Abstract
Integrin α5-null embryos die in mid-gestation from severe defects in cardiovascular morphogenesis, which stem from defective development of the neural crest, heart and vasculature. To investigate the role of integrin α5β1 in cardiovascular development, we used the Mesp1Cre knock-in strain of mice to ablate integrin α5 in the anterior mesoderm, which gives rise to all of the cardiac and many of the vascular and muscle lineages in the anterior portion of the embryo. Surprisingly, we found that mutant embryos displayed numerous defects related to the abnormal development of the neural crest such as cleft palate, ventricular septal defect, abnormal development of hypoglossal nerves, and defective remodeling of the aortic arch arteries. We found that defects in arch artery remodeling stem from the role of mesodermal integrin α5β1 in neural crest proliferation and differentiation into vascular smooth muscle cells, while proliferation of pharyngeal mesoderm and differentiation of mesodermal derivatives into vascular smooth muscle cells was not defective. Taken together our studies demonstrate a requisite role for mesodermal integrin α5β1 in signaling between the mesoderm and the neural crest, thereby regulating neural crest-dependent morphogenesis of essential embryonic structures.
Key index words: integrin alpha5, mesoderm, neural crest, aortic arch artery development
Introduction
Tissue recombination studies and genetic experiments in chick and mice demonstrated an intricate dependence of craniofacial and pharyngeal morphogenesis on signaling between the anterior mesoderm and the neural crest (Itasaki et al., 1996; Noden and Trainor, 2005; Trainor and Krumlauf, 2000). Neural crest ablation experiments in chick established the requisite role of the neural crest in patterning of facial muscles, elongation of the cardiac outflow tract, positioning of the great vessels relative to the cardiac chambers, morphogenesis of the thymus, and development of the thyroid and parathyroid glands (Evans et al., 2010; Hutson and Kirby, 2007; Tzahor and Evans, 2011). Similarly, tissue recombination experiments in chick and gene knockout studies in mice indicated that signals from cranial and paraxial mesoderm regulate neural crest patterning and cell fate (Chen et al., 2012; Ferguson and Graham, 2004; Gammill et al., 2007; Gammill et al., 2006; Itasaki et al., 1996; Trainor and Krumlauf, 2000). However, there is only scarce information on genetic and molecular regulation of mesoderm-neural crest interactions. In the context of the developing cardiovascular system and facial muscles, cranial neural crest plays an essential role in regulating the balance of Fgf and BMP signals sensed by the anterior mesoderm. In addition, neural crest-mesoderm interactions regulate migration, proliferation and differentiation of mesodermal cells that give rise to the cardiac outflow tract myocardium, arch artery vascular smooth muscle cells and facial muscles (Hutson et al., 2010; Hutson et al., 2006; Michailovici et al., 2014; Rinon et al., 2007; Tirosh-Finkel et al., 2010). At the genetic level, the expression of transcription factor Twist1 or activation of the canonical Wnt signaling in the cranial neural crest regulates patterning of the pharyngeal arch mesoderm in a non cell-autonomous manner (Rinon et al., 2007), while expression of Jagged 1 in the pharyngeal endothelial cells or VEGFR2, Fgf8 or Tbx1 in the anterior mesoderm regulates neural crest cell survival, migration, differentiation and patterning (Aggarwal et al., 2010; Chen et al., 2012; High et al., 2008; Milgrom-Hoffman et al., 2014; Zhang et al., 2006). Together, craniofacial and cardiovascular abnormalities constitute the most common birth defects in humans (Gorlin et al., 1990; Roger et al., 2011); therefore, a deeper understanding of the regulatory mechanisms mediating interactions between the anterior mesoderm and the neural crest is needed.
Communications between the neural crest and anterior mesoderm play obligate roles in morphogenesis of the aortic arch arteries, a system of blood vessels that routes oxygenated blood to various destinations within the systemic circulation. Abnormal morphogenesis of the aortic arch arteries is a common manifestation of human congenital heart disease (Moon, 2008; Moon, 2006). The development of this vascular tree is incredibly complex and intimately depends on signaling between all germ layers and the neural crest, reviewed by (Astrof, 2013; Rentschler et al., 2010). Integrins are a major class of cellular receptors that mediate signaling by extracellular matrix proteins, allowing cells to sense and respond to chemical and mechanical stimuli in their microenvironment (Schwartz, 2010). Thus, integrins are ideal candidates to mediate inter-tissue interactions during organ morphogenesis. There are 18 known integrin alpha chains and 8 known integrin beta chains in mammals, which combine to form 24 known heterodimers with distinct and overlapping specificities for ligands (Hynes, 2002). Integrin α5 forms the α5β1 heterodimer and is a major receptor for the extracellular matrix protein fibronectin (Hynes, 2002; Hynes and Naba, 2012). In this paper, we show that integrin α5β1 expressed by the anterior mesoderm facilitates arch artery morphogenesis by regulating neural crest cell fate.
Integrin α5β1 plays pleiotropic roles in vertebrate embryogenesis. Mouse mutants with global deletion of integrin α5 die by mid-gestation and exhibit multiple defects (Yang et al., 1993). During gastrulation, integrin α5 is required for development of the definitive endoderm and for morphogenesis of the node and the notochord, regulating development of the left-right body axis in mice (Pulina et al., 2014; Pulina et al., 2011; Villegas et al., 2013). Following gastrulation, integrin α5 plays requisite roles in the development of somites, the neural crest and the cardiovascular system (Goh et al., 1997; Julich et al., 2005; Julich et al., 2009; Mittal et al., 2013; Mittal et al., 2010; Yang et al., 1999; Yang et al., 1993). However, mid-gestation lethality along with severe morphogenetic defects in the global integrin α5-null embryos makes it difficult to discern causes of various malformations from their consequences, and thus the mechanisms whereby integrin α5β1 regulates cardiovascular development in vivo are not understood.
Our recent studies demonstrated that the heart does not form normally in integrin α5-null embryos. In particular, the outflow tract and the right ventricle, structures that are derived from the second heart field, were malformed in these mutants (Mittal et al., 2013). Since integrin α5 is expressed in cardiomyocytes, we used three different lines of mice to conditionally ablate integrin α5 in: 1) the myocardium, using cTNT-Cre transgenic mice, 2) in the second heart field, using Mef2C-AHF-Cre transgenic mice or 3) in the earliest known cardiac progenitors and their descendants, using Mesp1Cre knock-in mice (Jiao et al., 2003; Saga et al., 2000; Saga et al., 1999; Verzi et al., 2005). While ablation of integrin α5 using cTNT-Cre or Mef2C-AHF-Cre mice did not disrupt cardiac and embryonic development, the use of the Mesp1Cre strain to ablate integrin α5 gave rise to late embryonic and perinatal lethality. Surprisingly, mutant embryos exhibited numerous morphogenetic abnormalities related to defective neural crest development. In the studies reported in this paper, we investigated mechanisms contributing to defective development of the aortic arch arteries in the Mesp1Cre conditional mutants, and discovered that mesodermal expression of integrin α5 regulates proliferation of neural crest-derived cells in the fourth pharyngeal arch and their subsequent differentiation into vascular smooth muscle cells (VSMCs).
Materials and Methods
Mouse strains and genotyping
All experiments involving vertebrate animals were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University, and were performed in accordance with federal guidelines for humane care of animals. Integrin α5+/− mice (Yang et al., 1993) and integrin α5flox/flox mice (van der Flier et al., 2010) were obtained from Dr. Richard Hynes. Mesp1Cre knock-in mice (Saga et al., 2000; Saga et al., 1999) were obtained from Dr. Yumiko Saga. Trangenic cTnt-Cre mice, Tg(Tnnt2-cre)5Blh, were a gift from Dr. Brigit Hogan (Jiao et al., 2003). Tie2-Cre, Tg(Tek-cre)1Ywa, (Kisanuki et al., 2001), Rosa-mT/mG mice, Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo, (Muzumdar et al., 2007) were purchased from Jackson labs and Mef2C-AHF-Cre mice (Verzi et al., 2005) were purchased from Mutant Mouse Regional Resource Center. Integrin α5+/− mice were crossed with Cre-containing mice to generate integrin α5+/−; Cre+ males. For all experimental crosses, integrin α5flox/flox females were crossed with α5+/−; cTnt-Cre, α5+/−; Mef2C-AHF-Cre, α5+/−; Tie2-Cre or α5+/−;Mesp1Cre males. Genotyping was performed using PCR primers as described previously (Mittal et al., 2010; van der Flier et al., 2010; Yang et al., 1993).
Optical projection tomography (OPT) and 3D reconstruction
Embryos were isolated at E14.5, fixed in 4% paraformaldehyde (PFA) at 4°C overnight, and washed thoroughly with phosphate buffered saline (PBS). The heart and the connecting vasculature were dissected and embedded in 1% agarose. The agarose block was dehydrated using a methanol series and cleared with BABB (a mixture of benzyl benzoate and benzyl alcohol at the ratio of 2:1) until transparent. Specimens were scanned using Bioptonics OPT Scanner 3001M as described (Degenhardt et al., 2013). The 3-dimensional views were reconstructed using OsiriX software (http://www.osirix-viewer.com/).
India Ink Injection
Embryos were isolated at E10.5, fixed in 4% PFA overnight, and washed with PBS. Water insoluble India ink was injected into the right ventricle through a pulled glass micropipette until vessels were filled. Embryos were photographed using Zeiss Stemi 2000-C steromicroscope and Zeiss AxioCam ERc 5s camera.
Histology and Immunohistochemistry
Embryos were fixed overnight with 4% PFA at 4°C. For paraffin sections, embryos were dehydrated through graded series of ethanol, transferred to xylene and embedded in paraffin. For frozen sections, embryos were mounted in optimal cutting temperature (OCT) compound (Tissue-Tek® Sakura Finetek) and stored at −80 °C before sectioning. Frozen sections were used for detecting integrin α5 (1:50, cat # 553319, BD) and VEGFR2 (1:100, cat # AF644, R&D). For immunohistochemistry on paraffin section, the sections were deparaffinized, rehydrated and pretreated with 10 mM sodium citrate (pH 6.0) for 20 minutes in the microwave for antigen retrieval and then cooled to room temperature. After incubation in blocking buffer containing PBST (phosphate buffered saline with 0.05% Tween20) with 10% normal donkey serum (Sigma), for 1 hour at room temperature, sections were treated with primary antibodies overnight at 4 °C. The following primary antibodies and their dilutions in blocking buffer were used: rabbit polyclonal anti-FN antibody was a gift from Richard Hynes, MIT (George et al., 1993), chicken anti-GFP (1:500, Catalog # GFP-1020, Aves labs), mouse anti-αSMA (1:300, Catalog # A5228, Sigma), rabbit anti-cleaved caspase-3 (1:100, Catalog # 9661, Cell signaling technology), rabbit anti-phospho Histone H3 (1:100, Catalog # 9701, Cell signaling technology), and mouse anti-BrdU (1:500, cat # G3G4, Iowa Developmental Studies Hybridoma Bank). Slides were washed in PBST and treated with secondary antibodies diluted in blocking buffer for two hours at room temperature. The following secondary antibodies were used: donkey anti-mouse, anti-goat, or anti-rabbit conjugated to Alexa-488, Alexa-555, or to Alexa-647 (1:300, Invitrogen). Anti-chicken secondary antibodies were from Jackson Immunoresearch. Nuclei were either stained with DAPI (1:1000, Sigma) or with DRAQ5 (1:1000, cat # 4084, Cell Signaling Technology), and slides were mounted with ProLong® Gold antifade (Invitrogen). Images were captured with Olympus FV500 confocal microscope.
Whole mount immunostaining
Detection of integrin α5
Embryos were fixed in 4% PFA and incubated with 3% H2O2 in PBS with 0.1% Triton at room temperature for one hour with rocking, and then blocked overnight at 4 °C with blocking solution described above. Embryos were incubated with avidin and biotin blocking solutions (cat # SP-2001, Vector Laboratories) for 1 hour each with rocking at 4 °C. Embryos were then incubated with anti-integrin α5 primary antibody (1:100) overnight at 4 °C. After washing with PBS-0.1% Triton X, embryos were incubated with biotinylated secondary antibody (1:200, Jackson Immunoresearch) overnight at 4 °C. After washing with PBS-0.1% Triton X for 48 hours, the staining was developed using the ABC and DAB kits (Vector laboratories). Color was developed under the microscope. Detection of NF160. After incubating embryos in blocking solution overnight at 4 °C, embryos were incubated with 1:100 dilution of NF160 antibody (cat # 2H3, Iowa Developmental Studies Hybridoma Bank) overnight at 4 °C. After washing with PBS-0.1% TritonX, embryos were incubated with secondary antibody (1:300, anti-mouse Alexa-488) overnight at 4 °C and washed. Embryos were imaged using Olympus fluorescence stereomicroscope and Leica DFC450 camera.
In situ hybridization
In situ hybridization (ISH) was performed following the procedure described by (Henrique et al., 1995) obtained from Dr. Janet Rossant’s lab’s website (http://www.sickkids.ca/research/rossant/custom/protocols.asp). In all experiments, mutant and control embryos were kept in the same vial during all stages of the ISH and color development to avoid differences due to handling. The ISH probes were synthesized using previously described plasmids: Pitx2c (Liu et al., 2001), Fgf8 (Crossley and Martin, 1995), Hoxa3 (Manley et al., 2004), Hoxd4 (Gaunt et al., 1989) and Mkp3 (Mittal et al., 2013).
Quantification of smooth muscle coverage of aortic arch arteries
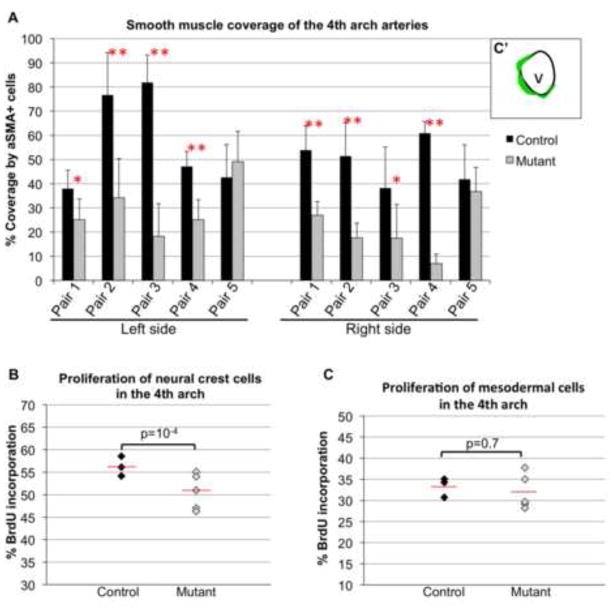
These studies were performed using serial coronal sections from control (n=5) and mutant (n=5) embryos isolated at E11.5. We focused on the left and right 4th pharyngeal arch arteries. Following staining and imaging as described above, we measured the perimeter of the 4th arch artery in each section; 5 sections spaced about 10 microns from each other were examined from each embryo. Then we measured the length of smooth muscle staining along the vessel wall (as drawn in Fig. 8A). Smooth muscle coverage was calculated as the fraction of vessel perimeter occupied by smooth muscle cells in each section. 25 sections per genotype were quantified.
Figure 8. Mesodermal expression of integrin α5 is required for proliferation and differentiation of neural crest-derived cells but not pharyngeal mesoderm.
A. Proliferation of neural crest cells (Mesp1-lineage negative pharyngeal mesenchyme) was evaluated following BrdU injection. Each data point represents individual embryos. N=3 control (5454 cells) and n=5 mutant (9344 cells) embryos were evaluated. B. Proliferation of Mesp1-derived mesoderm in the 4th pharyngeal arches. N=3 controls (690 cells) and n=5 mutants (1124 cells) were evaluated. Unpaired Student’s t-test probability (p) is reported for A – B. Red lines mark the means. C. Smooth muscle differentiation around aortic arch arteries was evaluated in 5 pairs of control and mutant littermate embryos at E11.5. C′. To quantify vessel coverage by αSMA+ smooth muscle cells, we measured the length of the vessel wall covered by αSMA+ cells (green) and divided that number by the perimeter of the vessel wall (4th arch artery) in each coronal section. Left and right 4th pharyngeal arches were examined, 5 serial sections per side per genotype were evaluated in each embryo. * Unpaired Student’s t-test probability (p) < 0.05; ** p< 0.005.
Quantification of proliferation of neural crest cells and Mesp1-derived pharyngeal mesoderm
Embryos for these studies were isolated at E10.5. All embryos carried Rosa-mT/mG reporter to identify Mesp1-derived pharyngeal mesoderm. For BrdU labeling, 2 mg of BrdU (10 mg/ml solution in PBS) per mouse was injected i.p. 20 min prior to sacrifice. Following antigen retrieval in citric acid as described above, paraffin embedded coronal sections from control (n=3) and mutant (n=5) embryos were stained using anti-BrdU andtibody. Anti-GFP antibody was used to detect Mesp1-derived mesodermal cells. Mesenchymal cells in the arches that were GFP – negative were presumed to be of neural crest origin. For proliferation of neural crest cells, we calculated and for proliferation of pharyngeal mesoderm cells, we calculated and plotted as percentages. Overall, 5454 neural crest cells were examined from 25 serial sections from 3 control embryos and 9344 neural crest cells were examined from 46 serial sections from 5 mutant embryos. 690 and 1124 GFP+, Mesp1-derived pharyngeal mesodermal cells were examined in controls and mutants, respectively. This was done in the same sections that were used for quantification of the neural crest. Proliferation of pharyngeal neural crest cells was also evaluated using anti-phospho histone H3 (pHH3) antibody with similar results as shown in Fig. 7A.
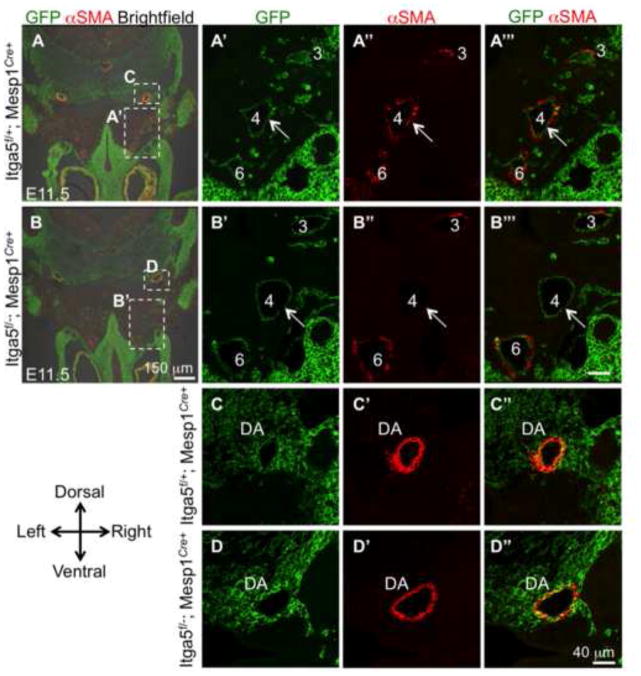
Figure 7. Integrin α5flox/−; Mesp1Cre+ mutants manifest defective differentiation of neural crest-derived cells into vascular smooth muscle cells.
Descendants of Mesp1+ lineage are marked green due to the expression of GFP from the ROSA-mT/mG reporter locus. Pharyngeal arch arteries are numbered. A – B‴. Note depletion of αSMA levels around the 4th arch arteries in the mutant compared with control (quantified in Fig. 7C). The majority of αSMA+ cells are GFP − (A‴) and are derived from the neural crest. Expression of αSMA is not affected in the dorsal aorta (C – D″).
Quantitative real time PCR
Control and mutant embryos isolated at 31–32 somite stage were used for these experiments. The regions containing either the left or the right pharyngeal arches 3–6 were dissected and stored in liquid N2 until use. RNA was isolated using RNeasy plus micro kit (Qiagen) and cDNA was synthesized using SuperScript VILO cDNA synthesis kit (Life Technologies). The PCR was performed using PerfeCTa SYBR green supermix with low ROX (cat #95056-100, Quanta Biosciences) and Stratagene Mx3000P instrument (Agilent Technologies). The primers for detecting 18S RNA were 5′-GTAACCCGTTGAACCCCATT-3′ and 5′-CCATCCAATCGGTAGTAGCG-3′, the primers for detecting integrin α5 were 5′-CCTTTTTGGCTTCTCCGTGG-3′ and 5′-ACCACCTTGCAGTACACCTG-3′, and the primers for detecting Tbx1 were 5′-TCAAGGCTCCGGTGAAGAAG-3′ and 5′-TGGAACGTGGGGAACATTC-3′.
Results
Expression of integrin α5β1 in the anterior mesoderm is required for cardiovascular development and morphogenesis of neural crest-dependent embryonic structures
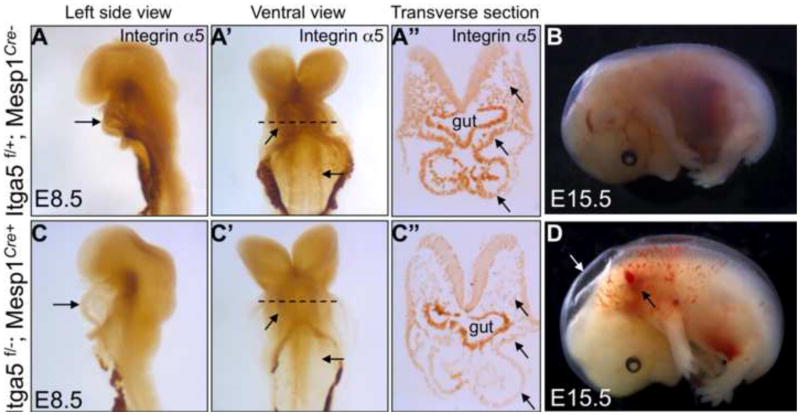
Integrin α5β1 is required for cardiac development (Mittal et al., 2013). Therefore, in order to determine whether signaling by integrin α5 in the cardiac mesoderm was required for heart development, we ablated integrin α5 using the transgenic cTnT-Cre mice (Jiao et al., 2003). This strategy led to efficient deletion of integrin α5 in the myocardium (but not endocardium) by embryonic day (E) 8.5 (Sup. Fig. 1A, B), however, mutant integrin α5flox/−; cTnT-Cre+ mice were born in normal proportions and were viable (Table 1). Given the severe defects in cardiogenesis observed in integrin α5-null embryos, especially in the development of the outflow tract (Mittal et al., 2013), we tested whether the expression of integrin α5 in the second heart field was required for cardiac development. To perform this experiment, we conditionally ablated integrin α5 using Mef2C-AHF-Cre transgenic mice (Verzi et al., 2005). However, α5flox/−; Mef2C-AHF-Cre+ mutants were born in normal proportions and survived at least until 3 weeks of age, even though integrin α5 was depleted in the cardiac outflow tract and the right ventricle (Sup. Fig. 1C, D), Table 1. Finally, we ablated integrin α5 in the anterior mesoderm, including myocardial, endocardial and endothelial progenitors and their descendants (Milgrom-Hoffman et al., 2014). To accomplish this, we used a Mesp1Cre knock-in strain, in which Cre is active in the earliest known cardiac progenitors, which originate from the anterior primitive streak at E6.5 (Saga et al., 2000). We found that ablation of integrin α5 using a Mesp1Cre knock-in strain led to depletion of integrin α5 expression in the anterior mesoderm including the heart (Fig. 1A, C) and was embryonic/perinatal lethal (Table 2). Many of α5flox/−; Mesp1Cre+ mutants exhibited edema and hemorrhage at E15.5, similar to the recently described Itga5Pdgfrb-cre mutants (Turner et al., 2014) (Fig. 1B, D). Early cardiac development appeared grossly normal at E9.5 and E10.5 in α5flox/−; Mesp1Cre+ mutants (Sup. Fig. 2). However, the majority of mutant hearts at E14.5 contained ventricular septal defects (VSD, n=12/14) at the cranial aspect of the heart (Fig. 2A, E; summarized in Table 3). These types of VSDs are sometimes referred to as membranous septal defects, e.g. (Gao et al., 2010). Taken together, our studies indicate that expression of integrin α5 in cardiomyocyte progenitors, cardiomyocytes, endocardium or in endothelium (van der Flier et al., 2010) is not required for cardiogenesis and baseline cardiac functions. Furthermore, our results suggest that the role for integrin α5 in early heart development is due to its requirement in lineages other than those derived from Mesp1-expressing cells. Alternatively, expression of integrin α5 in a combination of lineages is necessary for early cardiogenesis.
Table 1.
Myocardial expression of integrin α5 is not required for viability
| Genotypes | Integrin α5f/+ | Integrin α5f/+; cTNT-Cre+ | Integrin α5f/− | Integrin α5f/−; cTNT-Cre+ | Total # |
|---|---|---|---|---|---|
| Stages | |||||
| E7.5–E9.5 | 9 | 17 | 10 | 12 (25%) | 48 |
| At weaning (P21) | 29 | 17 | 28 | 26 (26%) | 100 |
| Integrin α5f/+ | Integrin α5f/+; Mef2C-AHF- Cre+ | Integrin α5f/− | Integrin α5f/−; Mef2C-AHF- Cre+ | Total # mice | |
| At weaning (P21) | 19 | 18 | 12 | 14 (22%) | 63 |
Figure 1. Conditional ablation of integrin α5 using Mesp1Cre.
Expression of integrin α5 at E8.5 in control embryos (A–A′) and mutants (C–C′). Arrows point at the expression of integrin α5 in the heart and dorsal aorta in controls and the depletion of integrin α5 in these structures in the mutants. Dashed lines in A′ and C′ indicate planes of section shown in A″, C″. Arrows in A″, C″ point at paraxial mesoderm (top arrow), splanchnic mesoderm (middle arrow) and the heart (bottom arrow). B. Control embryo at E15.5 and mutant embryo (D). About 50% of α5flox/−; Mesp1Cre+ mutants degenerate or show gross hemorrhage (black arrow) and edema (white arrow) by E15.5. Abbreviation: Itga5 – integrin α5. Images of controls and mutants were taken at the same magnification.
Table 2.
Requirement of integrin α5 in Mesp1-derived cells for embryonic and neonatal viability
| Genotypes | Integrin α5f/+; Mesp1+/+ | Integrin α5f/+; Mesp1Cre+ | Integrin α5f/−; Mesp1+/+ | Integrin α5f/−; Mesp1Cre+ | Total # |
|---|---|---|---|---|---|
| Stages | |||||
| E8.5-E11.5 | 71 (23.8%) | 84 (28.1%) | 67 (22.4%) | 77 (25.8%) | 299 |
| E14.5-E15.5 | 44 (29.1%) | 42 (27.8%) | 38 (25.2%) | 20 (14%)* | 150 |
| E16.5 | 14 (28.5%) | 22 (45%) | 10 (20.5%) | 3 (6%)+,** | 49 |
| At weaning | 44 (34%) | 49 (38%) | 33 (25%) | 4 (3%)*** | 130 |
Additional 6 severely degenerating mutant embryos were found; +Additional 2 severely degenerating mutant embryos were found;
p = 0.03, Fisher exact test;
p = 5×10 −6, Chi-square test.
Figure 2. Expression of intergrin α5 in Mesp1-derived cells mediates morphogenesis of the aortic arch arteries and interventricular septum.
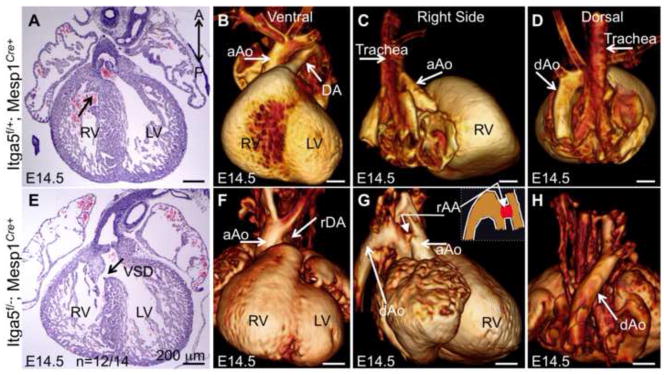
A–D control and E–H mutant hearts. A and E – H&E stained coronal sections. Arrow in A points to the closed inter-ventricular septum in the control embryo, arrow in E points at ventricular septum defect (VSD) in the mutant. B–D and F–H OPT views of control (B–D) and mutant (F–H) hearts. Mutant heart shown in F–H manifests right ductus arteriosus (rDA in F) and right-sided hypoplastic aortic arch (rAA in G). In control (B and Sup Movie 1), DA is directed toward the left. rDA is directed toward the right in the mutant (F and Sup Movie 2). rAA in G is hypoplastic and connects the ascending aorta with the descending aorta. The inset in G is a schematized drawing of the vessels on the right side, rAA is marked in red. Note the descending aorta is on the left in control (D) and on the right in the mutant embryo (H). aAo – ascending aorita, dAo- descending aorta. LV – left ventricle, RV – right ventricle. Scale bars are 200 μm.
Table 3.
Phenotypes of integrin α5f/−; Mesp1Cre+ mutant embryos (E14.5–E16.5)
| Genotypes | Controls (three groups) | Mutants |
|---|---|---|
| Phenotypes | ||
| Edema | 17% (3/18) | 73% (16/22) |
| Hemorrhage | 6% (1/18) | 59% (13/22) |
| Arch artery defects* | 0% (0/18) | 38% (9/24) |
| Membranous ventricular septal defects | 15% (2/13) | 86% (12/14) |
| Hypoglossal nerve defects | 0% (0/5) | 40% (2/5) |
| Thymus defects | 0% (0/18) | 58% (14/24) |
| Widely open palate (E14.5) | 0% (0/2) | 67% (2/3) |
| Cleft palate (≥E15.5) | 0% (0/7) | 56% (5/9) |
Arch artery defects: right ductus arteriosus (3/24), double or right-sided aortic arch (2/24), hypoplastic or interrupted aortic arch type B (3/24), retroesophageal right subclavian artery (6/24).
Cardiovascular defects are a major cause of embryonic and perinatal lethality (Chin et al., 2012; Roger et al., 2011), and since integrin α5flox/−; Mesp1Cre+ mutants exhibit late embryonic/perinatal lethality, we examined these mutants for signs of congenital heart disease as well as other phenotypes that could lead to perinatal lethality. To accomplish this, we used microdissection, histology and optical projection tomography (OPT) to examine the structures of the heart and the associated vasculature at E14.5 and E15.5. These studies indicated that close to 40% of integrin α5flox/−; Mesp1Cre+ mutants developed a variety of aortic arch artery defects (n=9/24, e.g. Fig. 2B–D, F–H; Sup. Fig. 3 and Sup. Movies 1–3). We found that the majority of mutants had ventricular septal defects (n=12/14) (Fig. 2E, arrow; summarized in Table 3). In addition, half of the mutants developed a cleft palate (Figs. 3A, B, E, F and Table 3). Together or individually, these defects account for perinatal mortality observed in integrin α5flox/−; Mesp1Cre+ mutants.
Figure 3. Expression of intergrin α5 in Mesp1-derived cells mediates development of the palate, thymus and hypoglossal nerves.
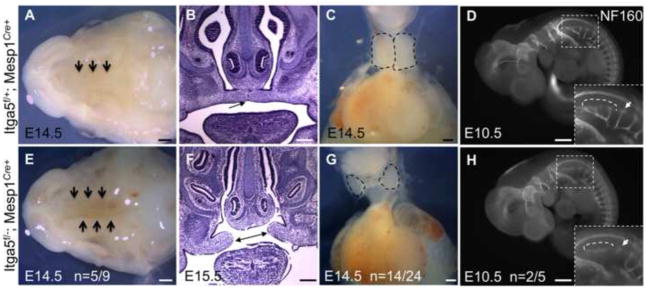
The palate closes in control embryos by E14.5 (arrows in A). B. Coronal section through the head shows closed palatal shelf (arrow in B). E–F. Mutant embryos manifest open palate (arrows in E–F). C. Normal position and shape of thymus seen in control embryos is distorted in mutants, G. Thymus lobes are outlined in C, G. D, control and H, mutant embryos stained with antibody to NF160 to detect neurons. Development of hypoglossal nerves (boxed) and the first cervical spinal nerve, arrow in H are defective in mutants. Note normal development of all other NF160+ nerves in the mutants. Scale bars are 500 μm in A, E, D, and H, and 200 μm in B, C, F, G.
Additional abnormalities in the mutants included defects in thymus development, n=14/24 (Fig. 3C, G) and abnormal development of the hypoglossal nerves, the nerves that innervate the tongue, n=2/5 (Fig. 3D, H) and Table 3. Variability in phenotypes observed with integrin α5flox/−; Mesp1Cre+ mutants is not unusual, and could be explained by several factors, one of which is a mixed C57BL6/J and 129S4 background. In addition, variability in aortic arch artery defects is common in mutants with defective function of the neural crest (Bockman et al., 1987; Conway et al., 2003; Stoller and Epstein, 2005). In such mutants, the stereotypical remodeling of the symmetrical pharyngeal arch arteries is altered, leading to randomized regression or persistence of these blood vessels (Hutson and Kirby, 2007). The randomness with which arch arteries regress gives rise to an assortment of defects in morphogenesis of the aortic arch artery tree, similar to the phenotypes observed in our mutants (Fig. 2, Sup Fig. 3, and Sup. Movies 1–3; summarized in Table 3). These defects include double aortic arch, right-sided aortic arch and right ductus arteriosus, interrupted or hypoplastic aortic arch and retroesophageal right subclavian artery (RERSA), a vascular ring surrounding the esophagus and trachea (Table 3 and Sup. Fig. 3G). RERSA was the most common arch artery abnormality observed in the mutants (Table 3).
The bulk of the palate is derived from the neural crest (Ito et al., 2003) and Sup Fig. 4A–B, and cleft palate commonly arises due to neural crest dysfunction, e.g. (Ito et al., 2003). Likewise, at E15.5, the cardiac ventricular septum at the cranial aspect of the heart is mainly derived from the neural crest (Gao et al., 2010) and Sup. Fig 4C, arrow. Defects in neural crest development lead to defective closure of the interventricular septum at the cranial aspect of the heart (Gao et al., 2010; High et al., 2007), and are analogous to the septal defects observed in our mutants (Fig. 2E). Similarly, development of the hypoglossal nerves and the thymus are dependent on the neural crest (Bockman and Kirby, 1984; Huettl and Huber, 2011). Anterior mesoderm and the neural crest develop in close proximity with each other (e.g. Fig. 4A) (Noden and Trainor, 2005) and development of their derivatives is intricately dependent on signaling between the two populations (Ferguson and Graham, 2004; Itasaki et al., 1996; Michailovici et al., 2014; Milgrom-Hoffman et al., 2014; Rinon et al., 2007). While individually, each of the neural crest-related defects observed in integrin α5flox/−; Mesp1Cre+ mutants was not fully penetrant, when taken in combination, these phenotypes suggest that mesodermal integrin α5 plays essential roles in regulating neural crest development. In order to test this hypothesis, we focused on elucidating mechanisms underlying defective development of the aortic arch arteries.
Figure 4. Mesp1Cre fate map.
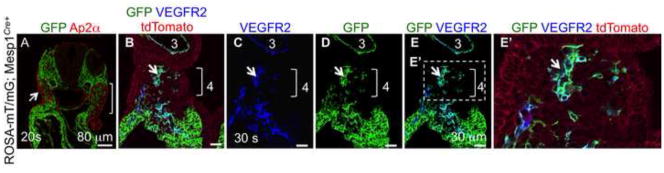
A. Descendants of Mesp1 lineage (green, GFP positive) give rise to the cranial and cardiac mesoderm and are in close contact with the neural crest cells and their derivatives (red, Ap2α). Ap2α is also expressed in the surface ectoderm (arrow in A). Note the absence of double labeled cells. B–E. Derivatives of Mesp1+ mesoderm give rise to endothelial progenitors, cells that are double positive for GFP and VEGFR2, brackets in B–E, magnified in E′. Note that the already formed 3rd arch artery endothelium is derived from Mesp1+ descendants. The presence of ROSA-mT/mG reporter in all embryos allows visualization of cell fate (GFP) in animals carrying the Cre transgene.
Defective morphogenesis of the aortic arch arteries is due to defective remodeling of the symmetrical pharyngeal arch arteries into their final asymmetrical form
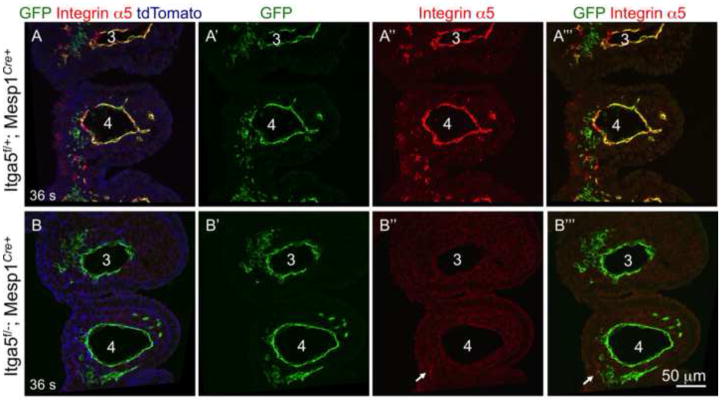
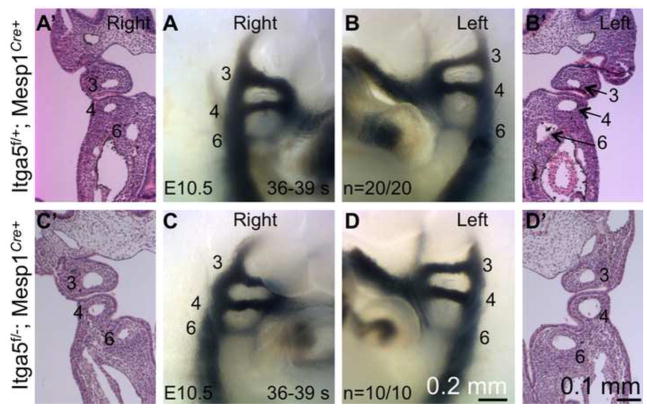
Cells derived from Mesp1 lineage give rise to endothelial cells of the pharyngeal arch arteries (Figs. 4–5) and the online Figure IIB in (Papangeli and Scambler, 2013). Consistent with these data, we found that integrin α5 was efficiently depleted from arch artery endothelium in integrin α5flox/−; Mesp1Cre+ mutants (Fig. 5B”–B’”). Thus the abnormal morphogenesis of aortic arch arteries in integrin α5flox/−; Mesp1Cre+ mutants could be due to defective formation or aberrant remodeling of the pharyngeal arch arteries (Conway et al., 2003). In order to test the role of mesodermal integrin α5 in arch artery development, we assayed formation of pharyngeal arch arteries by injection of black India ink into the hearts of control and mutant embryos isolated at the 36–39 somite stage, E10.5 (Kaufmann, 1992). At this stage, the arch arteries 3, 4, and 6 are well-formed in controls (n=20) and α5flox/−; Mesp1Cre+ mutants (n=10) (Fig. 6). Therefore, our experiments indicate that mesodermal expression of integrin α5 is not required for the formation of the pharyngeal arch arteries and suggest that the defective aortic arch artery tree observed at E15.5 in the mutants resulted from defective remodeling of these initially symmetrical blood vessels. This phenotype is consistent with the idea that mesodermal integrin α5 impacts neural crest cell functions, since defects or deficiencies in the cranial neural crest are commonly associated with defective remodeling of the pharyngeal arch arteries but not with their formation (Hutson and Kirby, 2007).
Figure 5. Efficient depletion of intergrin α5 in the 4th arch artery endothelium using Mesp1Cre knock-in mice.
Conditional ablation of integrin α5 in pharyngeal arch arteries. Note expression of integrin α5 (red) in the 3rd and 4th arch artery endothelium in sections from controls A–A‴ and depletion of endothelial integrin α5 in mutants, B–B‴. Arrow points at the remaining expression of integrin α5 in GFP-negative cells (B″–B‴). The presence of ROSA-mT/mG reporter in all embryos allows visualization of cell fate (GFP) in animals carrying the Cre transgene. Magnification is the same in all panels.
Figure 6. Pharyngeal arch arteries form normally in integrin α5flox/−; Mesp1Cre+ mutants.
India ink injections (A – D) and histological analyses (A’ –D’) demonstrate that arch arteries form in mutants as well as they do in controls. Arch arteries are numbered. Images of controls and mutants were taken at the same magnification.
Mesodermal expression of integrin α5 is required for neural crest development and smooth muscle differentiation
It is unlikely that defective cardiac function contributed to arch artery defects in α5flox/−; Mesp1Cre+ mutants observed at E15.5 because our experiments using cTnT-Cre and Mef2C-AHF-Cre transgenic mice showed that expression of integrin α5 in the myocardium was not required for baseline cardiac functions during embryogenesis or in adult. Aberrant remodeling of the pharyngeal arch arteries could stem from defective establishment or maintenance of the left-right body plan (Nonaka et al., 2002). However, the heart looped normally in all integrin α5flox/−; Mesp1Cre+ mutants examined (n>20) and the expression of Pitx2c was confined to the left side of the lateral plate mesoderm at E9.5 (n=4/4), suggesting that the expression of integrin α5 in Mesp1-derived lineage was not required for the establishment or maintenance of the left-right body axis (Sup Fig. 5A, A′, F, F′). Therefore, defective remodeling of pharyngeal arch arteries in these conditional mutants was due to a different cause.
Maturation and remodeling of blood vessels including that of aortic arch arteries is intimately dependent on the development of vascular smooth muscle cells (VSMCs) (Bergers and Song, 2005; Keyte and Hutson, 2012; Majesky, 2007). VSMCs of the pharyngeal arch arteries are derived from the cardiac neural crest, which is a sub-population of the cranial neural crest originating in the dorsal neural tube between the otic vesicle and the 4th somite (Bockman et al., 1987; Chan et al., 2004; Hutson and Kirby, 2007; Keyte and Hutson, 2012; Leatherbury et al., 1990). Lineage tracing using Mesp1Cre+ showed that the majority of smooth muscle cells around pharyngeal arch arteries are Mesp1-lineage negative (Fig. 7A′–A‴), and are derived from the neural crest, arrows in Sup. Fig. 4D–D’ and (High and Epstein, 2008; Hutson and Kirby, 2007; Jiang et al., 2000).
Prior studies indicated that severe deficiency in cardiac neural crest cell numbers or defective differentiation of neural crest-derived cells into VSMCs gave rise to pharyngeal arch artery remodeling defects comparable with those observed in our α5flox/−; Mesp1Cre+ mutants (Dai et al., 2013; High and Epstein, 2008; High et al., 2007; Hutson and Kirby, 2007; Li et al., 2005; Porras and Brown, 2008). Factors such as deficient proliferation, deficient differentiation or increased cell death of the neural crest-derived cells could affect development of the VSMC coat around the pharyngeal arch arteries. In order to examine these issues, we first evaluated differentiation of neural crest-derived cells into VSMCs around the 4th and 6th pharyngeal arch arteries. To accomplish this, we stained serial sections from control (n=5) and mutant (n=5) embryos isolated at E11.5 with antibody to αSMA. Overall, 50 tissue sections from control and mutant embryos were evaluated. These studies indicated a severe defect in smooth muscle coverage around pharyngeal arch arteries in 4 out of 5 mutants (Fig. 7 and Fig. 8A for individual comparison of each mutant and its littermate control), with the overall p<2×10−6 (unpaired Student’s t test) when all of the mutants were compared with all of the controls. Differentiation of neural crest-derived cells into VSMCs was also diminished around the 6th arch arteries in 3 out of 5 mutants (data not shown). Interestingly, we found that VSMC coverage of dorsal aortae was not affected in our mutants (Fig. 7C–C″, D–D″). This is because unlike VSMCs of pharyngeal arch arteries, which are derived from the neural crest, VSMCs of the dorsal aortae are derived from Mesp1+ mesoderm (Fig. 7C″) and (Majesky, 2007). Our studies are consistent with the recently published data demonstrating that integrin α5 was not required for the development of VSMCs of mesodermal origin (Turner et al., 2014).
In order to examine proliferation and cell death of neural crest-derived cells in the pharyngeal arches, we evaluated over 5000 mesenchymal, Mesp1-lineage-negative cells in the 4th pharyngeal arches of E10.5 mutants and controls (see Methods). The vast majority of these cells are of neural crest origin (Jiang et al., 2000) and Sup. Fig. 4D. While we did not observe any significant cell death in our mutants, we noted a slight (10%) but reproducible and statistically significant defect in proliferation of NC-derived mesenchyme in the 4th pharyngeal arches at E10.5 (Fig. 8B). Since we have deleted integrin α5 in the pharyngeal mesoderm, we also evaluated proliferation of mesodermal, Mesp1-derived cells within the 4th pharyngeal arches. For these studies, over 600 pharyngeal mesodermal cells were evaluated, some of which were endothelial cells within the 4th pharyngeal arch arteries. Interestingly, proliferation of Mesp1-derived mesodermal cells (including the endothelial cells) in the 4th pharyngeal arches was unaffected in integrin α5flox/−; Mesp1Cre+ mutants (Fig. 8C), suggesting that integrin α5 expressed by Mesp1-derived mesodermal cells is specifically required for the proliferation of neural crest-derived cells. Taken together, our data indicate a specific role of mesodermal integrin α5 in proliferation and differentiation of neural crest-derived cells.
In order to investigate potential mechanisms regulating the mesoderm-neural crest communications disrupted in our mutants, we evaluated expression of fibronectin (FN) around aortic arch arteries, since FN is enriched around these vessels and is expressed throughout the mesenchyme of pharyngeal arches 3–6 (Sup. Fig. 6A–A″) and (Mittal et al., 2010). However, localization of FN protein in mutant arches (Fig. 6B–B″) was comparable with that of controls, likely because mesodermal and endothelial cells within pharyngeal arches express other integrin heterodimers that bind FN, e.g. those containing integrin αv subunits (van der Flier et al., 2010). We also investigated expression of genes known to regulate neural crest proliferation and patterning in the pharyngeal arches. For example, signaling by Fgf8 regulates neural crest proliferation, however, we did not observe changes in the expression of Fgf8 or its downstream target Mkp3 in our mutants (Sup. Fig. 5B, C, G, H). Furthermore, we evaluated whether Mesp1Cre – mediated deletion of integrin α5 affected the expression of Hox genes in the mesenchyme of the posterior pharyngeal arches. However, expression of Hoxa3 and Hoxd4 mRNAs was not significantly affected in our mutants (Sup Fig. 5D, E, I, J). Finally, given the similarity between gross morphological phenotypes in our model relative to abnormalities observed in DiGeorge patients and in Tbx1-mutant mice, we assayed expression levels of Tbx1 mRNA in the pharyngeal arches of our mutants and controls using quantitative real time PCR. However, levels of Tbx1 mRNA were unchanged in α5flox/−; Mesp1Cre+ mutants relative to controls (Sup. Fig. 5K). This is not entirely surprising, since Tbx1 regulates formation of the 4th and 6th arch arteries (Arnold et al., 2006; Lindsay et al., 2001; Zhang et al., 2006), while mesodermal integrin α5 is required for the remodeling of these blood vessels but not for their formation. Taken together, our studies indicate that mesodermal integrin α5 plays an integral role in regulating differentiation and proliferation of neural crest-derived cells in the 4th pharyngeal arches, and suggest a model wherein integrin α5β1 expressed by the Mesp1-derived mesoderm regulates inter-cellular communications between the mesoderm and the neural crest (Fig. 9).
Figure 9. Model.
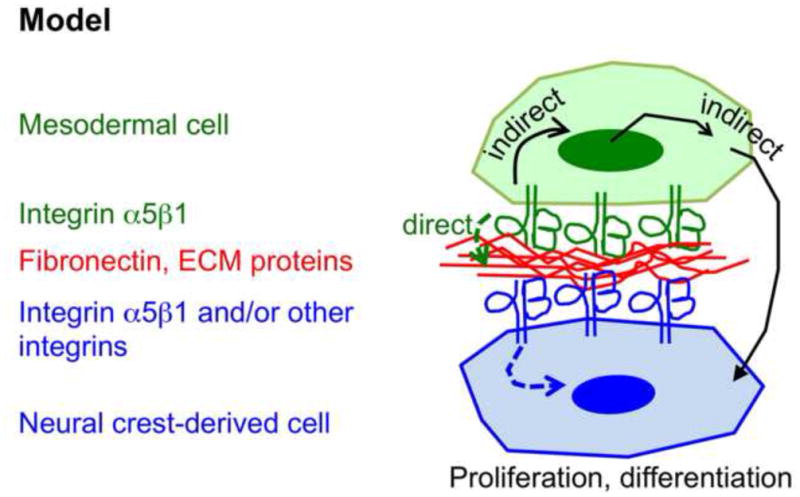
Mesodermal integrin α5β1 mediates signaling between the anterior mesoderm and the neural crest and influences neural crest development. Dashed arrows indicate the idea that binding of mesodermal integrin α5β1 to ECM modifies ECM properties, which are then sensed by the neural crest-derived cells. Solid arrows schematize the idea that signaling by mesodermal integrin α5β1 could lead to expression of soluble or cell-associated factors that could in turn influence neural crest development.
Discussion
Signaling interactions between anterior mesoderm and the neural crest influence development of the face, thymus, thyroid and cardiovascular system (Keyte and Hutson, 2012; Tzahor and Evans, 2011). However, molecular regulation of these interactions is not well understood. In this work, we demonstrate that integrin α5β1 expressed by the anterior mesoderm regulates morphogenesis of tissues mainly derived from the neural crest, such as the cranial segment of the cardiac ventricular septum and the palate. The palate is mainly comprised of the neural crest-derived cells, while Mesp1-derived mesoderm contributes only to a minority of cells within the palate, and we found that these cells comprise palatal vasculature (Sup. Fig. 4B′ – B‴). Therefore, it is possible that cleft palate in our mutants is caused by defective vascularization of the embryonic palatal mesenchyme. Alternatively, mesodermal integrin α5 could regulate mesoderm-neural crest interactions earlier in the development of these lineages, at the time of neural crest migration and close intermingling with cranial and/or pharyngeal mesoderm. We favor the latter hypothesis, since deletion of integrin α5 in endothelium using Tie2-Cre transgenic mice did not give rise to cleft palate (van der Flier et al., 2010). Neural crest also contributes to the mesenchyme of the thymus, Schwann cells of hypoglossal nerves, and vascular smooth muscle cells of aortic arch arteries. All of these tissues manifested morphogenetic defects in the integrin α5flox/−; Mesp1Cre+ mutants with variable penetrance; however, in combination, such defects implicate mesodermal α5β1 in regulating neural crest development.
In this study, we focused our attention on the development of the aortic arch arteries. These three pairs of pharyngeal arch arteries form by E10.5 in the mouse (e.g. Fig. 6) and then become remodeled in a highly stereotypical fashion to form the adult-like, asymmetrical vascular tree by E13.5. Defects in this remodeling process give rise to common forms of human congenital heart disease (Stoller and Epstein, 2005). Formation and remodeling of the 4th pharyngeal arch arteries are especially sensitive to genetic and environmental insults, for reasons that are not yet well understood. In our studies, the majority of arch artery defects were due to defective development of the 4th pharyngeal arch arteries. Examples of such defects are retroesophageal right subclavian artery (RERSA), the right-sided or double-sided aortic arch, and interrupted or hypoplastic aortic arch (Stoller and Epstein, 2005). All of these defects were observed in our mutants (Fig. 2, Sup Fig. 3 and Table 3). The aortic arch is derived from the left 4th pharyngeal arch artery, and the regression of this arch artery gives rise to the interrupted aortic arch type B, while its incomplete regression results in a hypoplastic aortic arch. RERSA is due to premature regression of the right 4th arch artery; The right-sided aortic arch is due to regression of the left 4th pharyngeal arch artery and ectopic persistence of the right 4th arch artery, while double aortic arch is due to ectopic persistence of the right 4th pharyngeal arch artery. We also observed embryos with right ductus arteriosus (Fig. 2, Sup. Fig. 3 and Movies 2–3), an abnormality that results from aberrant regression of the left 6th pharyngeal arch artery and ectopic persistence of the right 6th arch artery (Stoller and Epstein, 2005). Collectively, these phenotypes fall into a category of defects characterized by the unpredictability with which some arteries regress while others persist. This is in contrast with the ordered, stereotypical remodeling process in wild-type mice, which results in the stereotypical arrangement of the aortic arch and its branches. This unpredictability of arch artery remodeling is a classical manifestation of defective development of the cardiac neural crest (Hutson and Kirby, 2007). The cardiac neural crest gives rise to VSMCs of pharyngeal arch arteries, and studies using numerous mouse genetic models of neural crest dysfunction as well as neural crest ablation studies in chickens indicated that such irregular patterns of arch artery remodeling were due to defective development of VSMCs around pharyngeal arch arteries (High and Epstein, 2008; Hutson and Kirby, 2007; Kaartinen et al., 2004; Kirby, 2007; Li et al., 2005; Vallejo-Illarramendi et al., 2009).
Mesp1-derived mesoderm gives rise to endothelial cells of all pharyngeal arch arteries (Figs. 4–5, 7), and conditional deletion of integrin α5 using a Mesp1Cre knock-in strain resulted in efficient depletion of integrin α5 from Mesp1-derived endothelial cells. However, we did not observe defects in the formation of the pharyngeal arch arteries or in proliferation of Mesp1-derived mesodermal and endothelial cells in the mutants. Instead, we observed a marked decrease in the numbers of VSMCs and consequently, aberrant remodeling of the symmetrical pharyngeal arch arteries. We think that this decrease in VSMCs is likely due to defects in differentiation of neural crest-derived cells into VSMCs. Even though we observed a slight decrease in neural crest proliferation at E10.5, the appearance and the cellularity of pharyngeal arches at E10.5 and at E11.5 were similar in controls and in mutants (Figs. 5 – 7). Similar to controls, mutant arch arteries were surrounded with comparable numbers of neural crest-derived cells at E10.5 and at E11.5 (Figs. 5 and 7A–B). Thus neural crest-derived cells closely apposing arch artery endothelium in integrin α5flox/−; Mesp1Cre+ mutants were defective in their differentiation into VSMCs.
It is well established that the majority of VSMCs around pharyngeal arch arteries are derived from the neural crest (Hutson and Kirby, 2007); however, we observed that a small population of pharyngeal arch αSMA+ VSMCs (<10% at E11.5) was derived from cells of Mesp1+ lineage. While it is possible that ablation of integrin α5 in Mesp1 lineage contributed to defective VSMC development around the arch arteries, the decrease in the VSMC coverage of the 4th pharyngeal arch arteries was far greater than 10%. Indeed, we observed about 34% depletion in VSMC coverage in the mild case (pair 1, left 4th arch in Fig. 8A) and 89% depletion of VSMC coverage in the most severe case (pair 4, right 4th arch, Fig. 8A). Moreover, we did not observe a decrease in VSMC differentiation around the dorsal aortae: in this vascular bed, endothelial and smooth muscle cells are derived from Mesp1+ progenitors (Majesky, 2007) and Fig. 7C″, D″. Thus deletion of integrin α5 in mesodermal descendants of Mesp1-expressing cells is not required for differentiation of mesoderm-derived cells into VSMCs, a finding consistent with the recently published studies (Turner et al., 2014). Taken together, these data underscore the specific role of mesodermal integrin α5 in neural crest development and differentiation into VSMCs.
It is possible that expression of integrin α5β1 by endothelial cells of the 4th and 6th pharyngeal arch arteries regulates differentiation of the adjacent neural crest cells into VSMCs. However, deletion of integrin α5 in the endothelium using a Tie2-Cre transgenic strain did not result in embryonic vascular defects or in defective remodeling of the symmetrical pharyngeal arch arteries into the asymmetrical vascular tree (van der Flier et al., 2010). This suggests that expression of integrin α5 in the anterior or in the pharyngeal mesoderm prior to formation of the pharyngeal vasculature affected neural crest development. This idea is consistent with pleiotropic neural crest-related defects caused by the ablation of integrin α5 in Mesp1 lineage; these defects were not limited to the neural crest-derived cells in the immediate vicinity of the 4th or 6th arch artery endothelium.
How does mesodermal integrin α5β1 regulate neural crest development? Integrins are bi-directional signal transducers, which allow cells to sense and to respond to chemical and mechanical changes in their surrounding microenvironment (Hynes, 2002; Larsen et al., 2006; Schwartz, 2010). Mesodermal and neural crest-derived cells express a variety of integrins that can bind extracellular matrix (ECM) proteins and signal (Delannet et al., 1994; Strachan and Condic, 2004; Testaz et al., 1999; van der Flier et al., 2010; Xu et al., 2006). One could envision two general ways by which mesodermal integrin α5β1 could function in neural crest development (Fig. 9). One possibility is that binding of mesodermal integrin α5α1 to its major ECM ligand fibronectin within the pharyngeal arch mesenchyme influences neural crest cells by modifying their immediate extracellular microenvironment, e.g. its composition, structure and/or compliance (Larsen et al., 2006). Neighboring neural crest cells could sense these changes via their cell surface integrins and respond accordingly (this possibility is schematized by dashed arrows in Fig. 9). The binding of mesodermal integrin α5α1 to fibronectin could also affect association and/or activation of ECM-bound growth factors, e.g. (Fontana et al., 2005; Wurdak et al., 2005), which could then signal to the neural crest. Binding of integrin α5β1 to other ligands could also be involved, e.g. (Ossowski and Aguirre-Ghiso, 2000). Another possibility is a more upstream role, whereby signaling by integrin α5β1 expressed by mesodermal cells induces transcriptional responses in the mesoderm leading to the expression of soluble or cell-associated ligand(s) (e.g. Jagged1) that can bind and signal to the adjacent neural crest cells (solid arrow in Fig. 9).
In conclusion, our studies identified an important mesodermal regulator, integrin α5β1 that facilitates signaling between the anterior mesoderm and the neural crest. Future investigations into mechanisms whereby mesodermal integrin α5β1 regulates neural crest development would provide important insights into craniofacial and cardiovascular morphogenesis.
Supplementary Material
Highlights.
Ablation of mesodermal integrin α5β1 leads to craniofacial & cardiovascular defects;
Remodeling of aortic arch arteries is one of these defects;
These birth defects arise due to dysfunction in neural crest-derived cells;
Mesodermal integrin α5β1 regulates neural crest proliferation and differentiation.
Acknowledgments
We thank Drs Yumiko Saga for Mesp1Cre mice, Brigit Hogan for cTNT-Cre mice, Mef2C-AHF-Cre strain was generated by Dr. Brian Black. We are grateful to Dr. Richard Hynes for anti-FN antibody and for providing integrin α5 floxed mice prior to their publication. We are grateful to Dr. Jon Epstein for the use of his lab’s OPT scanner; Drs James Martin, Gail Martin, Nancy Manley and Denis Duboule for plasmids used for in situ hybridization, and Carlie McGinnis and Ahab Dababneh for genotyping. We thank Nathan Astrof and Dongying Chen for insightful comments, and Kelley Chen for editing the manuscript. The G3G4 and 2H3 monoclonal antibodies were developed by Drs S.J. Kaufman and T. M. Jessel respectively, and were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at the University of Iowa, Department of Biology, Iowa City, IA 52242. This work was supported by the grants from the NIH HL094763 to KD and HL103920 to SA, the American Heart Association Innovative Research Grant 12IRG9130012 and the Louis and Fannie Tolz Program for Weizmann Institute-Thomas Jefferson University Collaboration to S.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal VS, Carpenter C, Freyer L, Liao J, Petti M, Morrow BE. Mesodermal Tbxl is required for patterning the proximal mandible in mice. Developmental Biology. 2010;344:669–681. doi: 10.1016/j.ydbio.2010.05.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JS, Werling U, Braunstein EM, Liao J, Nowotschin S, Edelmann W, Hebert JM, Morrow BE. Inactivation of Tbxl in the pharyngeal endoderm results in 22qllDS malformations. Development. 2006;133:977–987. doi: 10.1242/dev.02264. [DOI] [PubMed] [Google Scholar]
- Astrof S. Interactions between neural crest-derived cells and extracellular microenvironment during cardiovascular development. In: Desimone DW, Mecham RP, editors. Extracellular Matrix in Development. Springer Verlag; Berlin: 2013. pp. 105–131. [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7:452–464. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman DE, Kirby ML. Dependence of thymus development on derivatives of the neural crest. Science. 1984;223:498–500. doi: 10.1126/science.6606851. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Redmond ME, Waldo K, Davis H, Kirby ML. Effect of neural crest ablation on development of the heart and arch arteries in the chick. The American journal of anatomy. 1987;180:332–341. doi: 10.1002/aja.1001800403. [DOI] [PubMed] [Google Scholar]
- Chan WY, Cheung CS, Yung KM, Copp AJ. Cardiac neural crest of the mouse embryo: axial level of origin, migratory pathway and cell autonomy of the splotch (Sp2H) mutant effect. Development. 2004;131:3367–3379. doi: 10.1242/dev.01197. [DOI] [PubMed] [Google Scholar]
- Chen Y, Moon AM, Gaufo GO. Influence of mesodermal Fgf8 on the differentiation of neural crest-derived postganglionic neurons. Dev Biol. 2012;361:125–136. doi: 10.1016/j.ydbio.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin AJ, Saint-Jeannet JP, Lo CW. How insights from cardiovascular developmental biology have impacted the care of infants and children with congenital heart disease. Mechanisms of development. 2012;129:75–97. doi: 10.1016/j.mod.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway SJ, Kruzynska-Frejtag A, Kneer PL, Machnicki M, Koushik SV. What cardiovascular defect does my prenatal mouse mutant have, and why? Genesis. 2003;35:1–21. doi: 10.1002/gene.10152. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- Dai X, Jiang W, Zhang Q, Xu L, Geng P, Zhuang S, Petrich BG, Jiang C, Peng L, Bhattacharya S, Evans SM, Sun Y, Chen J, Liang X. Requirement for integrin-linked kinase in neural crest migration and differentiation and outflow tract morphogenesis. BMC biology. 2013;11:107. doi: 10.1186/1741-7007-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt K, Singh MK, Aghajanian H, Massera D, Wang Q, Li J, Li L, Choi C, Yzaguirre AD, Francey LJ, Gallant E, Krantz ID, Gruber PJ, Epstein JA. Semaphorin 3d signaling defects are associated with anomalous pulmonary venous connections. Nature medicine. 2013;19:760–765. doi: 10.1038/nm.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delannet M, Martin F, Bossy B, Cheresh DA, Reichardt LF, Duband JL. Specific roles of the alpha V beta 1, alpha V beta 3 and alpha V beta 5 integrins in avian neural crest cell adhesion and migration on vitronectin. Development. 1994;120:2687–2702. doi: 10.1242/dev.120.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Yelon D, Conlon FL, Kirby ML. Myocardial lineage development. CircRes. 2010;107:1428–1444. doi: 10.1161/CIRCRESAHA.110.227405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CA, Graham A. Redefining the head-trunk interface for the neural crest. Dev Biol. 2004;269:70–80. doi: 10.1016/j.ydbio.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Fontana L, Chen Y, Prijatelj P, Sakai T, Fassler R, Sakai LY, Rifkin DB. Fibronectin is required for integrin alphavbeta6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEBJ. 2005;19:1798–1808. doi: 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Gonzalez C, Bronner-Fraser M. Neuropilin 2/semaphorin 3F signaling is essential for cranial neural crest migration and trigeminal ganglion condensation. Dev Neurobiol. 2007;67:47–56. doi: 10.1002/dneu.20326. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Gonzalez C, Gu C, Bronner-Fraser M. Guidance of trunk neural crest migration requires neuropilin 2/semaphorin 3F signaling. Development. 2006;133:99–106. doi: 10.1242/dev.02187. [DOI] [PubMed] [Google Scholar]
- Gao Z, Kim GH, Mackinnon AC, Flagg AE, Bassett B, Earley JU, Svensson EC. Etsl is required for proper migration and differentiation of the cardiac neural crest. Development. 2010;137:1543–1551. doi: 10.1242/dev.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt SJ, Krumlauf R, Duboule D. Mouse homeo-genes within a subfamily, Hox-1.4, -2.6 and -5.1, display similar anteroposterior domains of expression in the embryo, but show stage- and tissue-dependent differences in their regulation. Development. 1989;107:131–141. doi: 10.1242/dev.107.1.131. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Goh KL, Yang JT, Hynes RO. Mesodermal defects and cranial neural crest apoptosis in alpha5 integrin-null embryos. Development. 1997;124:4309–4319. doi: 10.1242/dev.124.21.4309. [DOI] [PubMed] [Google Scholar]
- Gorlin RJ, Cohen MM, Levin LS. Syndromes of the head and neck. Oxford University Press; Oxford, UK: 1990. [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9:49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- High FA, Lu MM, Pear WS, Loomes KM, Kaestner KH, Epstein JA. Endothelial expression of the Notch ligand Jagged1 is required for vascular smooth muscle development. Proceedings of the National Academy of Sciences %R. 2008;105:1955–1959. doi: 10.1073/pnas.0709663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS, Epstein JA. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J Clin Invest. 2007;117:353–363. doi: 10.1172/JCI30070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettl RE, Huber AB. Cranial nerve fasciculation and Schwann cell migration are impaired after loss of Npn-1. Dev Biol. 2011;359:230–241. doi: 10.1016/j.ydbio.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Hutson MR, Kirby ML. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol. 2007;18:101–110. doi: 10.1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson MR, Zeng XL, Kim AJ, Antoon E, Harward S, Kirby ML. Arterial pole progenitors interpret opposing FGF/BMP signals to proliferate or differentiate. Development. 2010;137:3001–3011. doi: 10.1242/dev.051565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson MR, Zhang P, Stadt HA, Sato AK, Li YX, Burch J, Creazzo TL, Kirby ML. Cardiac arterial pole alignment is sensitive to FGF8 signaling in the pharynx. Developmental biology. 2006;295:486–497. doi: 10.1016/j.ydbio.2006.02.052. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itasaki N, Sharpe J, Morrison A, Krumlauf R. Reprogramming Hox expression in the vertebrate hindbrain: influence of paraxial mesoderm and rhombomere transposition. Neuron. 1996;16:487–500. doi: 10.1016/s0896-6273(00)80069-0. [DOI] [PubMed] [Google Scholar]
- Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Jr, Nakajima A, Shuler CF, Moses HL, Chai Y. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development. 2003;130:5269–5280. doi: 10.1242/dev.00708. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julich D, Geisler R, Holley SA. Integrinalpha5 and delta/notch signaling have complementary spatiotemporal requirements during zebrafish somitogenesis. Dev Cell. 2005;8:575–586. doi: 10.1016/j.devcel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Julich D, Mould AP, Koper E, Holley SA. Control of extracellular matrix assembly along tissue boundaries via Integrin and Eph/Ephrin signaling. Development. 2009;136:2913–2921. doi: 10.1242/dev.038935. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–3490. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- Kaufmann MH. The Atlas of Mouse Development. Elsevier Ltd; London: 1992. [Google Scholar]
- Keyte A, Hutson MR. The neural crest in cardiac congenital anomalies. Differentiation. 2012;84:25–40. doi: 10.1016/j.diff.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ML. Cardiac Development. Oxford University Press; New York: 2007. [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Larsen M, Artym VV, Green JA, Yamada KM. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol. 2006;18:463–471. doi: 10.1016/j.ceb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Leatherbury L, Gauldin HE, Waldo K, Kirby ML. Microcinephotography of the developing heart in neural crest-ablated chick embryos. Circulation. 1990;81:1047–1057. doi: 10.1161/01.cir.81.3.1047. [DOI] [PubMed] [Google Scholar]
- Li J, Zhu X, Chen M, Cheng L, Zhou D, Lu MM, Du K, Epstein JA, Parmacek MS. Myocardin-related transcription factor B is required in cardiac neural crest for smooth muscle differentiation and cardiovascular development. Proc Natl Acad Sci U S A. 2005;102:8916–8921. doi: 10.1073/pnas.0503741102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay EA, Vitelli F, Su H, Morishima M, Huynh T, Pramparo T, Jurecic V, Ogunrinu G, Sutherland HF, Scambler PJ, Bradley A, Baldini A. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- Liu C, Liu W, Lu MF, Brown NA, Martin JF. Regulation of left-right asymmetry by thresholds of Pitx2c activity. Development. 2001;128:2039–2048. doi: 10.1242/dev.128.11.2039. [DOI] [PubMed] [Google Scholar]
- Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- Manley NR, Selleri L, Brendolan A, Gordon J, Cleary ML. Abnormalities of caudal pharyngeal pouch development in Pbx1 knockout mice mimic loss of Hox3 paralogs. Dev Biol. 2004;276:301–312. doi: 10.1016/j.ydbio.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Michailovici I, Harrington HA, Azogui HH, Yahalom-Ronen Y, Plotnikov A, Ching S, Stumpf MP, Klein OD, Seger R, Tzahor E. Nuclear to cytoplasmic shuttling of ERK promotes differentiation of muscle stem/progenitor cells. Development. 2014;141:2611–2620. doi: 10.1242/dev.107078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom-Hoffman M, Michailovici I, Ferrara N, Zelzer E, Tzahor E. Endothelial cells regulate neural crest and second heart field morphogenesis. Biology open. 2014 doi: 10.1242/bio.20148078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal A, Pulina M, Hou S, Astrof S. Fibronectin and integrin alpha 5 play requisite roles in cardiac morphogenesis. Dev Biol. 2013;381:73–82. doi: 10.1016/j.ydbio.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal A, Pulina M, Hou SY, Astrof S. Fibronectin and integrin alpha 5 play essential roles in the development of the cardiac neural crest. Mech Dev. 2010;127:472–484. doi: 10.1016/j.mod.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon A. Mouse models of congenital cardiovascular disease. Curr Top Dev Biol. 2008;84:171–248. doi: 10.1016/S0070-2153(08)00604-2. [DOI] [PubMed] [Google Scholar]
- Moon AM. Mouse models for investigating the developmental basis of human birth defects. Pediatr Res. 2006;59:749–755. doi: 10.1203/01.pdr.0000218420.00525.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. J Anat. 2005;207:575–601. doi: 10.1111/j.1469-7580.2005.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–99. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- Ossowski L, Aguirre-Ghiso JA. Urokinase receptor and integrin partnership: coordination of signaling for cell adhesion, migration and growth. CurrOpin Cell Biol. 2000;12:613–620. doi: 10.1016/s0955-0674(00)00140-x. [DOI] [PubMed] [Google Scholar]
- Papangeli I, Scambler PJ. Tbxl genetically interacts with the transforming growth factor-beta/bone morphogenetic protein inhibitor Smad7 during great vessel remodeling. Circ Res. 2013;112:90–102. doi: 10.1161/CIRCRESAHA.112.270223. [DOI] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras D, Brown CB. Temporal-spatial ablation of neural crest in the mouse results in cardiovascular defects. Dev Dyn. 2008;237:153–162. doi: 10.1002/dvdy.21382. [DOI] [PubMed] [Google Scholar]
- Pulina M, Liang D, Astrof S. Shape and position of the node and notochord along the bilateral plane of symmetry are regulated by cell-extracellular matrix interactions. Biology open. 2014 doi: 10.1242/bio.20148243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulina MV, Hou SY, Mittal A, Julich D, Whittaker CA, Holley SA, Hynes RO, Astrof S. Essential roles of fibronectin in the development of the left-right embryonic body plan. Dev Biol. 2011;354:208–220. doi: 10.1016/j.ydbio.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentschler S, Jain R, Epstein JA. Tissue-tissue interactions during morphogenesis of the outflow tract. Pediatr Cardiol. 2010;31:408–413. doi: 10.1007/s00246-009-9611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinon A, Lazar S, Marshall H, Buchmann-Moller S, Neufeld A, Elhanany-Tamir H, Taketo MM, Sommer L, Krumlauf R, Tzahor E. Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development. 2007;134:3065–3075. doi: 10.1242/dev.002501. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga Y, Kitajima S, Miyagawa-Tomita S. Mesp1 expression is the earliest sign of cardiovascular development. Trends Cardiovasc Med. 2000;10:345–352. doi: 10.1016/s1050-1738(01)00069-x. [DOI] [PubMed] [Google Scholar]
- Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller JZ, Epstein JA. Cardiac neural crest. Semin Cell Dev Biol. 2005;16:704–715. doi: 10.1016/j.semcdb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Strachan LR, Condic ML. Cranial neural crest recycle surface integrins in a substratum-dependent manner to promote rapid motility. J Cell Biol. 2004;167:545–554. doi: 10.1083/jcb.200405024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testaz S, Delannet M, Duband J. Adhesion and migration of avian neural crest cells on fibronectin require the cooperating activities of multiple integrins of the (beta)1 and (beta) 3 families. J CellSci. 1999;112(Pt 24):4715–4728. doi: 10.1242/jcs.112.24.4715. [DOI] [PubMed] [Google Scholar]
- Tirosh-Finkel L, Zeisel A, Brodt-Ivenshitz M, Shamai A, Yao Z, Seger R, Domany E, Tzahor E. BMP-mediated inhibition of FGF signaling promotes cardiomyocyte differentiation of anterior heart field progenitors. Development. 2010;137:2989–3000. doi: 10.1242/dev.051649. [DOI] [PubMed] [Google Scholar]
- Trainor P, Krumlauf R. Plasticity in mouse neural crest cells reveals a new patterning role for cranial mesoderm. Nat Cell Biol. 2000;2:96–102. doi: 10.1038/35000051. [DOI] [PubMed] [Google Scholar]
- Turner CJ, Badu-Nkansah K, Crowley D, van der Flier A, Hynes RO. Integrin-alpha5beta1 is not required for mural cell functions during development of blood vessels but is required for lymphatic-blood vessel separation and lymphovenous valve formation. Dev Biol. 2014;392:381–392. doi: 10.1016/j.ydbio.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, Evans SM. Pharyngeal mesoderm development during embryogenesis: implications for both heart and head myogenesis. Cardiovascular research. 2011;91:196–202. doi: 10.1093/cvr/cvr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Zang K, Reichardt LF. Focal adhesion kinase is required for neural crest cell morphogenesis during mouse cardiovascular development. J Clin Invest. 2009;119:2218–2230. doi: 10.1172/JCI38194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier A, Badu-Nkansah K, Whittaker CA, Crowley D, Bronson RT, Lacy-Hulbert A, Hynes RO. Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development. 2010;137:2439–2449. doi: 10.1242/dev.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Villegas SN, Barrios-Llerena ME, Pulina M, Hadjantonakis AK, Le Bihan T, Astrof S, Brickman JM. PI3K/Akt1 signalling specifies foregut precursors by generating regionalized extra-cellular matrix. eLife. 2013;2:e00806. doi: 10.7554/eLife.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurdak H, Ittner LM, Lang KS, Leveen P, Suter U, Fischer JA, Karlsson S, Born W, Sommer L. Inactivation of TGF{beta} signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev. 2005;19:530–535. doi: 10.1101/gad.317405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Francis R, Wei CJ, Linask KL, Lo CW. Connexin 43-mediated modulation of polarized cell movement and the directional migration of cardiac neural crest cells. Development. 2006;133:3629–3639. doi: 10.1242/dev.02543. [DOI] [PubMed] [Google Scholar]
- Yang JT, Bader BL, Kreidberg JA, Ullman-Cullere M, Trevithick JE, Hynes RO. Overlapping and independent functions of fibronectin receptor integrins in early mesodermal development. Dev Biol. 1999;215:264–277. doi: 10.1006/dbio.1999.9451. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Huynh T, Baldini A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development. 2006;133:3587–3595. doi: 10.1242/dev.02539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.