Abstract
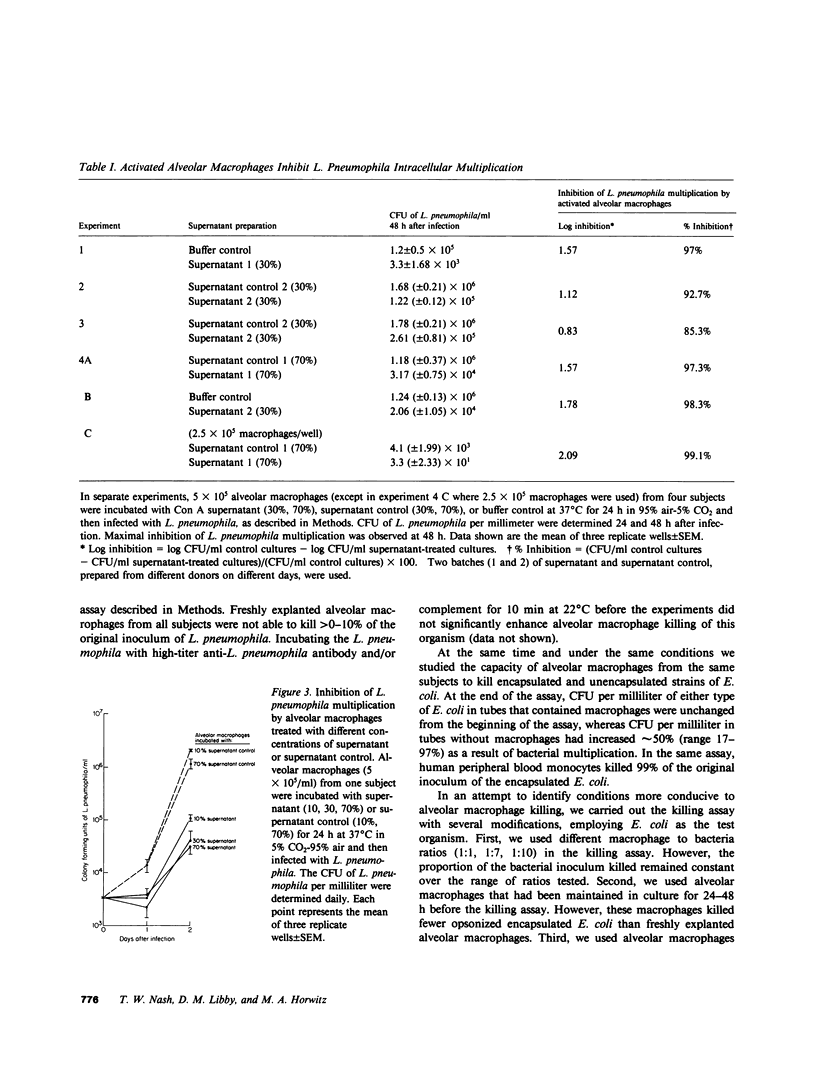
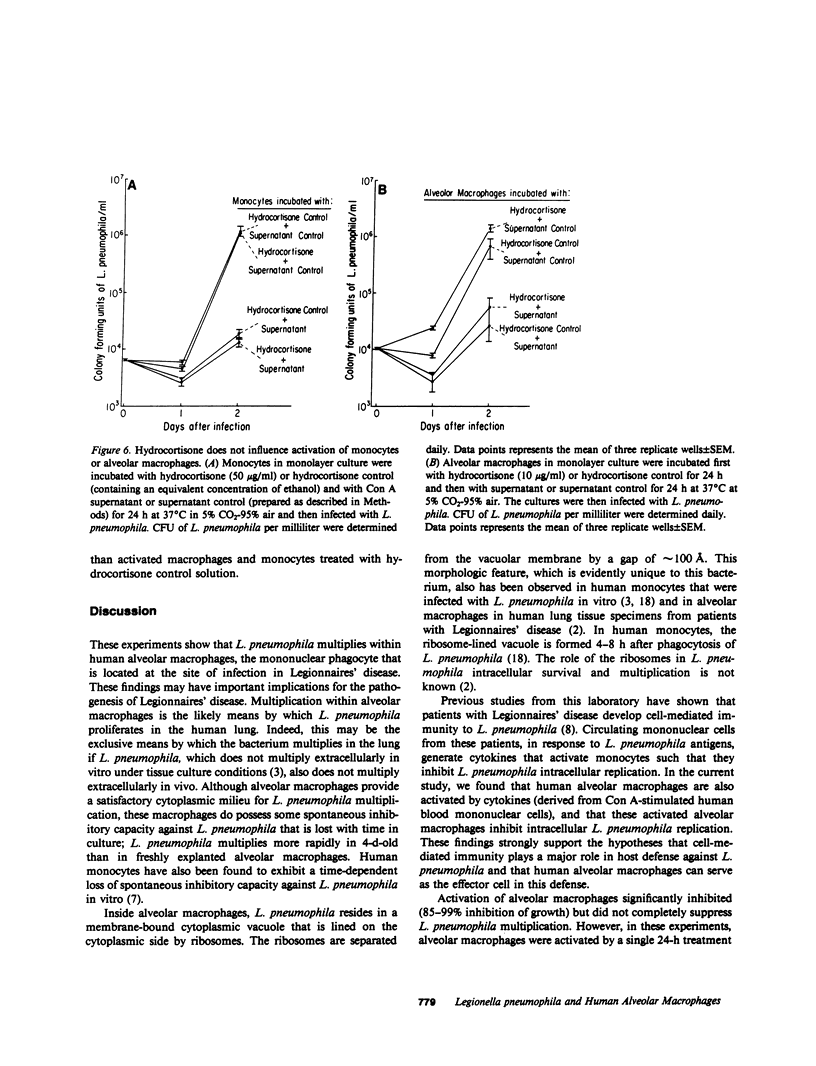
We have studied the interaction between virulent Legionella pneumophila and human alveolar macrophages, the resident phagocytes at the site of infection in Legionnaires' disease. L. pneumophila multiplied 2.5-5 logs within 3 d, as measured by colony forming units, when incubated with freshly explanted alveolar macrophages in monolayer culture. At the peak of bacterial multiplication, the alveolar macrophage monolayers were destroyed. L. pneumophila multiplied more rapidly in 4-d-old than in freshly explanted alveolar macrophages. Inside alveolar macrophages, L. pneumophila were located within membrane-bound vacuoles whose cytoplasmic sides were studded with ribosomes. Alveolar macrophages that were incubated with concanavalin A (Con A) stimulated human mononuclear cell supernatants (cytokines), inhibited L. pneumophila multiplication, and the degree of inhibition was proportional to the concentration of Con A supernatant added. Anti-L. pneumophila antibody in conjunction with complement promoted phagocytosis of L. pneumophila by alveolar macrophages. By electron microscopy, most (75%) of the phagocytized L. pneumophila were intracellular. However, freshly explanted alveolar macrophages were able to kill only 0-10% of an innoculum of L. pneumophila even in the presence of antibody and complement. At the same time, alveolar macrophages also killed opsonized Escherichia coli poorly. Increasing the ratio of macrophages to bacteria, adhering the macrophages to microcarrier beads, or preincubating the macrophages for 24 or 48 h with Con A supernatants failed to augment alveolar macrophage killing of opsonized E. coli. Corticosteroids appear to increase patient susceptibility to Legionnaires' disease. However, pretreatment of alveolar macrophages and monocytes with hydrocortisone had no influence on intracellular multiplication of L. pneumophila or on the inhibition of that multiplication by activated alveolar macrophages or monocytes. Hydrocortisone did impair cytokine-induced aggregation of alveolar macrophages. These findings demonstrate that L. pneumophila multiplies in human alveolar macrophages and that they do so within a ribosome-lined phagosome; that freshly explanted alveolar macrophages kill few L. pneumophila even in the presence of antibody and complement; that activated alveolar macrophages inhibit L. pneumophila multiplication; and that steroids do not exert a direct suppressive effect on the anti-L. pneumophila activity of activated or nonactivated alveolar macrophages. Our findings indicate that alveolar macrophages may play a central role in both the pathogenesis of Legionnaires' disease and in host defense against it. This paper shows that human resident macrophage can be activated to a higher state of antimicrobial capacity and that the human alveolar macrophage can serve as an effector call in call-mediated immunity.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnow P. M., Chou T., Weil D., Shapiro E. N., Kretzschmar C. Nosocomial Legionnaires' disease caused by aerosolized tap water from respiratory devices. J Infect Dis. 1982 Oct;146(4):460–467. doi: 10.1093/infdis/146.4.460. [DOI] [PubMed] [Google Scholar]
- Balow J. E., Rosenthal A. S. Glucocorticoid suppression of macrophage migration inhibitory factor. J Exp Med. 1973 Apr 1;137(4):1031–1041. doi: 10.1084/jem.137.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman B. L., Smathers M. Interaction of Nocardia asteroides with cultured rabbit alveolar macrophages. Infect Immun. 1976 Apr;13(4):1126–1131. doi: 10.1128/iai.13.4.1126-1131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood L. L., Pennington J. E. Dose-dependent effect of glucocorticosteroids on pulmonary defenses in a steroid-resistant host. Am Rev Respir Dis. 1982 Dec;126(6):1045–1049. doi: 10.1164/arrd.1982.126.6.1045. [DOI] [PubMed] [Google Scholar]
- Blussé van Oud Alblas A., van der Linden-Schrever B., Mattie H., van Furth R. The effect of glucocorticosteroids on the kinetics of pulmonary macrophages. J Reticuloendothel Soc. 1981 Jul;30(1):1–14. [PubMed] [Google Scholar]
- Cantey J. R., Hand W. L. Cell-mediated immunity after bacterial infection of the lower respiratory tract. J Clin Invest. 1974 Nov;54(5):1125–1134. doi: 10.1172/JCI107856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler F. W., Cole R. M., Hicklin M. D., Blackmon J. A., Callaway C. S. Ultrastructure of the Legionnaires' disease bacterium. A study using transmission electron microscopy. Ann Intern Med. 1979 Apr;90(4):642–647. doi: 10.7326/0003-4819-90-4-642. [DOI] [PubMed] [Google Scholar]
- Cohen A. B., Cline M. J. The human alveolar macrophage: isolation, cultivation in vitro, and studies of morphologic and functional characteristics. J Clin Invest. 1971 Jul;50(7):1390–1398. doi: 10.1172/JCI106622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D., Yoneda K. Detection and partial characterization of antibacterial factor(s) in alveolar lining material of rats. J Clin Invest. 1983 Jan;71(1):129–141. doi: 10.1172/JCI110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Scibienski C., Beaman B. L. Interaction of alveolar macrophages with Nocardia asteroides: immunological enhancement of phagocytosis, phagosome-lysosome fusion, and microbicidal activity. Infect Immun. 1980 Nov;30(2):578–587. doi: 10.1128/iai.30.2.578-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domby W. R., Whitcomb M. E. The effects of corticosteroid administration on the bronchoalveolar cells obtained from guinea pigs by lung lavage. Am Rev Respir Dis. 1978 May;117(5):893–896. doi: 10.1164/arrd.1978.117.5.893. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C., Balow J. E. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976 Mar;84(3):304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaumer H. R., Salvaggio J. E., Weston W. L., Claman H. N. Cortisol inhibition of immunologic activity in guinea pig alveolar cells. Int Arch Allergy Appl Immunol. 1974;47(6):797–809. doi: 10.1159/000231271. [DOI] [PubMed] [Google Scholar]
- Goldstein E., Lippert W., Warshauer D. Pulmonary alveolar macrophage. Defender against bacterial infection of the lung. J Clin Invest. 1974 Sep;54(3):519–528. doi: 10.1172/JCI107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M. The J. Burns Amberson Lecture--in defense of the lung. Am Rev Respir Dis. 1970 Nov;102(5):691–703. doi: 10.1164/arrd.1970.102.5.691. [DOI] [PubMed] [Google Scholar]
- Gump D. W., Frank R. O., Winn W. C., Jr, Foster R. S., Jr, Broome C. V., Cherry W. B. Legionnaires' disease in patients with associated serious disease. Ann Intern Med. 1979 Apr;90(4):538–542. doi: 10.7326/0003-4819-90-4-538. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Schmeling D., Peterson P. K. Phagocytosis, bacterial killing, and metabolism by purified human lung phagocytes. J Infect Dis. 1981 Jul;144(1):61–71. doi: 10.1093/infdis/144.1.61. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A. Cell-mediated immunity in Legionnaires' disease. J Clin Invest. 1983 Jun;71(6):1686–1697. doi: 10.1172/JCI110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983 Oct 1;158(4):1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. doi: 10.1084/jem.154.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Interaction of the Legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. I. L. pneumophila resists killing by polymorphonuclear leukocytes, antibody, and complement. J Exp Med. 1981 Feb 1;153(2):386–397. doi: 10.1084/jem.153.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Interaction of the legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. II. Antibody promotes binding of L. pneumophila to monocytes but does not inhibit intracellular multiplication. J Exp Med. 1981 Feb 1;153(2):398–406. doi: 10.1084/jem.153.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunologic reactivity of the lung. III. Effects of corticosteroids on alveolar macrophage cytotoxic effector cell function. J Immunol. 1977 Jan;118(1):146–150. [PubMed] [Google Scholar]
- Johnson J. D., Hand W. L., King N. L., Hughes C. G. Activation of alveolar macrophages after lower respiratory tract infection. J Immunol. 1975 Jul;115(1):80–84. [PubMed] [Google Scholar]
- Juers J. A., Rogers R. M., McCurdy J. B., Cook W. W. Enhancement of bactericidal capacity of alveolar macrophages by human alveolar lining material. J Clin Invest. 1976 Aug;58(2):271–275. doi: 10.1172/JCI108468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- LaForce F. M., Kelly W. J., Huber G. L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am Rev Respir Dis. 1973 Oct;108(4):784–790. doi: 10.1164/arrd.1973.108.4.784. [DOI] [PubMed] [Google Scholar]
- Laforce F. M., Boose D. S. Sublethal damage of Escherichia coli by lung lavage. Am Rev Respir Dis. 1981 Dec;124(6):733–737. doi: 10.1164/arrd.1981.124.6.733. [DOI] [PubMed] [Google Scholar]
- Lin H. S., Kuhn C., 3rd, Chen D. M. Effects of hydrocortisone acetate on pulmonary alveolar macrophage colony-forming cells. Am Rev Respir Dis. 1982 Jun;125(6):712–715. doi: 10.1164/arrd.1982.125.6.712. [DOI] [PubMed] [Google Scholar]
- Masur H., Murray H. W., Jones T. C. Effect of hydrocortisone on macrophage response to lymphokine. Infect Immun. 1982 Feb;35(2):709–714. doi: 10.1128/iai.35.2.709-714.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod R., Estes R., Mack D. G., McLeod E. G. Effects of human alveolar macrophages and peripheral blood monocytes on Toxoplasma gondii. J Infect Dis. 1983 May;147(5):957–957. doi: 10.1093/infdis/147.5.957. [DOI] [PubMed] [Google Scholar]
- Moore V. L., Myrvik Q. N. Inhibition of normal rabbit alveolar macrophages by factor(s) resembling migration inhibition factor. J Reticuloendothel Soc. 1974 Jul;16(1):21–26. [PubMed] [Google Scholar]
- Nakagawara A., DeSantis N. M., Nogueira N., Nathan C. F. Lymphokines enhance the capacity of human monocytes to secret reactive oxygen intermediates. J Clin Invest. 1982 Nov;70(5):1042–1048. doi: 10.1172/JCI110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Brown A., Elder E. M., Shonnard J., Luft B. J., Remington J. S. IgM and IgG antibody response in two immunosuppressed patients with Legionnaires' disease. Evidence of reactivation of latent infection. Am J Med. 1982 Dec;73(6):791–794. doi: 10.1016/0002-9343(82)90759-8. [DOI] [PubMed] [Google Scholar]
- Nguyen B. Y., Peterson P. K., Verbrugh H. A., Quie P. G., Hoidal J. R. Differences in phagocytosis and killing by alveolar macrophages from humans, rabbits, rats, and hamsters. Infect Immun. 1982 May;36(2):504–509. doi: 10.1128/iai.36.2.504-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Chaplan S., Reesink M., Tydings J., Cohn Z. A. Trypanosoma cruzi: induction of microbicidal activity in human mononuclear phagocytes. J Immunol. 1982 May;128(5):2142–2146. [PubMed] [Google Scholar]
- Nugent K. M., Pesanti E. L. Chronic glucocorticosteroid therapy impairs staphylococcal clearance from murine lungs. Infect Immun. 1982 Dec;38(3):1033–1036. doi: 10.1128/iai.38.3.1033-1036.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofrio J. M., Toews G. B., Lipscomb M. F., Pierce A. K. Granulocyte-alveolar-macrophage interaction in the pulmonary clearance of Staphylococcus aureus. Am Rev Respir Dis. 1983 Mar;127(3):335–341. doi: 10.1164/arrd.1983.127.3.335. [DOI] [PubMed] [Google Scholar]
- Pennington J. E. Differential effects of cyclophosphamide and cortisone acetate on bronchoalveolar phagocytic cell populations. Am Rev Respir Dis. 1978 Aug;118(2):319–324. doi: 10.1164/arrd.1978.118.2.319. [DOI] [PubMed] [Google Scholar]
- Pennington J. E., Ehrie M. G. Pathogenesis of Pseudomonas aeruginosa pneumonia during immunosuppression. J Infect Dis. 1978 Jun;137(6):764–774. doi: 10.1093/infdis/137.6.764. [DOI] [PubMed] [Google Scholar]
- Pennington J. E., Harris E. A. Influence of immunosuppression on alveolar macrophage chemotactic activities in guinea pigs. Am Rev Respir Dis. 1981 Mar;123(3):299–304. doi: 10.1164/arrd.1981.123.3.299. [DOI] [PubMed] [Google Scholar]
- Saravolatz L. D., Burch K. H., Fisher E., Madhavan T., Kiani D., Neblett T., Quinn E. L. The compromised host and Legionnaires' disease. Ann Intern Med. 1979 Apr;90(4):533–537. doi: 10.7326/0003-4819-90-4-533. [DOI] [PubMed] [Google Scholar]
- Territo M. C., Golde D. W. The function of human alveolar macrophages. J Reticuloendothel Soc. 1979 Jan;25(1):111–120. [PubMed] [Google Scholar]
- Weston W. L., Claman H. N., Krueger G. G. Site of action of cortisol in cellular immunity. J Immunol. 1973 Mar;110(3):880–883. [PubMed] [Google Scholar]
- Winn W. C., Jr, Myerowitz R. L. The pathology of the Legionella pneumonias. A review of 74 cases and the literature. Hum Pathol. 1981 May;12(5):401–422. doi: 10.1016/s0046-8177(81)80021-4. [DOI] [PubMed] [Google Scholar]
- Zwet T. L., Thompson J., Furth R. Effect of glucocorticosteroids on the phagocytosis and intracellular killing by peritoneal macrophages. Infect Immun. 1975 Oct;12(4):699–705. doi: 10.1128/iai.12.4.699-705.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]






