Abstract
Purpose
Corneas with advanced Fuchs endothelial dystrophy that require endothelial keratoplasty manifest anterior corneal structural and cellular abnormalities that have been associated with visual deficits both before and after endothelial keratoplasty. In this study, we determined the onset of these abnormalities in the course of the disease.
Design
Cross-sectional study.
Participants
Sixty-three eyes (39 subjects) with a range of severity of Fuchs dystrophy, and 25 eyes (13 subjects) with normal corneas.
Methods
All corneas were examined by using slit-lamp biomicroscopy, ultrasonic pachymetry, and confocal microscopy. The clinical grade of Fuchs dystrophy was assessed according to the presence and extent of guttae and clinically evident edema, and was categorized as mild (grades 1–2), moderate (grades 3–4), or advanced (grades 5–6). Normal corneas were devoid of any central guttae (grade 0). Corneal backscatter (haze) was measured from the confocal image light intensity profile. Stromal cell density and number, and the presence of abnormal subepithelial cells, were determined from confocal images. Comparisons between groups were made by using generalized estimating equation models to account for any correlation between fellow eyes of the same subject.
Main Outcome Measures
Anterior corneal backscatter, stromal cell density and number, presence of subepithelial cells, central corneal thickness.
Results
Anterior corneal backscatter was 18–67% higher in moderate and advanced Fuchs dystrophy compared to normal (p≤0.003); a similar trend was noted in mild Fuchs dystrophy compared to normal (p=0.08). Stromal cell density and the absolute number of stromal cells in the anterior 10% of the stroma were approximately 20% and 27% lower, respectively, in Fuchs dystrophy (regardless of severity) compared to normal (p<0.001). Abnormal subepithelial cells were visible in 9%, 19%, and 30% of corneas with mild, moderate, and advanced Fuchs dystrophy, respectively. Only corneas with advanced Fuchs dystrophy were thicker than normal (p<0.001).
Conclusions
Anterior corneal cellular and structural abnormalities begin early in the course of Fuchs dystrophy, prior to the onset of clinically evident edema. The chronicity of these changes can explain their incomplete resolution after endothelial keratoplasty, and understanding the onset of these might help to determine the optimal time to intervene to achieve best outcomes.
Fuchs endothelial corneal dystrophy is a bilateral, progressive, inherited disease of the corneal endothelium characterized by guttata and corneal edema resulting in decreased vision.1,2 Advanced and untreated cases result in corneal scarring and vascularization, which were the anterior corneal manifestations originally described by Fuchs in 1910.3 Traditional staging of the disease, based on the extent of guttae and presence of clinically detectable corneal edema, was appropriate when penetrating keratoplasty was the treatment of choice for Fuchs dystrophy. However, with the advances in corneal transplantation techniques enabling earlier surgical intervention4 and the future promise of non-surgical treatments,5,6 staging Fuchs dystrophy based on the presence or absence of clinical edema may no longer be adequate.7 Ideally, we would identify patients who would benefit from intervention before any changes in the host tissue that affect outcomes become irreversible.
Corneas with Fuchs dystrophy that require transplantation manifest abnormalities beyond guttae and edema. We previously found that anterior backscatter (haze) in such corneas was higher than normal, and this persisted at 2 years after restoring endothelial function by endothelial keratoplasty.8,9 Similarly, corneas with Fuchs dystrophy at the stage of transplantation also manifest abnormal subbasal and stromal nerves,10 depletion of anterior keratocytes,11 and the presence of abnormal subepithelial cells.12 The onset of these changes in the course of Fuchs dystrophy is unknown, but given that many of these changes do not resolve even at 3 years after endothelial keratoplasty,12 we hypothesized that they begin early in the course of the disease. Improving our understanding of the onset of corneal changes in Fuchs dystrophy might help determine the optimal time to intervene, especially as newer treatments are developed.6,13 In addition, quantitative analyses of corneas with Fuchs dystrophy could lead to objective methods of assessing disease severity and lead to a better understanding of the optical performance of these corneas before and after keratoplasty.
The goal of this study was to determine when corneal abnormalities become evident in the course of Fuchs dystrophy. Specifically, we examined corneas with a range of severity of Fuchs dystrophy by using confocal microscopy in vivo to measure corneal backscatter, stromal cell populations, and the presence of abnormal subepithelial cells.
METHODS
Subjects
Participants with Fuchs endothelial dystrophy and participants with normal corneas were recruited from patients at Mayo Clinic, Rochester, Minnesota. All patients with Fuchs dystrophy were examined by a corneal specialist (KHB or SVP); Fuchs dystrophy was defined as the presence of central or paracentral guttae by slit lamp examination, with or without clinically detectable edema. Eyes with Fuchs dystrophy were phakic, or pseudophakic with a posterior chamber intraocular lens, whereas eyes with normal corneas were all phakic. Exclusion criteria for all subjects included the presence of any corneal disease (except Fuchs dystrophy in the Fuchs dystrophy group), previous intraocular surgery (except phacoemulsification with posterior chamber intraocular lens implantation in the Fuchs dystrophy group), previous keratorefractive surgery, contact lens wear, pregnancy, diabetes, ocular hypertension or glaucoma, and use of topical medications (with the exception of artificial tears) or systemic medications known to affect the cornea. This study was prospectively approved by the Mayo Clinic Institutional Review Board and complied with the Health Insurance Portability and Accountability Act. The research adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients.
Clinical Grading
All subjects were examined by using slit-lamp biomicroscopy. Corneas without guttae were considered normal (grade 0). Corneas with central or paracentral guttae were considered to have Fuchs dystrophy and were graded according to the confluence of guttae and presence of corneal epithelial or stromal edema.7,14 Corneas with grades 1 or 2 were considered to have mild Fuchs dystrophy, with grades 3 or 4 were considered to have moderate Fuchs dystrophy, and with grades 5 or 6 were considered to have advanced Fuchs dystrophy. If corneas had clinically detectable corneal edema, they were assigned to grade 6 irrespective of the distribution of guttae.
Confocal Microscopy In Vivo
Subjects were examined by using a slit-scanning confocal microscope (ConfoScan 4, Nidek Technologies, Greensboro, NC). The central cornea was examined by using a through-focusing technique with a 40x objective15 and z-ring adapter to stabilize the cornea and determine the depth of the confocal plane in the cornea.16 Full-thickness confocal scans were acquired with a frame-to-frame step distance of 4 μm from endothelium to epithelium. Two to four scans were obtained and the best scan without axial movement of the cornea and minimal lateral movement was used for analysis. Prior to each confocal examination, corneal image intensity of a standard scattering solution (Amco Clear, GFS Chemicals) was measured to account for any fluctuations in the light source or detection system over time.17 The central corneal endothelium of subjects with Fuchs dystrophy was also examined with the same confocal microscope and a 20x non-contact objective for endothelial cell and guttate area analysis, which is an objective measure of disease severity.18
Corneal Backscatter
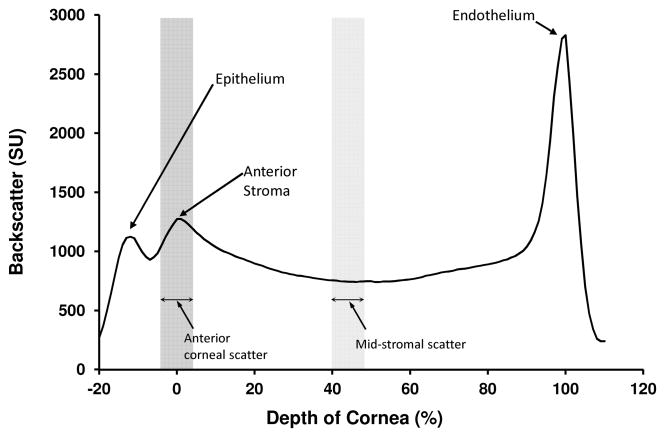
Corneal backscatter was determined from the intensity of confocal images in the central 167 μm × 169 μm area (300 × 300 pixel area), and all measurements were adjusted according to the intensity of the image brightness of a standard scattering solution scanned just before scanning the patient, to account for variations in the intensity of the light source or sensitivity of the detection system.17 Corneal backscatter was expressed as scatter units (SU), the mean intensity of an image of a solution of Amco Clear of known concentration (in nephelometric turbidity units) that gave the same image brightness as the confocal image of the cornea.17 A light intensity profile was generated for each cornea (Figure 1), and images corresponding to the epithelial surface, the anterior and posterior boundaries of the stroma, and the endothelium, were identified by an experienced reviewer (SVP) from the image brightness and appearance of the images. Because of variation in corneal thickness, image depth was scaled as a percentile of stromal thickness with the anterior stromal boundary as 0% and the endothelium as 100%.8,17 Anterior corneal backscatter was defined as the mean intensity of images spanning a 10- percentile range centered at the anterior stromal boundary, and mid-stromal backscatter was defined as the mean intensity of images spanning a 10-percentile range centered at the mid-stroma.
Figure 1.
Profile of backscatter through the normal cornea. Corneal backscatter profiles were generated from through-focus confocal images with the depth of the cornea scaled from 0 % (anterior stromal boundary) to 100 % (endothelium) of stromal thickness.15 Backscatter was measured in scatter units (SU), which were equivalent to the concentration of a turbidity standard that produced the same image brightness.
Stromal Cell Populations and Subepithelial Cells
Stromal cell density was determined from confocal images by using a custom automated program that identified bright objects (stromal cell nuclei).15 An experienced and masked observer (SVP) then manually corrected the automated analysis of images from corneas with Fuchs dystrophy because pathologic changes in the disease reduce the accuracy of cell identification by the automated program, particularly in the anterior 10% of the stroma where normal cell morphology had been disrupted.12 Cell density was determined in two frames selected from each of 5 layers representing the anterior 10%, 11–33%, 34–66%, 67–90%, and posterior 10% of the stroma.
Stromal cell density in edematous corneas with Fuchs dystrophy can be lower than normal because cells are redistributed in a larger tissue volume. To eliminate this effect of corneal edema, the absolute number of cells in a full-thickness section of stroma with frontal area of 1 mm2 was determined. This was equal to the sum of the number of cells in each layer represented by each frame selected to estimate cell density. The number of cells in each layer was the product of cell density and thickness of the layer, determined by the distance between the midpoints between the frame stromal depth and stromal depths of the two adjacent frames.11
Confocal scans were reviewed and the presence of abnormal subepithelial cells was noted.12 Because the small area of the confocal image can miss abnormal cells if they are sparsely distributed, all confocal scans for an eye were reviewed to determine if these cells were visible.
Effective endothelial cell density
Effective endothelial cell density (ECDe) was measured from the 20x confocal images of the endothelium as described in detail previously.18 Briefly, these images represented an endothelial area of 0.33 mm2, and ECDe was assessed from two or three images of the central cornea. The ECDe was an estimate of the number of cells in the entire image divided by the area of the image, and was determined by:
where ECDl was the local endothelial cell density, determined by using a variable frame technique for contiguous cells, and R was the fraction of the image covered by guttae and was determined by image filtering and thresholding.18
Corneal Thickness
Central corneal thickness was measured by using an ultrasonic pachymeter (DGH Pachette; DGH Technology, Exton, PA). After topical anesthetic (proparacaine hydrochloride 0.5%) was instilled, the pachymeter probe was placed in contact with the central cornea. Central corneal thickness was the mean of 25 measurements in rapid succession.
Statistical Analysis
The primary outcome of this study was anterior corneal backscatter. Based on previous anterior backscatter data in normal corneas and corneas with advanced Fuchs dystrophy,8 and assuming that the increase in anterior backscatter was linear with increasing severity of Fuchs dystrophy, we estimated that a difference of 370 scatter units17 (SU) would distinguish between consecutive groups (normal, mild, moderate, and advanced) of disease severity. A priori analysis to detect a difference of 330 SU between groups, assuming an average standard deviation of 450 SU, indicated that 20 eyes would be needed in each group (α=0.05, β=0.20, unpaired analysis, unequal variances). Differences in corneal backscatter between severities of disease were assessed by using generalized estimating equation (GEE) models to account for any correlation between fellow eyes of the same subject.19 Relationships between backscatter, number of stromal cells, clinical grade, ECDe, and central corneal thickness, were illustrated by using Pearson coefficients, and significances of the correlations were determined by using GEE models. Minimum detectable differences (MDDs) were calculated post hoc for non-significant comparisons (α=0.05, β=0.20).
RESULTS
Subjects
We examined 20 eyes (11 subjects) with advanced Fuchs dystrophy, 21 eyes (15 subjects) with moderate Fuchs dystrophy, 22 eyes (13 subjects) with mild Fuchs dystrophy, and 25 eyes (13 subjects) with normal corneas (Table 1). Normal subjects were younger than those with Fuchs dystrophy (p<0.001). Nine eyes in the Fuchs dystrophy group were pseudophakic; in all cases, previous uncomplicated phacoemulsification and endocapsular intraocular lens insertion was performed at our institution and none of these eyes had clinically significant corneal edema in the postoperative period.
Table 1.
Characteristics of enrolled subjects
| Fuchs Dystrophy
|
Normal | |||
|---|---|---|---|---|
| Mild | Moderate | Advanced | ||
| Number of eyes [subjects] | 22 [13] | 21 [15] | 20 [11] | 25 [13] |
| Age, Mean ± SD (years) [range] | 69 ± 13 [42–88] | 68 ± 11 [48–83] | 64 ± 11 [42–79] | 54 ± 6 [45–69] |
| Cornea Guttata (Grade) | 1 and 2 | 3 and 4 | 5 and 6 | 0 |
| Lenticular Status (number of eyes) | Phakic (18), PCIOL (4) | Phakic (18), PCIOL (3) | Phakic (18), PCIOL (2) | Phakic (25) |
SD, standard deviation; PCIOL, posterior chamber intraocular lens
Corneal Backscatter
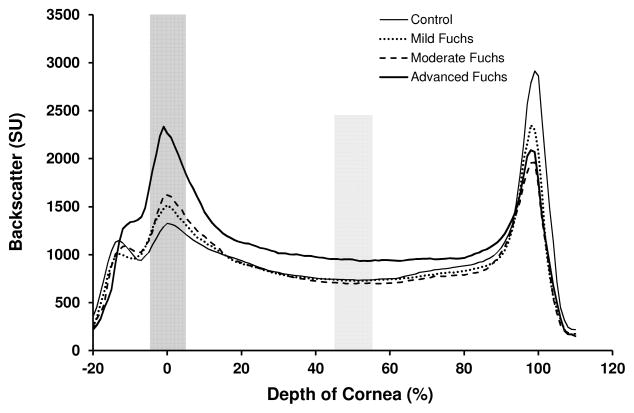
Corneal backscatter profiles had a similar shape between eyes with Fuchs dystrophy and normal eyes, with peaks corresponding to the subepithelial region and the endothelium (Figure 2). Anterior corneal backscatter was higher in eyes with moderate and advanced Fuchs dystrophy compared to normal (p≤0.003, Table 2, Figure 2), with a trend towards higher backscatter in mild Fuchs dystrophy compared to normal (p=0.08, Table 2, Figure 2). Mid-corneal backscatter was higher in advanced Fuchs dystrophy compared to normal and compared to mild and moderate Fuchs dystrophy (p<0.001, Figure 2, Table 2).
Figure 2.
Corneal backscatter by severity of Fuchs dystrophy. Backscatter from the anterior cornea differed between severities of Fuchs dystrophy, whereas backscatter from the mid-stroma only differed for advanced Fuchs dystrophy.
Table 2.
Corneal Backscatter and Central Corneal Thickness
| Fuchs Dystrophy
|
Normal | |||
|---|---|---|---|---|
| Mild | Moderate | Advanced | ||
| Anterior Corneal Backscatter (SU) | 1382 ± 282b,c,d | 1457 ± 227c | 2053 ± 579 | 1231 ± 230 |
| pa | 0.08 | 0.003 | <0.001 | - |
| Mid-Corneal Backscatter (SU) | 888 ± 160 | 893 ± 184 | 1109 ± 141e | 908 ± 205 |
| pa | 0.93 | 0.93 | <0.001 | - |
| Central Corneal Thickness (μm) | 566 ± 29 | 568 ± 37 | 588 ± 53 | 553 ± 30 |
| pa | 0.19 | 0.22 | 0.03 | - |
All values are mean ± standard deviation; SU, scatter units17
p-values are versus control, generalized estimating model equations
p=0.76 vs. moderate Fuchs dystrophy
p<0.001 vs. advanced Fuchs dystrophy
Minimum detectable difference compared to controls, 155 SU (α=0.05, β=0.20)
p<0.001 vs. mild and p<0.001 vs. moderate Fuchs dystrophy
Corneal Stromal and Subepithelial Cells
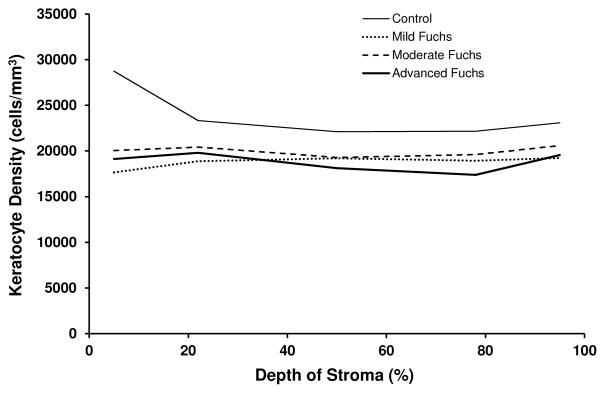
Cell density in the anterior 10% of the stroma was approximately 20% lower in each of the 3 severity groups (mild, moderate, and advanced) Fuchs dystrophy compared to normal (p<0.001, Table 3, Figures 3 and 4). There was a trend towards lower cell density in the deeper stroma of corneas with advanced Fuchs dystrophy compared to normal (Table 3). The absolute number of cells through the full thickness of the cornea in a section with frontal area of 1 mm2 was approximately 27% lower in mild, moderate, and advanced Fuchs dystrophy compared to normal (p<0.001, Table 3, Figure 4).
Table 3.
Density and Number of Stromal Cells
| Fuchs Dystrophy
|
Normal | |||
|---|---|---|---|---|
| Mild | Moderate | Advanced | ||
| Stromal Cell Density (cells/mm3) by stromal layer, % | ||||
| 0–10 (anterior) | 17,662 ± 3,975a | 20,041 ± 5,945b | 19,120 ± 4,805a | 26,206 ± 6,222 |
| 11–33 | 18,886 ± 3,646 | 20,426 ± 2,720 | 19,793 ± 3,005 | 21,236 ± 3,419 |
| 34–66 | 19,200 ± 4,073 | 19,272 ± 3,104 | 18,100 ± 3,341 | 20,374 ± 3,913 |
| 67–90 | 18,921 ± 3,189 | 19,602 ± 2,944 | 17,389 ± 3,958c,d | 19,897 ± 3,418 |
| 91–100 | 19,235 ± 3,230 | 20,591 ± 3,072 | 19,562 ± 4,854 | 20,220 ± 3,507 |
| Number of stromal cellse by stromal layer, % | ||||
| 0–10 (anterior) | 882 ± 211a | 982 ± 235a | 981 ± 245a | 1,355 ± 293 |
| 11–33 | 1,911 ± 479 | 2,045 ± 368 | 2,057 ± 394 | 2,023 ± 367 |
| 34–66 | 2,719 ± 656 | 2,820 ± 529 | 2,628 ± 506 | 2,760 ± 557 |
| 67–90 | 2,085 ± 390 | 2,139 ± 427 | 1,978 ± 370 | 2,095 ± 435 |
| 91–100 | 1,013 ± 213 | 1,050 ± 202 | 1,065 ± 268 | 1,040 ± 258 |
Only significant differences are indicated (generalized estimating equation models):
p<0.001,
p=0.005
p=0.05 vs. normal
p=0.05 vs. moderate Fuchs dystrophy
Absolute number of cells in a section of stroma with frontal area of 1 mm2
Figure 3.
Stromal cell density by severity of Fuchs dystrophy. Stromal cell density was significantly lower in the anterior 10% of the stroma in mild, moderate and advanced Fuchs dystrophy compared to normal (p<0.001). The lower cell density in the deeper stroma of Fuchs dystrophy compared to normal can be explained by corneal edema (see Table 3, which shows that the absolute number of cells in a section of cornea was only lower in the anterior 10% of the stroma and not deeper).
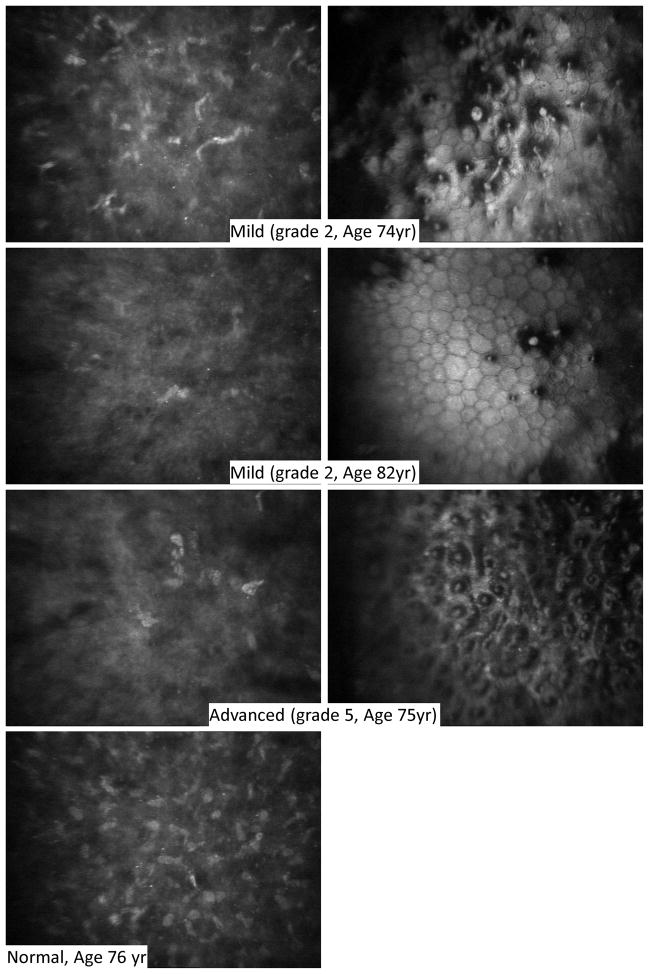
Figure 4.
Anterior stromal cells and associated endothelium in Fuchs dystrophy. The most anterior stromal cells (left) were depleted and extracellular reflectivity was higher in Fuchs dystrophy (upper three rows) compared to normal (bottom row). Anterior stromal cell depletion was evident even in mild cases of Fuchs dystrophy (upper two rows) in which the corresponding endothelial images (right) have scattered guttae. One case of advanced Fuchs dystrophy (third row) is also shown with depleted anterior stromal cells (left) and confluent guttae (right).
Abnormal subepithelial cells were visible in 12/63 eyes (19%) of all corneas with Fuchs dystrophy (Figure 5). These cells were visible in 2/22 (9%), 4/21 (19%), and 6/20 (30%) of corneas with mild, moderate, and advanced Fuchs dystrophy, respectively.
Figure 5.
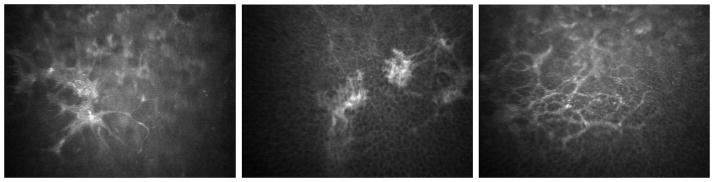
Confocal images of subepithelial cells in Fuchs dystrophy. Each image is from three different corneas and shows reticular networks of subepithelial cells (presumed fibroblasts). These were located just deep to the basal epithelial cells and were typically highly reflective, thus contributing to anterior corneal backscatter and presumably to an irregular anterior corneal surface.
Effective endothelial cell density
In Fuchs dystrophy, ECDe ranged from zero in corneas with large areas of confluent guttae to 2,946 cells/mm2 in corneas with few guttae. ECDe was 1,812 ± 657 cells/mm2 (mean ± standard deviation) in mild Fuchs dystrophy, 852 ± 574 cells/mm2 in moderate Fuchs dystrophy, and 59 ± 185 cells/mm2 in advanced Fuchs dystrophy.
Central Corneal Thickness
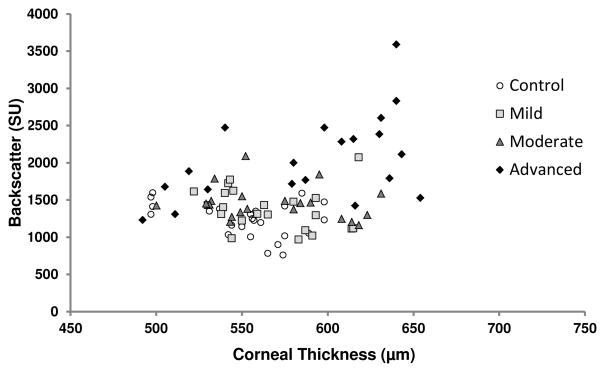
Corneas with advanced Fuchs dystrophy were thicker than normal (p<0.001), whereas corneas with mild and moderate Fuchs dystrophy did not differ from normal (mild, p=0.19, MDD= 24 μm; moderate, p=0.22, MDD=28 μm, Table 2). In normal corneas, there was little variation in anterior corneal backscatter across the range of central corneal thicknesses, whereas in corneas with Fuchs dystrophy, anterior corneal backscatter was more variable when central corneal thicknesses were in the normal range, and variability increased in corneas that were thicker than normal (Figure 6).
Figure 6.
Relationship between anterior corneal backscatter and central corneal thickness. In normal corneas, there was little variation in anterior corneal backscatter over a range of central corneal thicknesses. In corneas with Fuchs dystrophy, anterior corneal backscatter was more variable over the normal range of central corneal thickness, and the variability increased in thicker than normal corneas.
Relationships between anterior corneal abnormalities and disease severity
In Fuchs dystrophy, objective assessment of disease severity determined by the ECDe was strongly correlated with subjective assessment of disease severity determined by clinical grade (r= −0.84, p<0.001, n=63, Table 4). Central corneal thickness was not correlated with subjective grade or with ECDe (Table 4).
Table 4.
Relationships between anterior corneal abnormalities and parameters assessing severity of Fuchs dystrophy (n=63)
| ECDe | CCT | Number of stromal cells (anterior 10%) | Anterior Corneal Backscatter | |
|---|---|---|---|---|
| Clinical Grade | r= −0.84, p<0.001 | r= 0.25, p=0.13 | r=0.28, p=0.03 | r=0.56, p<0.001 |
| ECDe | - | r= −0.04, p=0.58 | r= −0.33, p=0.38 | r= −0.48, p<0.001 |
| CCT | - | - | r= −0.13, p=0.28 | r=0.32, p=0.01 |
| Number of stromal cells (anterior 10%) | - | - | - | r= −0.26, p=0.18 |
ECDe, Effective endothelial cell density; CCT, central corneal thickness
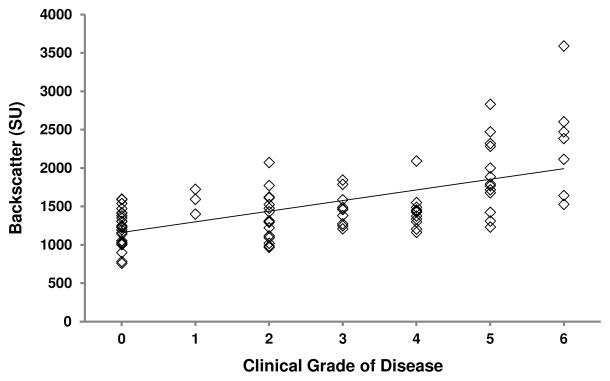
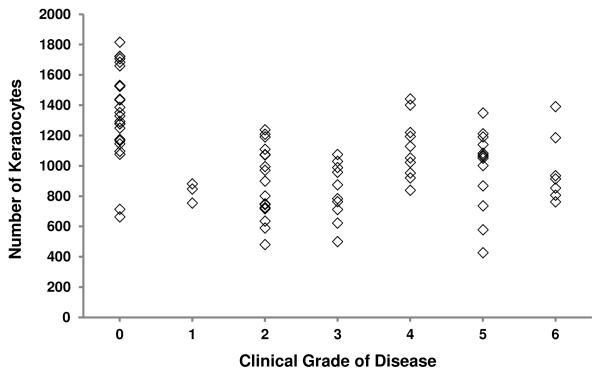
In all eyes, anterior corneal backscatter (r= 0.61, p<0.001, n=88, Figure 7) and the number of cells in the anterior 10% of the stroma (r= −0.36, p=0.002, n=88, Figure 8) were correlated with clinical grade. Relationships in eyes with Fuchs dystrophy are summarized in Table 4.
Figure 7.
Relationship between anterior corneal backscatter and grade of Fuchs dystrophy. In all eyes, anterior corneal backscatter increased with increasing grade of disease (r=0.61, p<0.001, n=88; regression line shown). In eyes with Fuchs dystrophy, a similar relationship was evident (r=0.56, p<0.001, n=63; regression line not shown for clarity).
Figure 8.
Relationship between number of keratocytes in the anterior 10% of the stroma and grade of Fuchs dystrophy. In all eyes, the number of keratocytes in the anterior 10% of the stroma decreased with increasing grade of disease (r= −0.36, p=0.002, n=88). In eyes with Fuchs dystrophy only, this trend was reversed and was weakly significant (r= 0.28, p=0.03). Regression lines are not shown for clarity.
DISCUSSION
Anterior cellular and structural abnormalities are known to be present in the advanced stages of Fuchs dystrophy,8,10,12 and in this study, we determined that these changes develop by the mild and moderate stages of the disease. Our findings indicate that the anterior cornea changes much earlier in the course of the disease than previously considered. Improving our understanding of these changes might help improve outcomes as newer treatments enable earlier intervention.
Fuchs dystrophy is considered to be a disease of the corneal endothelium that drives secondary changes in the anterior cornea. Traditionally, anterior corneal degradation, including corneal vascularization and scarring, was considered to be a late feature of the disease,1,2,20 but more recent studies have shown that subclinical and subtle clinical anterior corneal changes are present in Fuchs dystrophy corneas at the time of endothelial keratoplasty.8,12 In the present study, we found evidence that the structure and cellularity of corneas with Fuchs dystrophy becomes abnormal in the early stages of disease, explaining why the structure and optical function of the host corneas do not return to normal after endothelial keratoplasty.9,12 We are unaware of other studies that have found similar evidence of early anterior pathophysiologic changes in Fuchs dystrophy.
The clinical relevance of increased anterior corneal backscatter is its relationship to disability glare. Anterior corneal structural changes that result in increased anterior corneal backscatter also induce intraocular forward light scatter, which causes disability glare.21 Indeed, improvement in anterior corneal backscatter after endothelial keratoplasty has been associated with a concomitant improvement in disability glare.8 Because glare and increased corneal backscatter do not return to normal after successful endothelial keratoplasty, they cannot solely be explained by corneal edema. It is more likely that these findings are caused by chronic ultrastructural changes that persist in the anterior cornea. The present study supports this explanation, because abnormalities were present in the early stages of Fuchs dystrophy. In addition, disability glare is an early symptom of Fuchs dystrophy and can be explained by the presence of anterior corneal structural changes that result in increased anterior corneal backscatter. Although we were unable to show a statistical increase in anterior corneal backscatter in the earliest stages of Fuchs dystrophy in this study, there was a trend towards a difference that might have become significant with a larger sample size.
We previously found evidence of anterior stromal cell loss in Fuchs dystrophy by histology11 and by confocal microscopy in vivo before and after endothelial keratoplasty.12 Although the importance of stromal cell depletion in Fuchs dystrophy has not yet been established, the present study indicates that it occurs early, and possibly as early as clinical grades 1 and 2. We had expected to find a gradual decline in anterior stromal cells with increasing severity of disease, but in fact, anterior stromal cells were similarly depleted across the spectrum of disease (Figure 8). Although there was a very weak positive correlation between increasing subjective grade and anterior stromal cells in Fuchs dystrophy (Figure 8, Table 4), the same relationship was not apparent with objective assessment of disease severity by using the effective endothelial cell density (Table 4). With the significant variation in the number of anterior stromal cells by grade of disease (Figure 8), one cannot conclude that anterior stromal cells increase with increasing severity of disease based on this small sample. In addition, the variability of anterior stromal cellularity suggests that factors other than grade, such as age, disease chronicity, or genetic variation, affect cell loss. The mechanism of cell loss is not known, but might be similar to stromal cell loss after epithelial injury,22 or might represent a primary susceptibility to cell death.23 These cells are responsible for the repair of the corneal stroma and the maintenance of corneal curvature24; thus their depletion could contribute to the structural changes that increase corneal backscatter and irregularity, which affect visual outcomes after endothelial keratoplasty.4,25
Abnormal subepithelial cells were present in at least 9% of corneas with mild Fuchs dystrophy, and their presence increased with severity of the disease. We previously found these cells in two-thirds of corneas receiving endothelial keratoplasty, and they also persisted after successful endothelial keratoplasty.12 Their presence early in the course of the disease is further evidence of disease chronicity. Given the location of these cells just deep to the epithelium, they have been implicated as a cause of irregularity of the anterior corneal surface, which results in increased anterior corneal aberrations before and after endothelial keratoplasty.26 The increased anterior corneal aberrations are correlated with decreased visual acuity and quality after endothelial keratoplasty,26–29 sometimes requiring rigid contact lens correction for visual improvement.30
While Fuchs dystrophy clinically manifests as corneal guttae and edema, the anterior cornea also changes in other ways subtly and sub-clinically, possibly prior to the onset of a clinically detectable increase in corneal thickness. Nevertheless, corneas in the early stages of Fuchs dystrophy appear to be thicker than normal,7,31 suggesting that subclinical edema is present early in the disease because of early endothelial dysfunction. Thus, the anterior corneal abnormalities may be secondary to chronic subclinical corneal edema. This study was unable to identify the exact time and progression of the various anterior corneal changes, but we hypothesize that stromal cells are lost before, and this loss contributes to, the development of abnormal subepithelial cells and increased backscatter. Whether these anterior corneal changes can be used to identify stages of Fuchs dystrophy objectively remains to be determined.
This study had a relatively small sample size but was adequately powered to show meaningful differences between groups, even though our a priori assumptions varied slightly from what we actually found for corneal backscatter. Relationships between parameters and clinical grade should be interpreted cautiously because clinical grading is a subjective and morphologic assessment of the endothelium that can vary between specialists, and this traditional assessment is not based on measured criteria.7 In addition, the subjective grading scale might not be linearly related to objective metrics of the cornea. Despite these limitations, subjective grading correlates well with objective assessment of the disease by using the ECDe,18 and in this study we found that subjective grading as an estimate of disease severity was more closely related to ECDe than it was to central corneal thickness, which is not always a good measure of central corneal edema.7 While we attempted to age-match our subjects with Fuchs dystrophy and normal corneas, the mean age of the normal group was younger. Despite this limitation, increasing age is minimally associated with increased corneal backscatter,32 and the 4% per decade decrease in stromal cells33 would not explain the magnitude of the cell loss in this study. The stromal cell densities reported in this study appear lower than those in our previous study12 because unlike the previous study, we were unable to correct the densities for the effect of corneal edema (surgical intervention was performed in the previous study which enabled measurement of postoperative host stromal thickness). Nevertheless, we calculated absolute numbers of cells in sections of the cornea, which eliminated the effect of corneal edema, and confirmed loss of cells. Quantification of subepithelial cells was not possible because of regional variation and potential sampling errors with narrow-field confocal microscopy12; our estimate of the presence of subepithelial cells was therefore a minimum, and it is possible that more corneas actually exhibited this finding.
In summary, we found that the anterior cornea manifests abnormalities early in the course of Fuchs dystrophy, at stages where changes were previously considered insignificant. The notion that Fuchs dystrophy is only of clinical significance when it progresses to advanced stages with corneal edema, may have been appropriate when penetrating keratoplasty was the treatment of choice. With newer surgical techniques that enable earlier intervention, the early subclinical changes are more relevant to postoperative visual outcomes. Improving our understanding of the onset of corneal changes that affect outcomes might help determine the optimal time for intervention, especially as new surgical34 and medical5,13 interventions become available for the treatment of Fuchs dystrophy.
Acknowledgments
Support: Supported by the Mayo Clinic Center for Translational Science Activities (Grant UL1 TR000135 from the National Center for Advancing Translational Sciences [NCATS], a component of the National Institutes of Health); Research to Prevent Blindness, New York, NY (unrestricted departmental grant, and SVP as Olga Keith Wiess Special Scholar); Mayo Foundation, Rochester, MN. The funding organization had no role in the design or conduct of this research.
Footnotes
Financial Disclosure: None, All authors. Dr. Baratz has a patent application pending for “Assessing the risk of developing Fuchs Dystrophy”.
This work was and will be presented in part at the Association for Research in Vision and Ophthalmology Annual Meetings in Seattle, May 2013, and Orlando, May 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol. 1993;38:149–68. doi: 10.1016/0039-6257(93)90099-s. [DOI] [PubMed] [Google Scholar]
- 2.Wilson SE, Bourne WM. Fuchs’ dystrophy. Cornea. 1988;7:2–18. [PubMed] [Google Scholar]
- 3.Fuchs E. Dystrophia epithelialis corneae. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1910;76:478–508. [Google Scholar]
- 4.van der Meulen IJ, Patel SV, Lapid-Gortzak R, et al. Quality of vision in patients with Fuchs endothelial dystrophy and after Descemet stripping endothelial keratoplasty. Arch Ophthalmol. 2011;129:1537–42. doi: 10.1001/archophthalmol.2011.247. [DOI] [PubMed] [Google Scholar]
- 5.Koizumi N, Okumura N, Ueno M, et al. Rho-associated kinase inhibitor eye drop treatment as a possible medical treatment for Fuchs corneal dystrophy. Cornea. 2013;32:1167–70. doi: 10.1097/ICO.0b013e318285475d. [DOI] [PubMed] [Google Scholar]
- 6.Okumura N, Koizumi N, Kay EP, et al. The ROCK inhibitor eye drop accelerates corneal endothelium wound healing. Invest Ophthalmol Vis Sci. 2013;54:2493–502. doi: 10.1167/iovs.12-11320. [DOI] [PubMed] [Google Scholar]
- 7.Repp DJ, Hodge DO, Baratz KH, et al. Fuchs’ endothelial corneal dystrophy: subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology. 2013;120:687–94. doi: 10.1016/j.ophtha.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baratz KH, McLaren JW, Maguire LJ, Patel SV. Corneal haze determined by confocal microscopy two years after Descemet stripping with endothelial keratoplasty for Fuchs corneal dystrophy. Arch Ophthalmol. 2012;130:868–74. doi: 10.1001/archophthalmol.2012.73. [DOI] [PubMed] [Google Scholar]
- 9.Patel SV, Baratz KH, Hodge DO, et al. The effect of corneal light scatter on vision after Descemet stripping with endothelial keratoplasty. Arch Ophthalmol. 2009;127:153–60. doi: 10.1001/archophthalmol.2008.581. [DOI] [PubMed] [Google Scholar]
- 10.Ahuja Y, Baratz KH, McLaren JW, et al. Decreased corneal sensitivity and abnormal corneal nerves in Fuchs endothelial dystrophy. Cornea. 2012;31:1257–63. doi: 10.1097/ICO.0b013e31823f7888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecker LA, McLaren JW, Bachman LA, Patel SV. Anterior keratocyte depletion in Fuchs endothelial dystrophy. Arch Ophthalmol. 2011;129:555–61. doi: 10.1001/archophthalmol.2010.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel SV, McLaren JW. In vivo confocal microscopy of Fuchs endothelial dystrophy before and after endothelial keratoplasty. JAMA Ophthalmol. 2013;131:611–8. doi: 10.1001/jamaophthalmol.2013.799. [DOI] [PubMed] [Google Scholar]
- 13.Kim EC, Meng H, Jun AS. Lithium treatment increases endothelial cell survival and autophagy in a mouse model of Fuchs endothelial corneal dystrophy. Br J Ophthalmol. 2013;97:1068–73. doi: 10.1136/bjophthalmol-2012-302881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krachmer JH, Purcell JJ, Jr, Young CW, Bucher KD. Corneal endothelial dystrophy. A study of 64 families. Arch Ophthalmol. 1978;96:2036–9. doi: 10.1001/archopht.1978.03910060424004. [DOI] [PubMed] [Google Scholar]
- 15.McLaren JW, Bourne WM, Patel SV. Automated assessment of keratocyte density in stromal images from the ConfoScan 4 confocal microscope. Invest Ophthalmol Vis Sci. 2010;51:1918–26. doi: 10.1167/iovs.09-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaren JW, Nau CB, Patel SV, Bourne WM. Measuring corneal thickness with the ConfoScan 4 and z-ring adapter. Eye Contact Lens. 2007;33:185–90. doi: 10.1097/ICL.0b013e31802b3114. [DOI] [PubMed] [Google Scholar]
- 17.McLaren JW, Bourne WM, Patel SV. Standardization of corneal haze measurement in confocal microscopy. Invest Ophthalmol Vis Sci. 2010;51:5610–6. doi: 10.1167/iovs.10-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaren JW, Bachman LA, Kane KM, Patel SV. Objective assessment of the corneal endothelium in Fuchs’ endothelial dystrophy. Invest Ophthalmol Vis Sci. 2014;55:1184–90. doi: 10.1167/iovs.13-13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 20.Iwamoto T, DeVoe AG. Electron microscopic studies on Fuchs’ combined dystrophy. II. Anterior portion of the cornea. Invest Ophthalmol. 1971;10:29–40. [PubMed] [Google Scholar]
- 21.van den Berg TJ, Ijspeert JK. Clinical assessment of intraocular stray light. Appl Opt. 1992;31:3694–6. doi: 10.1364/AO.31.003694. [DOI] [PubMed] [Google Scholar]
- 22.Wilson SE, He YG, Weng J, et al. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62:325–7. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- 23.Li QJ, Ashraf MF, Shen DF, et al. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Arch Ophthalmol. 2001;119:1597–604. doi: 10.1001/archopht.119.11.1597. [DOI] [PubMed] [Google Scholar]
- 24.Muller LJ, Pels E, Vrensen GF. The specific architecture of the anterior stroma accounts for maintenance of corneal curvature. Br J Ophthalmol. 2001;85:437–43. doi: 10.1136/bjo.85.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dapena I, Yeh RY, Baydoun L, et al. Potential causes of incomplete visual rehabilitation at 6 months postoperative after Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2013;156:780–8. doi: 10.1016/j.ajo.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Patel SV, Baratz KH, Maguire LJ, et al. Anterior corneal aberrations after Descemet stripping endothelial keratoplasty for Fuchs endothelial dystrophy. Ophthalmology. 2012;119:1522–9. doi: 10.1016/j.ophtha.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 27.Koh S, Maeda N, Nakagawa T, et al. Characteristic higher-order aberrations of the anterior and posterior corneal surfaces in 3 corneal transplantation techniques. Am J Ophthalmol. 2012;153:284–90. doi: 10.1016/j.ajo.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 28.Rudolph M, Laaser K, Bachmann BO, et al. Corneal higher-order aberrations after Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2012;119:528–35. doi: 10.1016/j.ophtha.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi T, Negishi K, Yamaguchi K, et al. Effect of anterior and posterior corneal surface irregularity on vision after Descemet-stripping endothelial keratoplasty. J Cataract Refract Surg. 2009;35:688–94. doi: 10.1016/j.jcrs.2008.11.062. [DOI] [PubMed] [Google Scholar]
- 30.van Dijk K, Parker J, Liarakos VS, et al. Incidence of irregular astigmatism eligible for contact lens fitting after Descemet membrane endothelial keratoplasty. J Cataract Refract Surg. 2013;39:1036–46. doi: 10.1016/j.jcrs.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 31.Kopplin LJ, Przepyszny K, Schmotzer B, et al. Fuchs’ Endothelial Corneal Dystrophy Genetics Multi-Center Study Group. Relationship of Fuchs endothelial corneal dystrophy severity to central corneal thickness. Arch Ophthalmol. 2012;130:433–9. doi: 10.1001/archophthalmol.2011.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hillenaar T, Cals RH, Eilers PH, et al. Normative database for corneal backscatter analysis by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2011;52:7274–81. doi: 10.1167/iovs.11-7747. [DOI] [PubMed] [Google Scholar]
- 33.Patel SV, McLaren JW, Hodge DO, Bourne WM. Normal human keratocyte density and corneal thickness measurement by using confocal microscopy in vivo. Invest Ophthalmol Vis Sci. 2001;42:333–9. [PubMed] [Google Scholar]
- 34.Koizumi N, Okumura N, Kinoshita S. Development of new therapeutic modalities for corneal endothelial disease focused on the proliferation of corneal endothelial cells using animal models. Exp Eye Res. 2012;95:60–7. doi: 10.1016/j.exer.2011.10.014. [DOI] [PubMed] [Google Scholar]