Introduction
History of Telemedicine
Although the Institute of Medicine (IOM) first defined telemedicine in 1996 as the use of electronic information and communications technologies to provide and support health care when distance separates participants, the use of electronic communications technologies in medicine is not new [1]. In 1877, a group of physicians created a telephone exchange including local pharmacies in order to facilitate improved patient communications [2, 3]. In the 1970s, the National Aeronautics and Space Administration (NASA), Lockheed Martin, and the Indian Health Services teamed together to create the Space Technology Applied to Rural Papago Advanced Health Care (STARPACH) program—a telemedicine initiative involving real time video, data and voice-over-microwave interaction to extend healthcare to a rural setting [4].
Over the past two decades, telemedical systems have successfully connected rural and community hospitals to large urban centers with subspecialty expertise. These initiatives have improved care for specific patient subgroups including those in nursing homes, dermatological complaints, and trauma [2, 5]. In particular, the specialties of neurology and dermatology have employed telemedicine to extend their reach in resource poor settings. Telestroke employs a controlled video camera and monitor screen for a remote neurologist to help diagnose and manage emergency department patients with stroke while static photo technology and emerging live video devices have helped a remote dermatologist complete dermatology consults remotely [6–9].
Wearable Devices
The field of wearable devices includes a variety of sensors and displays, ranging from electrocardiogram (ECG) leads to wearable head-mounted computers like Google Glass (Table 1). Wearable devices provide novel methods for monitoring and potentially enhancing users’ health [10–13]. Each device, regardless of its specific niche, performs one or more of a limited set of tasks: actively recording and streaming biometric data, recording data for later collection, or providing real time feedback to the user.
Table 1.
Other types of wearables that record vital signs
| Wearable Device | Use | Commercially available examples |
|---|---|---|
| Fitness tracker | Monitor daily fitness, workouts | FitBit, Nike+ Fuelband, Samsung Gear, Jawbone |
| Heads up display | Head-mounted combination of video and static image functions | Google Glass, Vuzix |
| Watches | Integration with phone for notifications or as fitness device | Pebble, Androidwear |
| Clothing | Monitor heart rate, spirometer, fitness through clothing | FitnessSHIRT, BioMan AiQ, Sensoria SmartSock, Ravijour Smart Bra |
| Clip on Monitors | Devices clipped onto clothing or accessed to record vital signs | Scanadu, Lumo BodyLift |
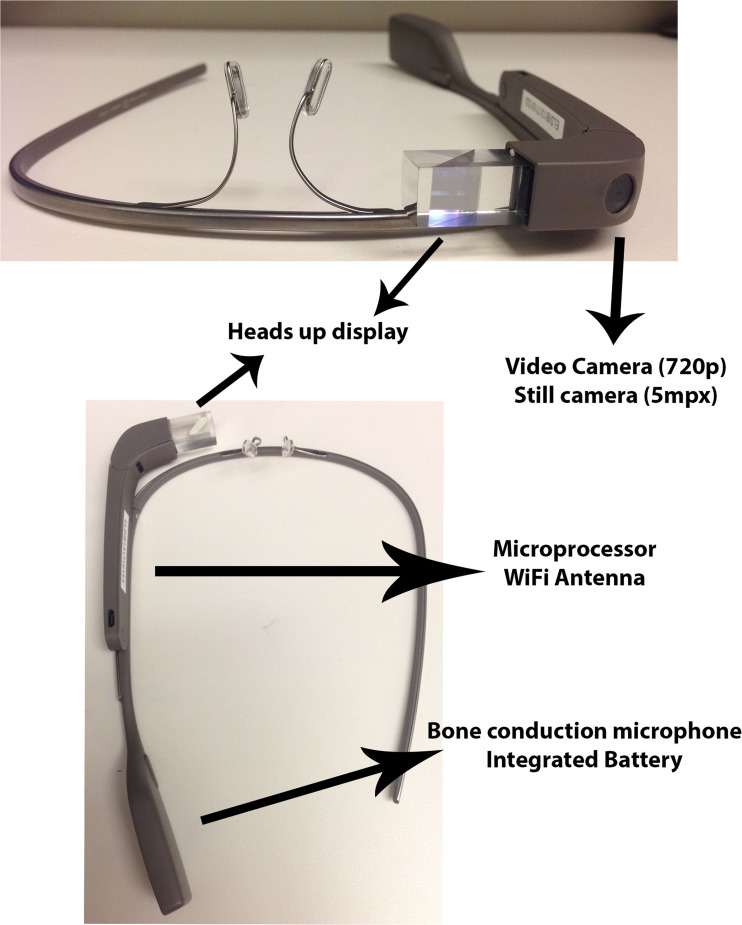
In 2012, Google introduced Google Glass, an innovative, wearable computer with a display that projects information at the wearer’s eye level, a head-mounted camera capable of transmitting live video, and the ability to respond to simple voice commands (Fig. 1) [14]. A head-mounted device is attractive to the emergency medicine provider as well as the medical toxicologist as it allows for hand-free assessment and video relay of the patient’s exam from the provider’s perspective [15, 16]. It also has the potential to be faster and easier to use than traditional telemedical systems. Intuitive voice controls embedded in Glass’s programming software eliminate the need for new computer training. Physicians may benefit from not having to boot up a device, focus a camera, or properly position a patient prior to a video consult [17]. Head-mounted wearable devices may improve care coordination not just in rural areas, but also in resource-rich environments such as academic medical centers.
Fig. 1.
Head-mounted camera capable of transmitting live video
Since 1996, the National Libraries of Medicine (NLM) in conjunction with the National Institutes of Health (NIH) have supported 19 multiyear grants for development of telemedical solutions in rural, urban, and suburban populations. In 1996, the total funding by the NIH for telemedicine research rose to $7.6 million. Since 2011, the NIH has released several grant mechanisms to support study of the efficacy and use of mobile health tools directed toward improvement in quality of care. Despite increasing funding, the potential benefits of a wearable device such as Glass for telemedicine have yet to be established, however, in either the research or clinical environment [2, 8]. Only minimal data supports the use of head-mounted technologies in telemedicine, in particular, and little literature addresses the optimal research methods for assessing their impact on patient care. Here, we highlight concepts and considerations involved in researching the use of emerging head-mounted wearable devices in toxicology.
Considerations for Implementing Head-Mounted Wearable Devices
Privacy and Security
The first major challenge to implementing wearable head-mounted technology in the clinical and research arena centers on the privacy and security of wearable devices. The Health Insurance Portability and Accountability Act (HIPAA) and its accompanying regulation about electronic medical information, the Health Information Technology for Economic and Clinical Health (HITECH) Act, define many of these concerns [18, 19]. Most crucial are the issues of encrypted data streams, the ability to store images or videos for later analysis (aka “store and forward”), and appropriate disclosures on research consent forms.
The HIPAA/HITECH acts require rigorous information technology (IT) encryptions standards to which wearable devices, as commercial products, do not adhere. For example, images and videos captured on the commercial version of Google Glass are uploaded to the cloud through Google servers. Users have the ability to post these images along with text and video recordings to various social media outlets. Such practice is not HIPAA compliant. Store and forward—the concept of taking a photograph or recording a video and saving it for documentation and future analysis—poses yet another wrinkle in the deployment of a novel wearable technology in the HIPAA/HITECH environment.
To ensure protection of patient privacy and to adhere to HIPAA/HITECH, secure servers in healthcare facilities should of course employ rigorous encryption methods for data storage [20]. Comparable encryption should also be employed in head-mounted wearable devices themselves. For instance, the programming embedded in commercially available Glass requires modification, such as by changing the native Google software so that users cannot remove patient data from the hospital’s secure server. Several commercial ventures are currently creating HIPAA-compliant software solutions that make these software modifications to Google Glass. Other wearable devices coming to market may require similar software modifications when applied to patient care settings. These processes must be evaluated in cooperation with the device manufacturer, software provider, and hospital IT security prior to implementation of a research study to ensure patient privacy.
Connectivity and Hospital Information Technology Considerations
Another barrier to studying the effectiveness of head‐mounted wearable devices for telehealth is connectivity. Unlike other mobile platforms, such as smart phones or tablets, which may run off of a 4G Long-Term Evolution (LTE) network, wearable devices in healthcare facilities require greater bandwidth than available on a mobile phone network. At present, head-mounted devices depend on Bluetooth tethering or robust wireless networks, potentially limiting their feasibility in healthcare environments with limited bandwidth.
Alternatives to standard Bluetooth and wireless networks do exist, although are not widely adopted in medicine. The Institute of Electrical and Electronics Engineers (IEEE) routinely establishes benchmarks for personal and shared wireless network bandwidth. The current existing benchmark for wireless device communication is listed as 802.15 [21]. Zigbee (San Ramon, CA), a low frequency 802.15 standard that uses less battery operating power than Bluetooth while providing an effective range of 10 to 100 m, is an attractive mechanism that has been studied to enable transmission of data from small medical devices such as remote electrocardiogram (ECG) monitors, pulse oximetry, or glucometers [22–24]. Given the low frequency of transmission, however, a high powered wearable device transmitting video or audio may require a higher bandwidth than is supported through Zigbee.
Existing hospital networks integrate a large number of devices, and as a result, connecting to a hospital network can be challenging. Integration of new devices requires approval by hospital IT committees that are very sensitive to the risks of overloading the hospital wireless network on which essential devices such depend. In addition, head-mounted devices that are used for video streaming introduce greater fluctuations in bandwidth than traditional devices. Moreover, increased connectivity is costly for hospitals.
Early involvement of hospital IT departments is critical for any research on head-mounted devices, in order to enable such devices to connect to the hospital network. After obtaining IT approval, researchers should create a map of wireless network signal strength throughout the hospital, to determine the “dead zones” where the device might fail. In our experience, working with hospital IT personnel to give the study device priority within the hospital network by creating a static IP address for the device (known as “whitelisting”) greatly enhances stability of data transmission.
As poor connectivity can introduce confounding factors into a study, solving these IT issues is a prerequisite for any meaningful research on head‐mounted devices.
Industry Partnership
The intercalation of commercial concerns into the mobile device industry, and the need for clinicians to collaborate with commercial entities, increases the risk of conflicts of interest as well as the need for the vigorous safeguards against these conflicts. Since 1995, the United States Department of Health and Human Services has defined any formal relationship with industry that involves financial stakes in stock, or equity as a financial and research conflict of interest (COI). Consequently, clinicians and researchers should assiduously disclose potential conflicts of interest in the planning and solicitation of funding for studies. In addition, researchers—whose lifeblood is publishing—should develop written agreements on publication and meaningful usage of data. These safeguards can promote mutually beneficial partnerships, ensure smooth implementation of the research project, and strengthen product development for future innovations, while protecting the rigor and trustworthiness of studies’ outcomes.
Research Goals
The rapid expansion of wearable head-mounted devices in the commercial and medical sector will provide opportunity for researchers to help determine which of these new devices will ultimately have a role in caring for our toxicology patients. As a first step, the feasibility of use for Glass in toxicology should be evaluated; this effort should focus on measuring the technological reliability of the device as well as the ease of integrating head-mounted wearable devices into the assessment of toxicology patients. Concurrently, the acceptability of the Glass by both clinicians and patients should be determined. An altered patient who is intoxicated or acutely poisoned may regard a physician wearing a head-mounted device as confrontational and may become more agitated. Whether poisoned patients or their family members are accepting of a head-mounted device to provide remote toxicology care has yet to be determined. Although our particular interest will be the extent to which wearable devices contribute to, or detract from, the clinical experience of managing toxicology patients, this line of investigation could also be extended to other areas where visual diagnosis remains a staple of clinical care.
Assuming the feasibility and acceptability can be established, the logical next step involves determining the impact of head-mounted wearable devices on clinical outcomes. Given that many of the theoretical benefits of wearable technologies lie in workflow improvements and cost reductions, studies should also focus on process measures such as length of stay and cost-effectiveness. While the excitement of novel technology might prompt initial trials of wearable technologies in healthcare, true improvement in the delivery of care, extension of clinical services into underserved environments, and improved reimbursement will ultimately drive adoption. Data from studies showing IT reliability, and improvement in satisfaction, clinical outcomes, workflow and cost are ultimately necessary for the long‐term integration of head-mounted wearable technologies into the clinical environment. When applied to medical toxicology, a live video feed of patients in the ED by a wearable head-mounted device may support timely clinical decision-making by the emergency medicine provider in conjunction with an expert toxicologist. An important part of toxicology is the physical exam, which requires visualization of the patient. Real time feedback can be crucial in the acutely poisoned patient and immediate access to a subtle physical exam can help guide management [25–27]. A head-mounted wearable device with live video capabilities may enhance such real-time assessment of toxidromes by an off-site consulting toxicologist. Head-mounted wearables may also allow off-site toxicologists to offer recommendations on antidote administration and management concurrently with clinicians’ delivery of medical care. Clinical outcome from time sensitive and expensive antidotes such as digoxin FAB, glucagon infusions, or crotalid antivenom can be observed remotely, and cost savings may be realized with a toxicologist providing real time feedback on management of these atypical poisonings [28–31].
Alternatively, Glass and future wearable technologies can function as triage devices for poisoned patients in determining whether transfer to a tertiary care center is required. Future research directions include improving care coordination, shortened time-to‐antidote administration, and enhanced clinician education. Other types of wearables that record vital signs, such as those listed in Table 1, may provide additional utility when integrated into wearable head-mounted devices for remote monitoring of potentially poisoned patients. One could imagine integration of a wearable heads up device that is able to stream vital signs from a monitor to the provider so they could monitor them while seeing another patient in the emergency department. These vitals or other critical information from laboratory data and imaging could be packaged through simple voice commands and transmitted to a Poison Control Center (PCC) or a medical toxicologist who is also using a head-mounted device, so they could have data to review while virtually being at the bedside of the poisoned patient. Recommendations for antidote administration, decontamination, or the need for advanced therapies such as dialysis or hyperinsulinemia-euglycemia, or intravenous lipid emulsion could be conveyed to the emergency department provider via a simple reply text to the head-mounted device with recommendations for dosing that can be recorded in an electronic medical record or forwarded for confirmation with the hospital pharmacy. Alternatively, wearable technologies may function as triage devices for poisoned patients, such as by determining whether transfer to a tertiary care center is required. At a time where funding for PCCs is decreasing, the integration of a wearable head-mounted device in the hospital setting may increase the consultative power of a regional PCC and provide more cost savings that what we already have.
Conclusion
Head-mounted wearable technologies such as Google Glass require additional research to validate their acceptability, efficacy, and cost-effectiveness. We have outlined concepts and considerations for research studies using such head-mounted wearables. Privacy, connectivity, potential conflict of interest, and research directions are all challenges when studying wearable technologies. Partnership with HIPAA-compliant software providers, close cooperation with hospital IT, and creating safeguards to prevent conflict of interest are potential solutions. Research will need to focus not only on clinical outcomes, but also satisfaction, workflow improvements, and cost-effectiveness. Many opportunities within toxicology exist to integrate wearable technologies.
Similar to the initial introduction of mobile technology, Google Glass and other head-mounted wearables may usher in a new era of communication through wearable platforms. After Steve Jobs introduced the world to the iPhone in 2007, he concluded his keynote presentation with a quote from Wayne Gretzky, “I skate to where the puck is going to be, not where it has been.” [32]. Wearable technology is likely here to stay. Our roles as clinicians and researchers will be to anticipate and shape the application of these new devices to improve the daily care of our patients.
Acknowledgments
Conflicts of interest
None.
Footnotes
Peter R Chai and Roger Y Wu share first author for this paper.
Contributor Information
Peter R. Chai, Email: peter.chai@umassmemorial.org
Roger Y. Wu, Email: rwu1@lifespan.org
References
- 1.Field MJ. Institute of medicine committee on evaluating clinical applications of telemedicine: a guide to assessing telecommunications in health care, National Academies Press (US) Washington (DC): National Academy of Sciences; 1996. [PubMed] [Google Scholar]
- 2.Mun SK, Turner JW. Telemedicine: emerging e-medicine. Annu Rev Biomed Eng. 1999;1:589–610. doi: 10.1146/annurev.bioeng.1.1.589. [DOI] [PubMed] [Google Scholar]
- 3.Starr P. The social transformation of American medicine. Basic: Books; 1984. [Google Scholar]
- 4.Freiburger G, Holcomb M, Piper D. The STARPAHC collection: part of an archive of the history of telemedicine. J Telemed Telecare. 2007;13(5):221–223. doi: 10.1258/135763307781458949. [DOI] [PubMed] [Google Scholar]
- 5.Switzer JA, Demaerschalk BM. Overcoming challenges to sustain atelestroke network. J Stroke Cerebrovasc Dis. 2012;21(7):535–540. doi: 10.1016/j.jstrokecerebrovasdis.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Bowns RI, Collins K, Walters SJ, McDonagh AJ. Telemedicine indermatology: a randomised controlled trial. Health Technol Assess. 2006;10(43):1–39. doi: 10.3310/hta10430. [DOI] [PubMed] [Google Scholar]
- 7.Czaplik M, Bergrath S, Rassaint R, Thelen S, Brodziak T, Valentin B, et al. Employment of telemedicine in emergency medicine. Clinical requirement analysis, system development and first test results. Methods Inf Med. 2014;53(2):99–07. doi: 10.3414/ME13-01-0022. [DOI] [PubMed] [Google Scholar]
- 8.Hess DC, Audebert HJ. The history and future of telestroke. Nat Rev Neurol. 2013;9(6):340–350. doi: 10.1038/nrneurol.2013.86. [DOI] [PubMed] [Google Scholar]
- 9.Silva GS, Farrell S, Shandra E, Viswanathan A, Schwamm LH. The status of telestroke in the United States: a survey of currently active stroke telemedicine programs. Stroke. 2012;43(8):2078–2085. doi: 10.1161/STROKEAHA.111.645861. [DOI] [PubMed] [Google Scholar]
- 10.Stoppa M, Chiolerio A. Wearable electronics and smart textiles: a critical review. Sensors. 2014;14(7):11957–11992. doi: 10.3390/s140711957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobkin BH. Wearable motion sensors to continuously measure real world physical activities. Curr Opin Neurol. 2013;26(6):602–608. doi: 10.1097/WCO.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane NA, Simmons MC, John D, Thompson DL, Bassett DR. Validity of the Nike+ device during walking and running. Int J Sports Med. 2010;31(2):101–105. doi: 10.1055/s-0029-1242810. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery Downs HE, Insana SP, Bond JS. Movement toward a novel activity monitoring device. Sleep Breath. 2012;16(3):913–917. doi: 10.1007/s11325-011-0585-y. [DOI] [PubMed] [Google Scholar]
- 14.Google. In: Google I/O 2012. 2012. https://developers.google.com/events/io/2012/. Accessed 30 May 2014.
- 15.Horng S, Porter PS, Samani K. Emergency providers see big potential for Google Glass. ED Manag. 2014;26(5):55–58. [PubMed] [Google Scholar]
- 16.Klonoff DC. New wearable computers move ahead: Google Glass and smart wigs. J Diabetes Sci Technol. 2014;8(1):3–5. doi: 10.1177/1932296813518858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muensterer OJ, Lacher M, Zoeller C, Bronstein M, Kubler J. Google Glass in pediatric surgery: an exploratory study. Int J Surg. 2014;12(4):281–289. doi: 10.1016/j.ijsu.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Library of Congress, Health Insurance Portability and Accountability Act of 1996, U.S. Congress, Editor. 1996: The Library of Congress.
- 19.Sebelius SK (2013) Modifications to the HIPAA Privacy, Security, Enforcement and Breach Notification Rules Under the Health Information Technology for Economic and Clinical Health Act and the Genetic Information Nondiscrimination Act; Other Modifications to the HIPAA Rule. Federal Register 5566–5702 [PubMed]
- 20.Guerra W. Legal and Policy In: Mobile Health Roadmap: mHealth Strategic Framework for Hospitals and Health Systems. 2012 http://www.himss.org/mobilehealthit/roadmap. Accessed 10 July 2014.
- 21.Institute of Electrical and Electronics Engineers, 802.15.1-2005-EEE Standard for Information technology Local and metropolitan area networks Specific requirements Part 15.1a: Wireless Medium Access Control (MAC) and Physical Layer (PHY) specifications for Wireless Personal Area Networks (WPAN). 2010: IEEE Standards Association.
- 22.Chen SK, Kao T, Chan CT, Huang CN, Chiang CY, Lai CY, et al. A reliable transmission protocol for ZigBee-based wireless patient monitoring. IEEE Trans Inf Technol Biomed. 2012;16(1):6–16. doi: 10.1109/TITB.2011.2171704. [DOI] [PubMed] [Google Scholar]
- 23.Frehill P, Chambers D, Rotariu C (2007) Using Zigbee to integrate medical devices. Conf Proc IEEE Eng Med Biol Soc 6718–6721 [DOI] [PubMed]
- 24.Mo L, Liu S, Gao RX, John D, Staudenmayer J, Freedson P (2011) ZigBee based wireless multi-‐sensor system for physical activity assessment. Conf Proc IEEE Eng Med Biol Soc 846–849 [DOI] [PubMed]
- 25.Maddry JK, Sessions D, Heard K, Lappan C, McManus J, Bebarta VS. Wartime toxicology: evaluation of a military medical toxicology telemedicine consults service to assist physicians serving overseas and in combat (2005–2012). J Med Toxicol. 2014. doi:10.1007/s13181-014-0398-z. [DOI] [PMC free article] [PubMed]
- 26.Skolnik A. Telemedicine and toxicology: back to the future? J Med Toxicol. 2013;9(3):217–219. doi: 10.1007/s13181-013-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischbein CB, Mueller GM, Leacock PR, Wahl MS, Aks SE. Digital imaging: a promising tool for mushroom identification. Acad Emerg Med. 2003;10(7):808–811. doi: 10.1111/j.1553-2712.2003.tb00079.x. [DOI] [PubMed] [Google Scholar]
- 28.Blizzard JC, Michels JE, Richardson WH, Reeder CE, Schulz RM, Holstege CP. Cost-benefit analysis of a regional poison center. Clin Toxicol (Phila) 2008;46(5):450–456. doi: 10.1080/15563650701616145. [DOI] [PubMed] [Google Scholar]
- 29.Vassilev ZP, Marcus SM. The impact of a poison control center on the length of hospital stay for patients with poisoning. J Toxicol Environ Health A. 2007;70(2):107–110. doi: 10.1080/15287390600755042. [DOI] [PubMed] [Google Scholar]
- 30.Zaloshnja E, Miller T, Jones P, Litovitz T, Coben J, Steiner C, et al. The potential impact of poison control centers on rural hospitalization rates for poisoning. Pediatrics. 2006;118(5):2094–2100. doi: 10.1542/peds.2006-1585. [DOI] [PubMed] [Google Scholar]
- 31.Skoutakis VA, Wojciechowski NJ, Carter CA, Hayes JM, Hudson BL, Martin JA. Drug Information Center network: need, effectiveness, and cost justification. Drug Intell Clin Pharm. 1987;21(1):49–56. doi: 10.1177/10600280870211p108. [DOI] [PubMed] [Google Scholar]
- 32.Block R. Live from Macworld 2007. In: Live from Macworld 2007: Steve Jobs keynote. 2007. http://www.engadget.com/2007/01/09/live from macworld 2007 steve jobs keynote/. Accessed 14 July 2014.