Abstract
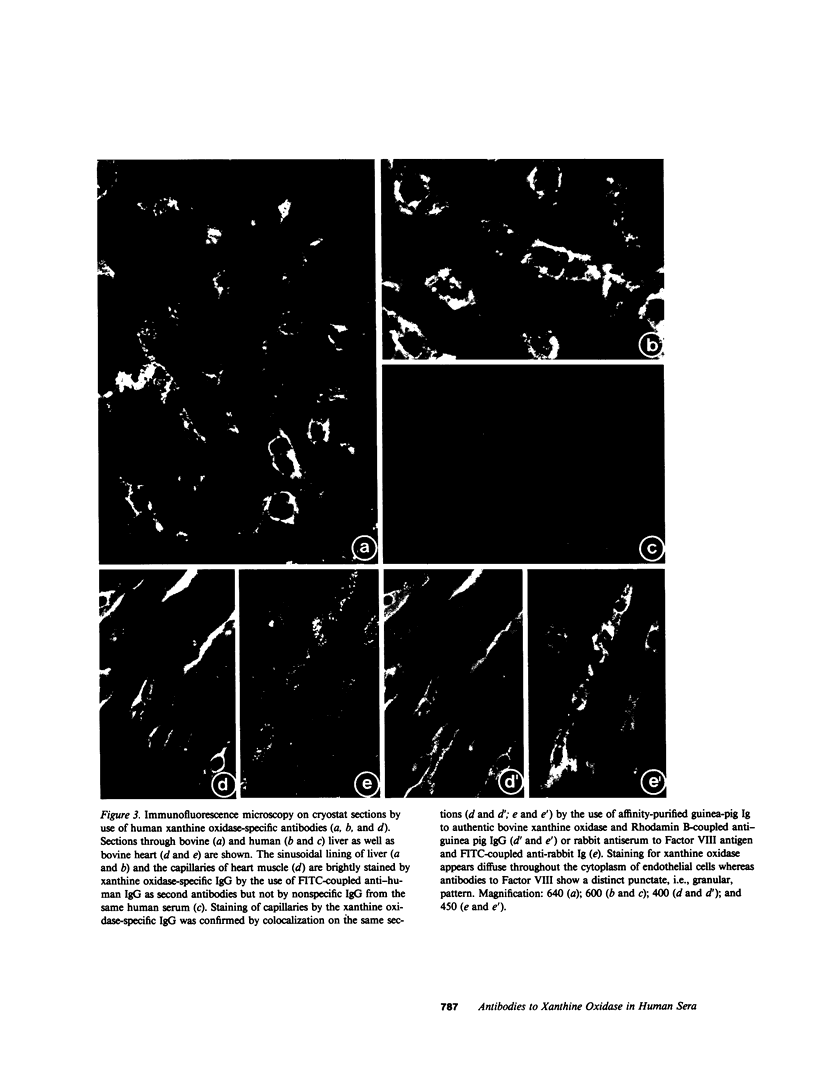
The widespread occurrence of antibodies (IgG) specific to xanthine oxidase in both normal (nonimmune) human and animal sera, and in antisera raised against a diversity of unrelated antigens is described. A study of sera from 81 humans revealed that xanthine oxidase-specific IgG represents a high proportion (1-8%) of total IgG. No obvious correlation to pathological events or symptoms of disease could be found. These xanthine oxidase-specific antibodies could be isolated by immunoaffinity chromatography on purified human or bovine xanthine oxidase and showed specific binding to the enzyme polypeptide of Mr 155,000 in immunoblotting experiments. By immunofluorescence microscopy they displayed the same cell type-specific reaction as experimentally induced antibodies, i.e., the staining of lactating mammary gland epithelium and capillary endothelium. The naturally occurring xanthine oxidase-specific antibodies consisted of polyclonal IgG of various subclasses. F(ab')2 preparations gave immune-reactions identical to those of IgG. The human xanthine oxidase-specific IgG cross-reacted with the bovine enzyme and both human and animal antibodies partially inhibited its activity. The xanthine oxidase activity of human milk lipid globules and supernatant fractions from various human tissues was extremely low when compared with that of the bovine antigen. The enzyme protein, however, was effectively precipitated from these sources by both the human and bovine antibodies. We suggest that the exceptionally high concentrations of antibodies against one protein, xanthine oxidase, are due to self-immunization to the xanthine oxidase antigen present in endothelial cells of capillaries. We do not exclude, however, nutritional contributions of bovine milk antigen to the appearance of xanthine oxidase antibodies in human sera. The possible biological functions of this immunological reaction are discussed.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Dighiero G., Lymberi P., Guilbert B. Studies on natural antibodies and autoantibodies. Ann Immunol (Paris) 1983 Jul-Aug;134D(1):103–113. [PubMed] [Google Scholar]
- Bruder G., Heid H. W., Jarasch E. D., Mather I. H. Immunological identification and determination of xanthine oxidase in cells and tissues. Differentiation. 1983;23(3):218–225. doi: 10.1111/j.1432-0436.1982.tb01286.x. [DOI] [PubMed] [Google Scholar]
- Bruder G., Heid H., Jarasch E. D., Keenan T. W., Mather I. H. Characteristics of membrane-bound and soluble forms of xanthine oxidase from milk and endothelial cells of capillaries. Biochim Biophys Acta. 1982 Mar 4;701(3):357–369. doi: 10.1016/0167-4838(82)90239-4. [DOI] [PubMed] [Google Scholar]
- Deeth H. C. Homogenized milk and atherosclerotic disease: a review. J Dairy Sci. 1983 Jul;66(7):1419–1435. doi: 10.3168/jds.S0022-0302(83)81956-0. [DOI] [PubMed] [Google Scholar]
- Dellagi K., Brouet J. C., Perreau J., Paulin D. Human monoclonal IgM with autoantibody activity against intermediate filaments. Proc Natl Acad Sci U S A. 1982 Jan;79(2):446–450. doi: 10.1073/pnas.79.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk H., Krepler R., Lackinger E., Artlieb U., Franke W. W. Biochemical and immunocytochemical analysis of the intermediate filament cytoskeleton in human hepatocellular carcinomas and in hepatic neoplastic nodules of mice. Lab Invest. 1982 Jun;46(6):584–596. [PubMed] [Google Scholar]
- Dighiero G., Guilbert B., Avrameas S. Naturally occurring antibodies against nine common antigens in humans sera. II. High incidence of monoclonal Ig exhibiting antibody activity against actin and tubulin and sharing antibody specificities with natural antibodies. J Immunol. 1982 Jun;128(6):2788–2792. [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Brown K. D., Birdwell C. R., Zetter B. R. Control of proliferation of human vascular endothelial cells. Characterization of the response of human umbilical vein endothelial cells to fibroblast growth factor, epidermal growth factor, and thrombin. J Cell Biol. 1978 Jun;77(3):774–788. doi: 10.1083/jcb.77.3.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbert B., Dighiero G., Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982 Jun;128(6):2779–2787. [PubMed] [Google Scholar]
- Hautanen A., Linder E. C3c-binding ELISA for the detection of immunoconglutinins and immunoglobulin aggregates. Methods Enzymol. 1981;74(Pt 100):588–607. doi: 10.1016/0076-6879(81)74041-2. [DOI] [PubMed] [Google Scholar]
- Hintner H., Steinert P. M., Lawley T. J. Human upper epidermal cytoplasmic antibodies are directed against keratin intermediate filament proteins. J Clin Invest. 1983 Oct;72(4):1344–1351. doi: 10.1172/JCI111090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A. Endothelial cells and the biology of factor VIII. N Engl J Med. 1977 Feb 17;296(7):377–383. doi: 10.1056/NEJM197702172960707. [DOI] [PubMed] [Google Scholar]
- Jarasch E. D., Bruder G., Keenan T. W., Franke W. W. Redox constituents in milk fat globule membranes and rough endoplasmic reticulum from lactating mammary gland. J Cell Biol. 1977 Apr;73(1):223–241. doi: 10.1083/jcb.73.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarasch E. D., Grund C., Bruder G., Heid H. W., Keenan T. W., Franke W. W. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981 Jul;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Krohne G., Debus E., Osborn M., Weber K., Franke W. W. A monoclonal antibody against nuclear lamina proteins reveals cell type-specificity in Xenopus laevis. Exp Cell Res. 1984 Jan;150(1):47–59. doi: 10.1016/0014-4827(84)90700-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lutz H. U., Wipf G. Naturally occurring autoantibodies to skeletal proteins from human red blood cells. J Immunol. 1982 Apr;128(4):1695–1699. [PubMed] [Google Scholar]
- Martin S. E., Martin W. J. Interspecies brain antigen detected by naturally occurring mouse anti-brain autoantibody. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1036–1040. doi: 10.1073/pnas.72.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather I. H., Jarasch E. D., Bruder G., Heid H. W., Mepham T. B. Protein synthesis in lactating guinea-pig mammary tissue perfused in vitro. I. Radiolabelling of membrane and secretory proteins. Exp Cell Res. 1984 Mar;151(1):208–223. doi: 10.1016/0014-4827(84)90369-0. [DOI] [PubMed] [Google Scholar]
- Mead G. M., Cowin P., Whitehouse J. M. Antitubulin antibody in healthy adults and patients with infectious mononucleosis and its relationship to smooth muscle antibody (SMA). Clin Exp Immunol. 1980 Feb;39(2):328–336. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Osborn M., Franke W. W., Weber K. Visualization of a system of filaments 7-10 nm thick in cultured cells of an epithelioid line (Pt K2) by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2490–2494. doi: 10.1073/pnas.74.6.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzucidlo S. J., Zikakis J. P. Correlation of dairy food intake with human antibody to bovine milk xanthine oxidase. Proc Soc Exp Biol Med. 1979 Apr;160(4):477–482. doi: 10.3181/00379727-160-40474. [DOI] [PubMed] [Google Scholar]
- Schmid E., Schiller D. L., Grund C., Stadler J., Franke W. W. Tissue type-specific expression of intermediate filament proteins in a cultured epithelial cell line from bovine mammary gland. J Cell Biol. 1983 Jan;96(1):37–50. doi: 10.1083/jcb.96.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senecal J. L., Rothfield N. F., Oliver J. M. Immunoglobulin M autoantibody to vimentin intermediate filaments. J Clin Invest. 1982 Mar;69(3):716–721. doi: 10.1172/JCI110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele E. J., Cunningham A. J. High proportion of Ig-producing cells making autoantibody in normal mice. Nature. 1978 Aug 3;274(5670):483–484. doi: 10.1038/274483a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]