Abstract
Rev-erbα is a nuclear receptor that links circadian rhythms to transcriptional control of metabolic pathways. Rev-erbα is a potent transcriptional repressor, and plays an important role in the core mammalian molecular clock while also serving as a critical regulator of clock output in metabolic tissues including liver and brown adipose tissue. Recent findings have shed new light on the role of Rev-erbα and its paralog Rev-erbβ in rhythm generation, as well as additional regulatory roles for Rev-erbα in other tissues that contribute to energy expenditure, inflammation, and behavior. This review highlights physiological functions of Rev-erbα and β in multiple tissues and discusses the therapeutic potential and challenges of targeting these pathways in human disease.
Keywords: Rev-erbα, Circadian Rhythm, Nuclear Receptor, Metabolism, Transcriptional Regulation
Rev-erbα: A nuclear receptor linking circadian rhythms and metabolism
Rev-erbα is a member of the nuclear receptor (NR) superfamily of ligand-regulated transcription factors (TF) [1]. It was discovered in 1989 and mapped to the reverse strand of the gene encoding another NR, thyroid hormone receptor α [2,3]. A highly similar factor, Rev-erbβ, was identified in 1994 by multiple labs [4–7], although the functional connection between these two paralogs is only now beginning to be understood. Recent studies involving a combination of genetic, genomic, biochemical and pharmacological techniques have revealed that Rev-erbα and β play critical roles in circadian rhythm generation [8,9], as well as in the normal function of many tissues [10–14]. Furthermore, the endogenous ligand for Rev-erbα is the metabolite heme [15,16], which is involved in mitochondrial respiration and cellular redox balance [17]. Thus, Rev-erbα also functions as a sensor for the metabolic state of the cell, and likely entrains the clock to metabolic cues [15,18]. The emergence of Rev-erbα as a transcriptional link from circadian rhythms to metabolism in multiple tissues is the main focus of this review.
NR proteins recruit co-regulator complexes to specific genomic regions, which in turn impact the epigenome and the recruitment of the core transcriptional machinery [19,20]. In particular, the nuclear co-repressor complex consists of core proteins such as Nuclear Corepressor 1 (NCoR), which directly interacts with NR proteins in a conformation-dependent manner [21,22], and epigenomic modifying enzymes such as Histone Deacetylase 3 (HDAC3), which deacetylates histone protein tails to create a repressive chromatin environment [23]. Recent work on Rev-erbα has revealed critical and genome-wide interactions with specific components of the co-repressor complex in the liver [10]. These findings and their potential implications on the mechanism of gene regulation by Rev-erbα in liver and other tissues and discussed below.
Rev-erbα is a dedicated repressor of transcription
Rev-erbα and β both lack the C-terminal helix that is critical for ligand dependent recruitment of coactivators and transcriptional activation by NRs [19]. As a result, Reverbα is a potent repressor of transcription [24], and interacts constitutively with the NR corepressor NCoR via its C-terminal ligand binding domain [25,26]. Rev-erbα has also been shown to interact with the closely related corepressor Silencing Mediator of Retinoid and Thyroid Receptors (SMRT, also known as NCoR2), although this requires distinct regions of the Rev-erbα C-terminus and does not occur on DNA under conditions where NCoR is bound to Rev-erbα [27], potentially because the SMRT interaction is weaker [28]. NCoR has several short (<20 amino acids) nuclear receptor interaction domains, referred to as corepressor-NR (CoRNR) boxes [29–31]. Rev-erbα binds preferentially to the more N-terminal CoRNR1 [28], which forms an antiparallel beta-sheet in the crystal structure of the complex [32].
Molecular heme functions as a diffusible, saturable ligand for Rev-erbα, and further stabilizes its interaction with full-length, endogenous NCoR [15,16]. The structure of heme bound to Rev-erbβ shows that heme binds in a prototypical NR ligand-binding pocket [33]. In addition to serving as a heme sensor, the oxidation state of the heme iron may regulate Rev-erb activity [33], although this has not been universally observed [15,16] and future work is needed to clarify this important point.
Rev-erbα recruits NCoR/SMRT to the genome by binding DNA in a sequence specific manner, with the preferred binding site consisting of a classical NR half-site AGGTCA flanked by an A/T-rich 5′ sequence (typically AANT) [34]. This binding site is often referred to as the RORE, as it is also bound by the Retinoic Acid Receptor-related Orphan Receptor (ROR), which opposes Rev-erbα function by activating transcription [6,35–37]. The Rev-erbα DNA-binding domain (DBD) binds in the major groove of the AGGTCA core sequence, while a C-terminal helical extension of the DBD makes minor groove contacts with the A/T-rich sequence of the RORE [38].
Two Rev-erbα molecules are required for a productive interaction with NCoR [27]. This can occur as two Rev-erbα monomers bound to independent ROREs or, more strongly and cooperatively, as a dimer bound to a direct repeat of the RORE separated by 2 base pairs, referred to as the RevDR2 [24,39]. ROR proteins are also capable of binding RevDR2 elements, primarily as a monomer, and drive transcriptional activation from these sites, similar to their role at the RORE [40]. Notably, Rev-erbα binds with greater stability at RevDR2 sites, while ROR binds with greater stability at monomeric RORE sites [41], suggesting that RevDR2 are dominantly controlled by Rev-erbα. Rev-erbα binding configurations and mechanisms of transcriptional regulation on DNA are shown in Figure 1.
Figure 1. Binding configurations of Rev-erbα.
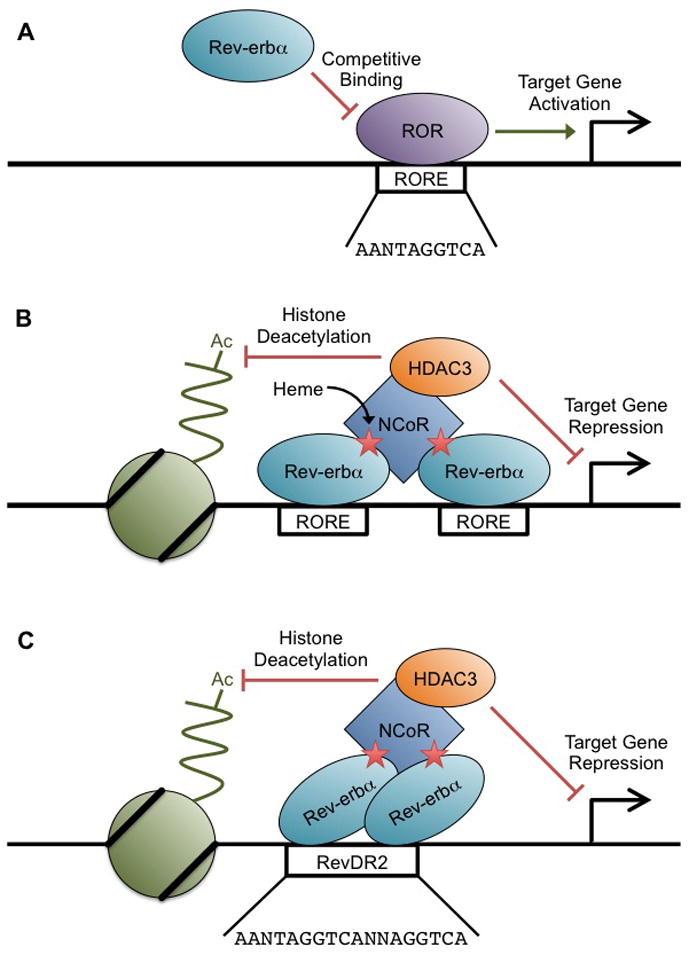
(A) At single RORE sites, Rev-erbα can inhibit transcription passively by competing for binding with ROR proteins. (B) Two Rev-erbα proteins bound independently to separate RORE motifs can recruit NCoR in a heme-dependent manner. The NCoR complex recruits HDAC3, which deacetylates surrounding histone tails and represses target gene transcription. (C) A Rev-erbα dimer bound to RevDR2 motif can also recruit NCoR, with similar regulatory effects as in (B).
Circadian expression and function of Rev-erbα
Shortly after its discovery, Rev-erbα was implicated in aspects of metabolism, including adipocyte differentiation [42] and myogenesis [43]. However, mice engineered to lack Rev-erbα appeared relatively normal [44]. A major breakthrough occurred in 1998, when Rev-erbα emerged at the top of the list of transcripts whose expression oscillated with a circadian rhythm in a cell-autonomous manner [45]. In mice, Rev-erbα mRNA levels were found to exhibit robust circadian oscillation in multiple tissues [37,46,47], and genetic ablation of Rev-erbα in mice was shown to shorten the period of behavioral rhythms by 0.5 hours in the absence of daily light cues [48]. These studies also showed that Rev-erbα was not strictly required for rhythm generation, leading to the suggestion that it serves in an auxiliary loop feeding back on the transcription of the core clock component Bmal1 to stabilize circadian oscillations [48]. Subsequent studies have implicated transcriptional repression by Rev-erbα in the rhythmicity of additional circadian regulators, including Clock [49], Cry1 [50], Nfil3 [51], and Npas2 [52]. Thus, Rev-erbα is a highly connected component of the molecular clock and has the potential to alter overall cellular oscillations through regulatory interactions with multiple genes.
Rev-erbα in the core molecular clock: Back-up by Rev-erbβ
Over-expression of Rev-erbα in mouse liver suppressed 90% of cycling transcripts, suggesting a potentially broader impact on circadian rhythm than predicted by the deletion of Rev-erbα [53]. More recent studies on the simultaneous disruption of Reverbα and β have now revealed an essential, though partially redundant, role for Reverbα in circadian rhythm generation. Rev-erbα and β are expressed with very similar circadian patterns, with both proteins peaking in mouse liver at Zeitgeber Time (ZT) 10, although Rev-erbα oscillation has a greater amplitude [8]. Depletion of either one of the Rev-erb proteins has a minimal effect on the cell-autonomous circadian clock in mouse embryonic fibroblasts, but loss of both Rev-erbα and β abrogated circadian gene expression in this system [8]. Moreover, genetic ablation of both Rev-erbα and β in adult mice resulted in arrhythmic wheel running behavior, in both the presence or absence of light entrainment cues [9]. These findings demonstrate that Rev-erbα and β are required, though redundant, components of the core clock machinery. Rev-erbα appears to be more important, because its absence results in mild disruptions to circadian rhythms, in contrast to the relatively inconsequential loss of Rev-erbβ alone [8,9,48].
Rev-erbα in liver: Orchestrating an epigenomic rhythm and lipid metabolism
The liver is a central tissue for whole body metabolic homeostasis, and Rev-erbα has long been known to regulate expression of the core clock gene Bmal1 in liver cells [48,54]. Studies within the past decade have also shown an important role for Rev-erbα in regulating whole body metabolism through control of cholesterol and bile acid metabolism in liver [51,55], as well regulation of apolipoprotein CIII [56,57]. More recently, the liver has become a key tissue for investigating the molecular mechanisms underlying gene regulation by Rev-erbα and remains a central tissue likely to mediate the effects of Rev-erbα on whole body metabolic homeostasis.
Rapid advances in genomic techniques have enabled mapping of the complete set of binding sites, or “cistrome”, for Rev-erbα in liver [10]. Rev-erbα binds to thousands of genomic locations at ZT10, when its expression was maximal, but to very few sites at ZT22, when its expression is nearly absent. Thus, the circadian expression of Reverbα drives its rhythmic binding genome-wide. Pathway analysis revealed a strong enrichment for Rev-erbα-bound genes involved in lipid metabolism and, correspondingly, Rev-erbα null mice were found to have hepatic steatosis [10].
The liver Rev-erbβ cistrome is very similar to that of Rev-erbα, and the binding sites for both Rev-erbs are highly enriched for the RORE and RevDR2 motifs [8]. Moreover, paralleling the partially redundant roles of Rev-erbα and β in the core clock, knock-down of Rev-erbβ in livers of Rev-erbα null mice caused a further increase in hepatic lipid content, although knock-down of Rev-erbβ had no effect on the livers of wild-type mice [8]. Inducible genetic ablation of both Rev-erbα and β in adult mice also disrupted circulating glucose, triglyceride, and free-fatty acid levels, demonstrating an effect on whole body metabolic homeostasis [9]. Thus, similar to their roles in the core clock, Rev-erbα and β appear to be partially redundant in the maintenance of hepatic lipid homeostasis, with Rev-erbα being of greater importance.
In liver, genome-wide location analysis of the Rev-erbα co-repressor components NCoR and HDAC3 revealed remarkable similarity to the Rev-erbα cistrome [10]. Thus, at ZT10 NCoR and HDAC3 binding overlapped at the vast majority of the thousands of sites of Rev-erbα binding, and the Rev-erbα dependent recruitment of HDAC3 generates a circadian rhythm of the epigenome at Rev-erbα binding sites, genome-wide [10]. Intriguingly, very little binding of NCoR and HDAC3 was observed at ZT22, which is quite surprising given that NCoR and HDAC3 are expressed throughout the day and the NCoR complex can, in principle, be recruited to many other NRs and TFs [19,21,58]. The tight connection between the liver genomic binding of Rev-erbα, NCoR, and HDAC3 reflects a shared function because liver-specific ablation of NCoR or HDAC3 phenocopies the changes in gene expression and hepatic lipid content resulting from the loss of Rev-erbα and β [8,59,60]. However, the dedication of NCoR and HDAC3 to Reverbα seems to be liver-specific, as recruitment of the corepressor complex to the genome is not restricted to Rev-erbα binding sites in macrophages [61–63].
Rev-erbα in adipose tissue: Fat storage and thermogenesis
Studies of adipogenic cell lines have suggested that Rev-erbα is required for adipocyte differentiation [42,64,65]. However, adipose tissue mass is normal or even increased in mice lacking Rev-erbα [44,66] suggesting some redundancy or compensation in vivo. Recently, a role for Rev-erbα was identified in brown adipose tissue (BAT) [13], which is a major site of thermogenesis in the body [67]. In mouse BAT, the circadian expression of Rev-erbα is similar to that in liver, peaking at ZT10. This is antiphase to the circadian rhythm of body temperature, and mice lacking Rev-erbα were shown to have an attenuated nadir in temperature oscillation due to derepression of Uncoupling Protein 1 (UCP1), which is a direct target of Rev-erbα in BAT [13]. Rev-erbα was also shown to play a key role in defending body temperature against cold challenge, such that mice either genetically lacking Rev-erbα or at the trough of normal Rev-erbα expression are protected from extreme cold.
The marked effect of Rev-erbα deletion on body temperature regulation suggests that Rev-erbβ is not redundant in BAT, although it remains to be determined whether the loss of both Rev-erbs would be even more dramatic as in liver. It should also be noted that strong Rev-erbα binding was observed at the UCP1 gene in BAT but not in liver, highlighting the tissue specificity of the genomic binding of Rev-erbα that is readily apparent from cistromic comparisons of BAT and liver.
Rev-erbα in skeletal muscle: A promoter of energy expenditure
Skeletal muscle is a critical peripheral tissue impacting metabolic homeostasis, insulin sensitization, and glucose disposal. A role for Rev-erbα in skeletal myocytes was first shown using C2C12 cultured cells, in which Rev-erbα repressed the expression of key genes required for muscle cell differentiation [43]. Rev-erbβ was later shown to share this role [68] as well as to regulate genes involved in lipid absorption in C2C12 myocytes [69]. Studies of Rev-erbα in primary skeletal muscle found preferential expression in specific fiber types, and differential composition of muscle fiber types in Rev-erbα null mice [70]. Expression profiling of NRs across multiple tissues found that Rev-erbα mRNA is expressed in a circadian manner in mouse skeletal muscle, with similar phase as in liver and adipose [47].
More recently, overexpression of Rev-erbα in C2C12 myocytes was shown to increase mitochondrial content and activity by modulating the AMP-activated protein kinase (AMPK) pathway, which also responds to changes in energy availability in the cell [12]. Similar findings were made in mouse skeletal muscle, and loss of Rev-erbα function was shown to reduce mitochondrial content and function, leading to an impaired exercise capacity [12]. Several genes involved in autophagy and mitophagy were shown to be direct targets of repression by Rev-erbα in muscle, suggesting that Rev-erbα inhibits autophagy of mitochondria in this tissue, thereby increasing mitochondrial content and oxidative capacity of myocytes [12]. These transcriptomic changes in muscle are not observed in liver or BAT, and presumably reflect muscle-specific sites of Rev-erbα recruitment to the genome, although this requires further cistromic analyses. Additionally, it remains to be determined whether Rev-erbα controls muscle mitochondrial content in a circadian manner.
Rev-erbα in endocrine pancreas function
Recent studies in mice have demonstrated a role for Rev-erbα in the function of both the insulin-producing β-cells and glucagon-producing α-cells of pancreatic islets. Rev-erbα mRNA is expressed in islets and oscillates with a circadian rhythm similar to that of liver [71–73]. Islets isolated at the time of peak Rev-erbα expression have higher levels of glucose-stimulated insulin secretion, and Rev-erbα regulates gene expression as well as insulin processing, exocytosis, and proliferation in primary β-cells and β-cell lines [73]. Rev-erbα has also been shown to promote glucagon-secretion from islet α-cells [74]. Intriguingly, Rev-erbα was also found to regulate lipogenic genes in mouse islets [73], similar to its role in liver [10], although the full set of genome-wide Rev-erbα targets remains to be mapped in the relevant islet cell types. Rev-erbβ is also expressed in both α-cells and β-cells [71], although its functional role remains to be investigated.
Rev-erbα in macrophages and inflammation
Rev-erbα was first shown to play a role in blocking pro-inflammatory signals in macrophages by repressing the Toll-Like Receptor 4 (TLR4) gene, which triggers the innate immune response to lipopolysaccharide (LPS) associated with gram-negative bacteria [75]. More recently, Rev-erbα was shown to mediate the circadian gating of the LPS-induced endotoxic response [11]. In human macrophages, pharmacological activation of Rev-erbα led to a decrease in production of the pro-inflammatory cytokine Interleukin 6 (IL-6), while loss of Rev-erbα increased IL-6. Thus, Rev-erbα provides a critical link between the circadian clock and immune function through direct repression of pro-inflammatory gene expression in macrophages. Recent mapping of the macrophage Rev-erbα and Rev-erbβ cistromes, together with the transcriptome changes in Reverbα/β double null macrophages suggests that Rev-erbα controls gene expression in macrophages through the repression of enhancer RNA transcription marked by lineage-determining TFs such as PU.1 [76].
Rev-erbα in brain and behavior
Rev-erbα expression is circadian in the suprachiasmatic nucleus (SCN) of the hypothalamus [48], which entrains other core clocks throughout the body [77], and this likely controls the phase-advance in locomotor activity characteristic of Rev-erbα null mice [48]. Rev-erbα null mice were also shown to exhibit postnatal developmental delays in the cerebellum, although this delay was overcome and no cerebellar dysfunction was apparent in adults [44].
Recently, Rev-erbα null mice were reported to exhibit behavioral abnormalities, including novelty-induced hyperactivity and impairment in memory formation, suggesting abnormalities in hippocampal function due to alterations in dopaminergic tone [14]. They also exhibited increased aggression, anxiety, and depression-associated behaviors indicative of dysfunctions in the midbrain dopaminergic neurons [78]. Rev-erbα expression in both the hippocampus [14] and midbrain [78] is circadian, but with much lower amplitude than in the SCN and other tissues. Rev-erbα was shown to repress several common targets in both hippocampus and midbrain, including tyrosine hydroxylase, which is the rate-limiting enzyme in dopamine biosynthesis [14,78]. Some of the additional behavioral changes did not appear to be circadian, indicating that Reverbα controls other behavioral functions in addition to rhythm generation, via multiple regions of the brain. Interestingly, many of the behaviors observed in Rev-erbα null mice parallel human behaviors observed in bipolar disorder [78], which is also linked to Rev-erbα by the molecular actions of lithium [79] (discussed in the next section).
Targeting Rev-erbα with small molecules
The activities of many NRs are regulated by direct binding of specific lipophilic molecules, including both endogenous compounds and drugs, which target the ligand-binding pocket of the NR protein [80]. The identification of heme as an endogenous ligand for Rev-erbα and β [15,16] demonstrated that Rev-erb activity could be regulated by ligand binding. The crystal structure of heme-bound Rev-erbβ identified the ligand-binding pocket [33], which is highly conserved in Rev-erbα and could also be the target of synthetic ligands. Indeed, several synthetic agonists, defined in the case of Rev-erb as molecules that increase corepressor interaction and repressive function, have been identified for Rev-erbα/β [81–85]. One study suggested that a Rev-erb agonist disrupts rhythmic wheel running behavior and circadian hypothalamic gene expression in mice, and can also alter whole-body metabolism [82]. The same synthetic agonist mimicked muscle-specific over-expression of Rev-erbα in promoting exercise capacity and muscle mitochondrial content [12]. These results suggest that Rev-erb agonists could promote metabolic health, although the pleiotropic tissue-specific effects of Rev-erbα, including disruptions to circadian rhythmicity [8,9,48] and increased thermogenesis upon loss of function [13], make Rev-erbα a complicated drug target. Thus, there is a need to further examine the function of Rev-erbs and their synthetic ligands in specific tissues, and extra attention must be paid to the time of day at which Rev-erbα ligands are administered. It may prove more effective to target Rev-erbα or downstream pathways in a tissue-specific manner.
Rev-erbα is also regulated at the level of protein stability, like other core components of the molecular clock [86]. E3 ligases, such as Arf-bp1 and Pam, ubiquitylate Rev-erbα to promote its proteasomal degradation[87], and glycogen synthase kinase 3 (GSK3) stabilizes Rev-erbα through N-terminal phosphorylation [79]. Lithium, which is commonly used in the treatment of bipolar disorder [88], inhibits phosphorylation by GSK3, thereby promoting the degradation of Rev-erbα [79]. Subsequently, several human genetic studies reported associations between polymorphisms at the Rev-erbα locus and responsiveness to lithium treatment among patients exhibiting bipolar disorder [89,90]. Thus, both the protein level and regulatory activity of Rev-erbα may be targeted with small molecules.
Concluding remarks and future perspectives
Within the last five years, it has become clear that Rev-erbα acts in a tissue-specific manner to regulate circadian rhythms as well as critical metabolic, inflammatory, and behavioral functions (Figure 2), in some cases uniquely and in others redundantly with Rev-erbβ. Thus, Rev-erbα is a key link between the core clock and numerous physiological processes, primarily studied in mice but likely to be relevant to human health. A number of open questions remain, however, particularly with regard to the contributions of Rev-erbα in each individual tissue, and the mechanisms underlying the similar—but not entirely overlapping—functions of Rev-erbα, Rev-erbβ, NCoR, and HDAC3 (Outstanding Questions Box). For example, the partial redundancy of Rev-erbα and Rev-erbβ raises the question of why Rev-erbβ compensation for Rev-erbα is variable across tissues and physiological processes. In addition, why the NCoR/HDAC3 complex is coupled tightly to Rev-erbα in liver but less so in other tissues is not well understood.
Figure 2. Tissue-specific rhythms and functions of Rev-erbα.
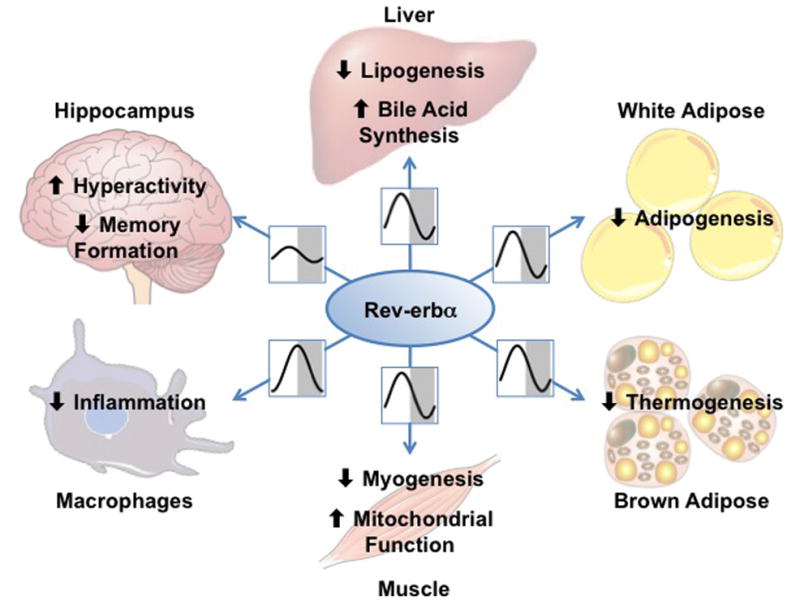
Diagram of Rev-erbα circadian expression patterns and regulatory targets, in various tissues.
A critical question remains whether Rev-erbα can be targeted for therapeutic purposes, particularly in metabolic, inflammatory, and neurological disorders. Recent studies of synthetic agonists have shown promising results in treating systemic metabolic dysfunction in mice, yet the recently discovered diverse roles of Rev-erbα in numerous tissues throughout the body has unveiled major complexity of Rev-erbα biology. It will be essential to probe the molecular and physiological tissue-specific effects of Rev-erbα agonists to fully assess their potential as human therapeutics.
OUTSTANDING QUESTIONS BOX.
What are the tissue-specific contributions of Rev-erbα to overall metabolic homeostasis?
What mechanisms underlie the partially redundancy of Rev-erbα and Rev-erbβ for some functions but perhaps not for others?
How can we explain the tight coupling of NCoR and HDAC3 Rev-erbs in liver?
Can synthetic Rev-erb agonists be used to safely treat metabolic, inflammatory, or behavioral disorders in humans?
HIGHLIGHTS.
Rev-erbα is an unusual nuclear receptor that is dedicated to transcriptional repression
NCoR and HDAC3 are important and functional corepressor partners of Rev-erbα
Rev-erbα is a key component of a core repressive loop of the molecular clock
Rev-erbα is a circadian regulator of metabolic pathways in multiple tissues
Acknowledgments
Research on Rev-erb in the Lazar laboratory is supported by NIH grant R01 DK45586 (MAL). LJE was supported by NIH training grant F32 DK095526.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mangelsdorf DJ, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lazar MA, et al. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA alpha transcriptional unit. Mol Cell Biol. 1989;9:1128–36. doi: 10.1128/mcb.9.3.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyajima N, et al. Two erbA homologs encoding proteins with different T3 binding capacities are transcribed from opposite DNA strands of the same genetic locus. Cell. 1989;57:31–9. doi: 10.1016/0092-8674(89)90169-4. [DOI] [PubMed] [Google Scholar]
- 4.Dumas B, et al. A new orphan member of the nuclear hormone receptor superfamily closely related to Rev-Erb. Mol Endocrinol. 1994;8:996–1005. doi: 10.1210/mend.8.8.7997240. [DOI] [PubMed] [Google Scholar]
- 5.Retnakaran R, et al. Identification of RVR, a novel orphan nuclear receptor that acts as a negative transcriptional regulator. Mol Endocrinol. 1994;8:1234–44. doi: 10.1210/mend.8.9.7838156. [DOI] [PubMed] [Google Scholar]
- 6.Forman BM, et al. Cross-talk among ROR alpha 1 and the Rev-erb family of orphan nuclear receptors. Mol Endocrinol. 1994;8:1253–61. doi: 10.1210/mend.8.9.7838158. [DOI] [PubMed] [Google Scholar]
- 7.Bonnelye E, et al. Rev-erb beta, a new member of the nuclear receptor superfamily, is expressed in the nervous system during chicken development. Cell Growth Differ. 1994;5:1357–65. [PubMed] [Google Scholar]
- 8.Bugge A, et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho H, et al. Regulation of circadian behaviour and metabolism by REVERB-α and REV-ERB-β. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng D, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science (80-) 2011;331:1315–1319. doi: 10.1126/science.1198125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs JE, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109:582–7. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woldt E, et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med. 2013;19:1039–46. doi: 10.1038/nm.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerhart-Hines Z, et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature. 2013;503:410–3. doi: 10.1038/nature12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jager J, et al. Behavioral Changes and Dopaminergic Dysregulation in Mice Lacking the Nuclear Receptor Rev-erbα. Mol Endocrinol. 2014;28:490–8. doi: 10.1210/me.2013-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin L, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science (80-) 2007;318:1786–9. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 16.Raghuram S, et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14:1207–13. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsiftsoglou AS, et al. Heme as key regulator of major mammalian cellular functions: molecular, cellular, and pharmacological aspects. Pharmacol Ther. 2006;111:327–45. doi: 10.1016/j.pharmthera.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Kaasik K, Lee CC. Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature. 2004;430:467–71. doi: 10.1038/nature02724. [DOI] [PubMed] [Google Scholar]
- 19.Xu L, et al. Coactivator and corepressor complexes in nuclear receptor function. Curr Opin Genet Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 20.Lonard DM, et al. Nuclear receptor coregulators and human disease. Endocr Rev. 2007;28:575–87. doi: 10.1210/er.2007-0012. [DOI] [PubMed] [Google Scholar]
- 21.Mottis A, et al. Emerging roles of the corepressors NCoR1 and SMRT in homeostasis. Genes Dev. 2013;27:819–35. doi: 10.1101/gad.214023.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson PJ, et al. Nuclear hormone receptor co-repressors: structure and function. Mol Cell Endocrinol. 2012;348:440–9. doi: 10.1016/j.mce.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Z, et al. Circadian Epigenomic Remodeling and Hepatic Lipogenesis: Lessons from HDAC3. Cold Spring Harb Symp Quant Biol. 2011;76:49–55. doi: 10.1101/sqb.2011.76.011494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harding HP, Lazar MA. The monomer-binding orphan receptor RevErb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol. 1995;15:4791–802. doi: 10.1128/mcb.15.9.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zamir I, et al. A nuclear hormone receptor corepressor mediates transcriptional silencing by receptors with distinct repression domains. Mol Cell Biol. 1996;16:5458–65. doi: 10.1128/mcb.16.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Downes M, et al. Two receptor interaction domains in the corepressor, N-CoR/RIP13, are required for an efficient interaction with Rev-erbA alpha and RVR: physical association is dependent on the E region of the orphan receptors. Nucleic Acids Res. 1996;24:4379–86. doi: 10.1093/nar/24.22.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamir I, et al. Stoichiometric and steric principles governing repression by nuclear hormone receptors. Genes Dev. 1997;11:835–46. doi: 10.1101/gad.11.7.835. [DOI] [PubMed] [Google Scholar]
- 28.Hu X, et al. Determinants of CoRNR-dependent repression complex assembly on nuclear hormone receptors. Mol Cell Biol. 2001;21:1747–58. doi: 10.1128/MCB.21.5.1747-1758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu X, Lazar MA. The CoRNR motif controls the recruitment of corepressors by nuclear hormone receptors. Nature. 1999;402:93–6. doi: 10.1038/47069. [DOI] [PubMed] [Google Scholar]
- 30.Perissi V, et al. Molecular determinants of nuclear receptor-corepressor interaction. Genes Dev. 1999;13:3198–208. doi: 10.1101/gad.13.24.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagy L, et al. Mechanism of corepressor binding and release from nuclear hormone receptors. Genes Dev. 1999;13:3209–16. doi: 10.1101/gad.13.24.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phelan CA, et al. Structure of Rev-erbalpha bound to N-CoR reveals a unique mechanism of nuclear receptor-co-repressor interaction. Nat Struct Mol Biol. 2010;17:808–14. doi: 10.1038/nsmb.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardee KI, et al. The structural basis of gas-responsive transcription by the human nuclear hormone receptor REV-ERBbeta. PLoS Biol. 2009;7:e43. doi: 10.1371/journal.pbio.1000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harding HP, Lazar MA. The orphan receptor Rev-ErbA alpha activates transcription via a novel response element. Mol Cell Biol. 1993;13:3113–21. doi: 10.1128/mcb.13.5.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giguère V, et al. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994;8:538–53. doi: 10.1101/gad.8.5.538. [DOI] [PubMed] [Google Scholar]
- 36.Bois-Joyeux B, et al. Modulation of the far-upstream enhancer of the rat alpha-fetoprotein gene by members of the ROR alpha, Rev-erb alpha, and Reverb beta groups of monomeric orphan nuclear receptors. DNA Cell Biol. 2000;19:589–99. doi: 10.1089/104454900750019344. [DOI] [PubMed] [Google Scholar]
- 37.Guillaumond F, et al. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20:391–403. doi: 10.1177/0748730405277232. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Q, et al. Structural elements of an orphan nuclear receptor-DNA complex. Mol Cell. 1998;1:849–61. doi: 10.1016/s1097-2765(00)80084-2. [DOI] [PubMed] [Google Scholar]
- 39.Adelmant G, et al. A functional Rev-erb alpha responsive element located in the human Rev-erb alpha promoter mediates a repressing activity. Proc Natl Acad Sci U S A. 1996;93:3553–8. doi: 10.1073/pnas.93.8.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raspè E, et al. Transcriptional regulation of human Rev-erbalpha gene expression by the orphan nuclear receptor retinoic acid-related orphan receptor alpha. J Biol Chem. 2002;277:49275–81. doi: 10.1074/jbc.M206215200. [DOI] [PubMed] [Google Scholar]
- 41.Harding HP, et al. Transcriptional activation and repression by RORalpha, an orphan nuclear receptor required for cerebellar development. Mol Endocrinol. 1997;11:1737–46. doi: 10.1210/mend.11.11.0002. [DOI] [PubMed] [Google Scholar]
- 42.Chawla A, Lazar MA. Induction of Rev-ErbA alpha, an orphan receptor encoded on the opposite strand of the alpha-thyroid hormone receptor gene, during adipocyte differentiation. J Biol Chem. 1993;268:16265–9. [PubMed] [Google Scholar]
- 43.Downes M, et al. Constitutive expression of the orphan receptor, Rev-erbA alpha, inhibits muscle differentiation and abrogates the expression of the myoD gene family. Mol Endocrinol. 1995;9:1666–78. doi: 10.1210/mend.9.12.8614403. [DOI] [PubMed] [Google Scholar]
- 44.Chomez P, et al. Increased cell death and delayed development in the cerebellum of mice lacking the rev-erbA(alpha) orphan receptor. Development. 2000;127:1489–98. doi: 10.1242/dev.127.7.1489. [DOI] [PubMed] [Google Scholar]
- 45.Balsalobre A, et al. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–37. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 46.Nuclear Receptor Signaling Atlas [Online] Available: http://www.nursa.org.
- 47.Yang X, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–10. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 48.Preitner N, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–60. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 49.Crumbley C, Burris TP. Direct regulation of CLOCK expression by REV-ERB. PLoS One. 2011;6:e17290. doi: 10.1371/journal.pone.0017290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ukai-Tadenuma M, et al. Delay in feedback repression by cryptochrome 1 is required for circadian clock function. Cell. 2011;144:268–81. doi: 10.1016/j.cell.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 51.Duez H, et al. Regulation of bile acid synthesis by the nuclear receptor Rev-erbalpha. Gastroenterology. 2008;135:689–98. doi: 10.1053/j.gastro.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 52.Crumbley C, et al. Characterization of the core mammalian clock component, NPAS2, as a REV-ERBalpha/RORalpha target gene. J Biol Chem. 2010;285:35386–92. doi: 10.1074/jbc.M110.129288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kornmann B, et al. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yin L, Lazar MA. The orphan nuclear receptor Rev-erbalpha recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol Endocrinol. 2005;19:1452–9. doi: 10.1210/me.2005-0057. [DOI] [PubMed] [Google Scholar]
- 55.Le Martelot G, et al. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7:e1000181. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coste H, Rodríguez JC. Orphan nuclear hormone receptor Rev-erbalpha regulates the human apolipoprotein CIII promoter. J Biol Chem. 2002;277:27120–9. doi: 10.1074/jbc.M203421200. [DOI] [PubMed] [Google Scholar]
- 57.Raspé E, et al. Identification of Rev-erbalpha as a physiological repressor of apoC-III gene transcription. J Lipid Res. 2002;43:2172–9. doi: 10.1194/jlr.m200386-jlr200. [DOI] [PubMed] [Google Scholar]
- 58.Bailey P, et al. The nuclear receptor corepressor N-CoR regulates differentiation: N-CoR directly interacts with MyoD. Mol Endocrinol. 1999;13:1155–68. doi: 10.1210/mend.13.7.0305. [DOI] [PubMed] [Google Scholar]
- 59.Sun Z, et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat Med. 2012;18:934–42. doi: 10.1038/nm.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Z, et al. Deacetylase-independent function of HDAC3 in transcription and metabolism requires nuclear receptor corepressor. Mol Cell. 2013;52:769–82. doi: 10.1016/j.molcel.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mullican SE, et al. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 2011;25:2480–8. doi: 10.1101/gad.175950.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghisletti S, et al. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23:681–93. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barish GD, et al. The Bcl6-SMRT/NCoR cistrome represses inflammation to attenuate atherosclerosis. Cell Metab. 2012;15:554–62. doi: 10.1016/j.cmet.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fontaine C, et al. The orphan nuclear receptor Rev-Erbalpha is a peroxisome proliferator-activated receptor (PPAR) gamma target gene and promotes PPARgamma-induced adipocyte differentiation. J Biol Chem. 2003;278:37672–80. doi: 10.1074/jbc.M304664200. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Lazar MA. Bifunctional role of Rev-erbalpha in adipocyte differentiation. Mol Cell Biol. 2008;28:2213–20. doi: 10.1128/MCB.01608-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Delezie J, et al. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012;26:3321–35. doi: 10.1096/fj.12-208751. [DOI] [PubMed] [Google Scholar]
- 67.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 68.Burke L, et al. Transcriptional repression by the orphan steroid receptor RVR/Rev-erb beta is dependent on the signature motif and helix 5 in the E region: functional evidence for a biological role of RVR in myogenesis. Nucleic Acids Res. 1996;24:3481–9. doi: 10.1093/nar/24.18.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramakrishnan SN, et al. Rev-erbbeta regulates the expression of genes involved in lipid absorption in skeletal muscle cells: evidence for cross-talk between orphan nuclear receptors and myokines. J Biol Chem. 2005;280:8651–9. doi: 10.1074/jbc.M413949200. [DOI] [PubMed] [Google Scholar]
- 70.Pircher P, et al. Aberrant expression of myosin isoforms in skeletal muscles from mice lacking the rev-erbAalpha orphan receptor gene. Am J Physiol Regul Integr Comp Physiol. 2005;288:R482–90. doi: 10.1152/ajpregu.00690.2003. [DOI] [PubMed] [Google Scholar]
- 71.Chuang J-C, et al. Research resource: nuclear hormone receptor expression in the endocrine pancreas. Mol Endocrinol. 2008;22:2353–63. doi: 10.1210/me.2007-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mühlbauer E, et al. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol. 2009;606:61–71. doi: 10.1016/j.ejphar.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 73.Vieira E, et al. The clock gene Rev-erbα regulates pancreatic β-cell function: modulation by leptin and high-fat diet. Endocrinology. 2012;153:592–601. doi: 10.1210/en.2011-1595. [DOI] [PubMed] [Google Scholar]
- 74.Vieira E, et al. Involvement of the clock gene Rev-erb alpha in the regulation of glucagon secretion in pancreatic alpha-cells. PLoS One. 2013;8:e69939. doi: 10.1371/journal.pone.0069939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fontaine C, et al. The nuclear receptor Rev-erbalpha is a liver X receptor (LXR) target gene driving a negative feedback loop on select LXR-induced pathways in human macrophages. Mol Endocrinol. 2008;22:1797–811. doi: 10.1210/me.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lam MTY, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–5. doi: 10.1038/nature12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dibner C, et al. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 78.Chung S, et al. Impact of Circadian Nuclear Receptor REV-ERBα on Midbrain Dopamine Production and Mood Regulation. Cell. 2014;157:858–68. doi: 10.1016/j.cell.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 79.Yin L, et al. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science (80-) 2006;311:1002–5. doi: 10.1126/science.1121613. [DOI] [PubMed] [Google Scholar]
- 80.Moore JT, et al. The nuclear receptor superfamily and drug discovery. Chem Med Chem. 2006;1:504–523. doi: 10.1002/cmdc.200600006. [DOI] [PubMed] [Google Scholar]
- 81.Grant D, et al. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbα. ACS Chem Biol. 2010;5:925–32. doi: 10.1021/cb100141y. [DOI] [PubMed] [Google Scholar]
- 82.Solt LA, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–8. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meng QJ, et al. Ligand modulation of REV-ERBalpha function resets the peripheral circadian clock in a phasic manner. J Cell Sci. 2008;121:3629–35. doi: 10.1242/jcs.035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shin Y, et al. Small molecule tertiary amines as agonists of the nuclear hormone receptor Rev-erbα. Bioorg Med Chem Lett. 2012;22:4413–7. doi: 10.1016/j.bmcl.2012.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trump RP, et al. Optimized chemical probes for REV-ERBα. J Med Chem. 2013;56:4729–37. doi: 10.1021/jm400458q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takahashi JS, et al. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–75. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yin L, et al. E3 ligases Arf-bp1 and Pam mediate lithium-stimulated degradation of the circadian heme receptor Rev-erb alpha. Proc Natl Acad Sci U S A. 2010;107:11614–9. doi: 10.1073/pnas.1000438107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lenox RH, et al. Endophenotypes in bipolar disorder. Am J Med Genet. 2002;114:391–406. doi: 10.1002/ajmg.10360. [DOI] [PubMed] [Google Scholar]
- 89.McCarthy MJ, et al. Functional genetic variation in the Rev-Erbα pathway and lithium response in the treatment of bipolar disorder. Genes Brain Behav. 2011;10:852–61. doi: 10.1111/j.1601-183X.2011.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Campos-de-Sousa S, et al. Nuclear receptor rev-erb-{alpha} circadian gene variants and lithium carbonate prophylaxis in bipolar affective disorder. J Biol Rhythms. 2010;25:132–7. doi: 10.1177/0748730410362713. [DOI] [PubMed] [Google Scholar]


