) patients receiving more than one type of treatment. Intravenous fluid resuscitation (62.0 %) and intubation (31.6 %) together accounted for the majority of treatments.
Table 44.
Supportive care (pharmacological)
| N (%)a | |
|---|---|
| Benzodiazepines | 1,170 (56.0) |
| Vasopressors | 206 (9.9) |
| Antipsychotics | 165 (7.9) |
| Anticonvulsants | 141 (6.7) |
| Opioids | 122 (5.8) |
| Glucose (concentration > 5 %) | 105 (5.0) |
| Albuterol (or other bronchodilator) | 48 (2.3) |
| Neuromuscular blockers | 41 (2.0) |
| Corticosteroids | 37 (1.8) |
| Antiarrhythmics | 19 (0.9) |
| Antihypertensives | 16 (0.8) |
| Vasodilators | 11 (0.5) |
| Beta blockers | 8 (0.4) |
aPercentages are out of the total number of treatments administered (2,089); 3.9 % of registry cases received more than one form of treatment.
Table 45.
Supportive care (nonpharmacological)
| N (%)a | |
|---|---|
| IV fluid resuscitation | 1,207 (62.0) |
| Intubation/ventilatory management | 615 (31.6) |
| CPR | 55 (2.8) |
| Pacemaker | 19 (1.0) |
| Transfusion | 15 (0.8) |
| Hyperbaric oxygen | 13 (0.7) |
| ECMO | 11 (0.6) |
| Cardioversion | 10 (0.5) |
| Organ transplantation | 2 (0.1) |
| Aortic balloon pump | 1 (0.1) |
aPercentages are out of the total number of treatments administered (1,948); 4.1 % of registry cases received more than one form of treatment
CPR Cardiopulmonary resuscitation, ECMO extracorporeal membrane oxygenation
Decontamination and enhanced elimination techniques were used in a small number of cases (Tables 46 and 47). Only 388 (4.5 %) patients received some form of decontamination, with 28 (0.3 %) receiving more than one type. Oral activated charcoal made up the majority (79.4 %) of decontamination therapies. Enhanced elimination therapies were provided to 219 (2.5 %) patients with 22 (0.3 %) receiving more than one type. Hemodialysis and continuous renal replacement accounted for 62 % of enhanced elimination entries. The remaining included urinary alkalinization and multiple-dose activated charcoal. There was one instance of exchange transfusion.
Table 46.
Decontamination
| N (%)a | |
|---|---|
| Activated charcoal | 332 (79.4) |
| Whole bowel irrigation | 48 (11.5) |
| Gastric lavage | 23 (5.5) |
| External irrigation | 15 (3.6) |
aPercentages are out of the total number of treatments administered (418); 28 registry cases received more than one form of treatment
Table 47.
Enhanced elimination
| N (%)a | |
|---|---|
| Hemodialysis (toxin removal) | 70 (29.7) |
| Urinary alkalinization | 61 (25.8) |
| Continuous renal replacement therapy | 41 (17.4) |
| Hemodialysis (other indication) | 36 (15.3) |
| Multiple-dose activated charcoal | 27 (11.4) |
| Exchange transfusion | 1 (0.4) |
aPercentages are out of the total number of treatments administered (236); 22 registry cases received more than one form of treatment
Adverse Drug Reactions
ADRs were the reason for medical toxicology consultation in 580 (4.8 %) of the Registry cases. A total of 177 drugs were implicated at least once. Table 48 lists the 15 most frequently encountered drugs associated with ADRs (≥10 occurrences), along with their percentage of the total number of ADR cases. More than one drug was involved in 129 (22 %) of these cases.
Table 48.
Most common drugs associated with ADRs
| N (%) | |
|---|---|
| Lithium | 40 (6.9) |
| Valproic acid | 26 (4.5) |
| Risperidone | 18 (3.1) |
| Phenytoin | 17 (2.9) |
| Bupropion | 14 (2.4) |
| Digoxin | 14 (2.4) |
| Citalopram | 13 (2.2) |
| Acetaminophen | 12 (2.1) |
| Haloperidol | 12 (2.1) |
| Oxycodone | 12 (2.1) |
| Lamotrigine | 11 (1.9) |
| Methylphenidate | 11 (1.9) |
| Sertraline | 11 (1.9) |
| Fentanyl | 10 (1.7) |
| Quetiapine | 10 (1.7) |
Limitations
Reporting bias is a potential limitation of any database dependent on spontaneous reporting. However, all participating sites agree, as a condition of participation, that all of their cases will be entered into the Registry thus minimizing such bias. Incomplete data entry is a limitation. As seen in Tables 2, 3, and 4, some case data were missing. This is an area of ongoing quality improvement for the Registry. When such data are missing, it is typically less than 5 % of the total cases for any given field, making it unlikely to significantly impact the results and trends. However, this problem was most notable in the fatal cases (Table 7), where a specific exposure was not documented in a substantial fraction of entries. Another limitation is that other than fatality, there is no mechanism to determine the relative severity of outcome. In fatal cases where toxic exposure is responsible for clinical symptoms or findings, it is likely that death is attributable to poisoning. However, the current data collection methods do not allow for distinguishing whether a secondary, nontoxic etiology is responsible for death.
The ToxIC Registry is not population based and has a specific ascertainment bias, which is present by design. The key inclusion criterion for entry into the Registry is the consultation by a medical toxicologist. Thus, Registry cases represent patients for whom there was a concern for significant toxicity. Cases of no, or mild, toxicity are likely to be underrepresented.
Discussion
This report of the ToxIC Registry serves as a comprehensive overview of cases involving medical toxicology consultations. The number of cases (Fig. 1) and number of participating institutions have grown steadily since the Registry’s inception in 2010.
For the most part, the patterns seen in this report are similar to the trends seen in prior years [3]. The pattern of ADRs deserves further discussion. Adverse drug reactions are a considerable patient safety issue. Overall, ADRs ranked as the fourth most common reason for consultation. It is interesting that of the most common drugs associated with ADRs, only three, acetaminophen, oxycodone, and fentanyl, belong to the top three agent classes found in Table 5. Rather ADRs were dominated by antidepressants, antipsychotics, and anticonvulsants. The reason for this pattern is not clear. However, the medication types presented in Table 48 may indicate a disproportionate representation of psychiatric patients in ADR consultations. Nevertheless, these medications have a significant intrinsic potential for toxicity. Since the ADRs represented in this Registry are likely to be serious, future studies should be focused on evaluating their causes and clinical syndromes in more detail.
There have been a number of upgrades to the Registry in 2014 which should be evident in next year’s annual report. Because most of the cases involve intentional pharmaceutical ingestions, we are evaluating the reasons for taking the medication in more detail. This includes an assessment of intent to determine if the medication was taken for therapeutic purposes, for attempts at self-harm, or for other reasons, such as to generate a pleasurable effect. Additional information about intent is being collected within each of these categories. Furthermore, this year, we are also beginning to obtain ethnic and racial data to refine the demographic profile for patients with the various kinds of toxicologic disease enumerated in this report.
Another factor of considerable interest to medical toxicologists involves the decision to terminate life support. This decision is especially complicated because of the difficulties in assessing brain death or neurological function when central nervous system-active medications are present, particularly in the high concentrations that are often found in these patients. In 2014, we began collecting additional information regarding the decision to terminate life support, and these data should be reflected in next year’s report.
Conclusions
Cases requiring medical toxicology consultation in 2013 predominantly involved pharmaceuticals and illicit drugs. Reasons for these drug exposures were diverse and included intentional overdose, unintentional exposure, withdrawal syndromes, and ADRs. Nonopioid analgesics, sedative-hypnotic agents, and opioids remained the most frequently encountered agent classes. While over half of cases required some form of medical treatment, fatalities were uncommon.
Acknowledgments
The authors express their sincere gratitude to the staff at the American College of Medical Toxicology for supporting the ToxIC Registry project. We very much appreciate the contributions to the Registry from each of the ToxIC sites. The following is a list of the principle coordinators from each site:
Boston, MA (Beth Israel Deaconess Medical Center)
Michael Ganetsky, MD
Boston, MA (Children’s Hospital of Boston)
Michele Burns-Ewald, MD
Charlotte, NC
Michael Beuhler, MD
Charlottesville, VA
Joshua King, MD
Chicago, IL
Steven Aks, DO
Cincinnati, OH
Shan Yin, MD
Dallas, TX
Kurt Kleinschmidt, MD
Paul Wax, MD
Denver, CO
Jeffrey Brent, MD
Eric Lavonas, MD
Evanston, IL
Jerold Leikin, MD
Fresno, CA
Rais Vohra, MD
Grand Rapids, MI
Bryan Judge, MD
Bradley Riley, MD
Haifa, Israel
Yedidia Bentur, MD
Harrisburg, PA
J. Ward Donovan, MD
Hartford, CT
Charles McKay, MD
Houston, TX
Spencer Greene, D
Indianapolis, IN
Daniel Rusyniak, MD
Kansas City, MO
Jennifer Lowry, MD
D. Adam Algren, MD
Los Angeles, CA
Michael Levine, MD
Manhasset, NY
Josh Nogar, MD
Milwaukee, WI
David Gummin, MD
Mark Kostic, MD
Morristown, NJ
Diane Calello, MD
New Brunswick, NJ
Ann-Jeanette Geib, MD
Newark, NJ
Steven Marcus, MD
New York, NY (Mt. Sinai Hospital)
Stephanie Hernandez, MD
Alex Manini, MD
New York, NY (NYU Langone Medical Center)
Silas Smith, MD
Lewis Nelson, MD
New York, NY (Staten Island University Hospital)
Nima Majlesi, DO
Omaha, NE
Ronald Kirschner, MD
Philadelphia (Hahnemann University Hospital)
David Vearrier, MD
Philadelphia (Einstein Medical Center)
Adam K. Rowden, DO
Phoenix, AZ
Michael Levine, MD
Anne-Michelle Ruha, MD
Pittsburgh, PA
Anthony Pizon, MD
Portland, OR
Nathaneal McKeown, DO
Richmond, VA
Brandon Wills, DO
Kirk Cumpston, DO
Rochester, NY
Timothy Wiegand, MD
Salt Lake City, UT
E. Martin Caravati, MD
San Antonio, TX
Shawn Varney, MD
Vikhyat Bebarta, MD
San Francisco, CA
Derrick Lung, MD
Craig Smollin, MD
St. Louis, MO
Evan Schwarz, MD
Thomas Kibby, MD
St. Paul, MN
Samuel Stellpflug, MD
Kristin Engebretsen, PharmD
Sydney West, Australia
Naren Gunga, MD
Worcester, MA
Sean Rhyee, MD, MPH
Conflict of Interest
The authors have no conflicts of interests to disclose relevant to the content of this manuscript. No outside funding was involved in the production of this manuscript.
Funding Sources
Support for the Registry in 2013 was derived from three sources: NINDS grant U01 NS083452-01S1, an unrestricted grant from BTG International Ltd., and the American College of Medical Toxicology, the sponsor of ToxIC.
Footnotes
Data contained in this manuscript has not been previously presented in any form.
References
- 1.Wax PM, Kleinschmidt KC, Brent J. The Toxicology Investigators Consortium (ToxIC) Registry. J Med Toxicol. 2011;7(4):259–265. doi: 10.1007/s13181-011-0177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brent J, Wax PM, Schwartz T, et al. The Toxicology Investigators Consortium Case Registry—the 2010 experience. J Med Toxicol. 2011;7(4):266–276. doi: 10.1007/s13181-011-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiegand T, Wax P, Smith E, et al. The Toxicology Investigators Consortium Case Registry—the 2012 experience. J Med Toxicol. 2013;9(4):380–404. doi: 10.1007/s13181-013-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiegand TJ, Wax PM, Schwartz T, et al. The Toxicology Investigators Consortium Case Registry—the 2011 experience. J Med Toxicol. 2012;8(4):360–377. doi: 10.1007/s13181-012-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]