Abstract
Prematurity and neonatal growth restriction (GR) are risk factors for autism and attention deficit hyperactivity disorder (ADHD). Leptin production is suppressed during periods of undernutrition, and we have shown that isolated neonatal leptin deficiency leads to adult hyperactivity while neonatal leptin supplementation normalizes the brain morphology of GR mice. We hypothesized that neonatal leptin would prevent the development of GR-associated behavioral abnormalities. From postnatal day 4–14, C57BL/6 mice were randomized to daily injections of saline or leptin (80 ng/g), and GR was identified by a weanling weight below the tenth percentile. The behavioral phenotypes of GR and control mice were assessed beginning at 4 months. Within the tripartite chamber, GR mice had significantly impaired social interaction. Baseline escape times from the Barnes maze were faster for GR mice (65+/−6 sec vs 87+/−7 sec for controls, p<0.05), but GR mice exhibited regression in their escape times on days 2 and 3 (56% regressed vs 22% of control saline mice, p<0.05). Compared to controls, GR mice entered the open arms of the elevated plus maze more often and stayed there longer (72+/−10 sec vs 36+/−5 sec, p<0.01). Neonatal leptin supplementation normalized the behavior of GR mice across all behavioral assays. In conclusion, GR alters the social interactions, learning and activity of mice, and supplementation with the neurotrophic hormone leptin mitigates these effects. We speculate neonatal leptin deficiency may contribute to the adverse neurodevelopmental outcomes associated with postnatal growth restriction, and postnatal leptin therapy may be protective.
Keywords: leptin, growth restriction, developmental origins, behavior, ADHD, autism
1. Introduction
Leptin has well described roles in the regulation of adult body composition and metabolism. Classically, increased food intake leads to increased adipocyte leptin production, which in turn suppresses appetite and stimulates metabolism, completing a negative feedback cycle. Unfortunately, the fetus lacks control over their own nutritional intake during a critical developmental window in which leptin exerts important neurotrophic effects. While transplacental delivery and endogenous leptin production typically support perinatal brain development, this system fails in the presence of maternal-fetal undernutrition or premature delivery, and both intrauterine growth restricted and premature infants have critically low circulating leptin levels [1] [2] and [3].
With advances in healthcare facilitating cardiopulmonary support at earlier gestational ages and lower birth weights, the survival of low birth weight and preterm infants has improved significantly over the past 30 years [4]. As a consequence, there is a growing population that may be vulnerable to the long-term effects of preterm birth. Despite advances in neonatal nutrition including the early provision of protein supplementation, postnatal growth restriction (GR) develops in a majority of premature infants, and together, prematurity and neonatal GR increase the risk of neurodevelopmental impairment, autism and attention-deficit hyperactivity disorder (ADHD) [5], [6], [7], [8], [9], [10], [11], and [12].
Rodents are born with neurodevelopmental immaturity. The first two postnatal weeks of life of a mouse approximates the third trimester of brain development of humans. This correlation allows modeling of the effects of prematurity associated neonatal GR on the developing brain. Our previous studies have shown that neonatal GR mice experience cardiovascular and metabolic sequelae, reminiscent of the phenotypes described in premature and otherwise GR populations [13]. We have further shown that GR mice have alterations in brain morphology that are mitigated by leptin supplementation, but classic tests for learning, autism-like and ADHD-like behavior were not performed [13]. Our most recent investigations revealed that otherwise well-nourished mice with isolated neonatal leptin deficiency have reduced adult brain volumes and increased adult locomotor activity [25]. We hypothesized that neonatal GR alters adult behavior in mice, and that these behavioral disturbances can be prevented with neonatal leptin supplementation.
2. Methods
2.1 Animal Model
All animal procedures were approved by the University of Iowa Animal Care and Use Committee. Utilizing an established model of neonatal GR [13], C57BL/6J mice were bred from initial stock (Jackson Laboratories, Bar Harbor, ME). Pups with appropriate intrauterine growth (birth weight >10th percentile) were cross fostered into litters of 6 or 12 from day of life 1 to 21 to obtain control and GR mice respectively. GR pups were randomized to daily intraperitoneal injections of leptin (80 ng/g) or vehicle alone (10 ml/kg normal saline), while control mice received daily normal saline injections (10 ml/kg). The injections encompassed the phase of leptin-dependent neurodevelopment extending from postnatal day 4 to 14. We previously demonstrated this leptin dose normalizes circulating leptin levels and brain morphology in GR mice [13]. Upon weaning on day of life 21, neonatal GR was confirmed by a weight <10th percentile (<7.1 g in males, <6.75 g in females); mice from litters of 12 pups that exceeded this cut-off were excluded from further investigation. Behavior testing was performed beginning at 4 months. Overall, 194 out of 225 mice (86%) underwent a single behavioral test, and no mouse underwent all three behavioral tests.
2.2. Social Interaction
Social interaction was assessed using a tripartite chamber using previously described methods [14], [15], and [16]. The apparatus is constructed of Plexiglas with dimensions 40cm wide × 22cm high × 22cm deep with three identical size chambers connected by 5cm × 5cm doors. Each test mouse was placed in the center chamber without access to the lateral chambers for a 5 minute habituation period. Then, a stranger mouse of the same sex and strain as the test mouse was placed under a wire enclosure in one lateral chamber and an identical empty wire enclosure was placed in the opposite lateral chamber. The test mouse was then allowed to freely explore for 10 minutes. The amount of time spent in each chamber was measured, along with the amount of time spent interacting with (sniffing) the stranger mouse versus empty enclosure. As a screen for impaired social interaction, the percentage of sniffing time directed towards the stranger mouse was determined, and poor social interaction was identified whenever this value was more than 1 standard deviation below the colony mean. Videos were scored by three independent investigators who were blinded to group assignment.
2.3. Spatial Learning
Spatial learning was assessed by Barnes maze using established methods [17]. The apparatus is a circular platform of 125 cm diameter with 40 potential escape holes of 5 cm in diameter. The escape hatch is a black enclosure 8 cm × 6 cm × 20 cm in dimensions that attaches below the target escape hole. Colored shapes were placed on the four walls of the secluded test room to allow orientation to the environment. Test mice underwent 3 minute trials in triplicate on 5 consecutive days and the amount of time taken to find the escape hole was measured using video tracking software (ViewPoint Live Sciences, Inc. Montreal, Canada). If a mouse did not find the escape hole during a particular trial, the mouse was placed next to the escape hole and allowed to escape spontaneously; latency to escape was then assigned a maximum value of 3 minutes. Trials were performed at 30 minute intervals.
2.4. Anxiety/Fear Response
Anxiety and fear were assessed using an elevated plus maze apparatus using established methods [18]. The maze had dimensions of 35 cm long by 5 cm wide for the open and closed arms, 5 cm by 5 cm center platform, and 10 cm height of the closed arm walls. The entire apparatus was elevated 50 cm above the ground. Testing was performed in a darkened room with a red spectrum light the only light source. The mice underwent a testing trial of 5 minutes by being placed on the central platform of the plus maze, and allowed to explore all arms of the maze freely. The amount of time spent in the open and closed arms was recorded using video tracking software (ANY Maze version 4.98, Stoelting Co.).
2.5. Statistical Analysis
One way ANOVA was used for between-group comparisons. Fisher’s exact test was used to compare categorical variables. Analysis was performed using Sigma Plot 12.0. A p value of <0.05 was considered significant.
3. Results
3.1. Animal Model
Control mice came from 26 litters, GR mice from 19 litters and GR-leptin mice from 23 litters. Weanling weight was not altered by neonatal leptin administration (GR-saline: 6.17+/−0.08g, N=73, GR-leptin: 6.05+/−0.10, N=72, P=0.33). By definition, both groups of GR mice had weanling weights significantly below control mice (control: 9.43+/−0.13, N=80, P<0.001). Both male and female mice were included in each of the behavioral assays, and no sex-specific effects were observed.
3.2. Social Interaction
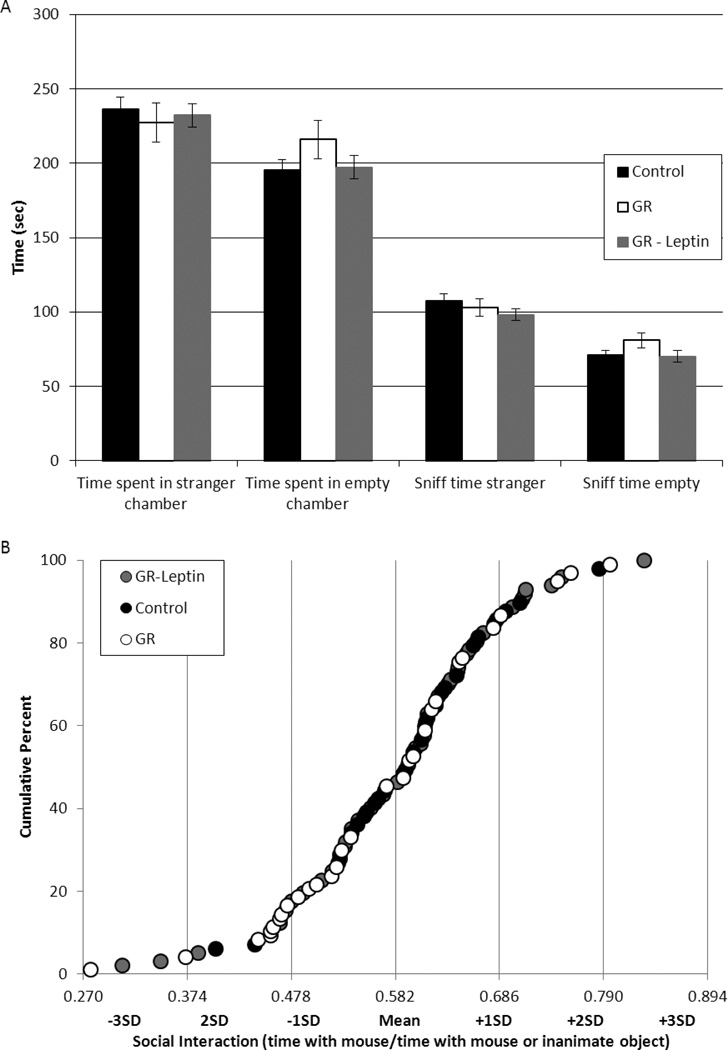
There were no significant between group differences in the number of times the test mice entered either side of the tripartite chamber (data not shown). Likewise, the total time spent in each lateral chamber and the overall interaction time (sniff time) was not significantly altered by neonatal GR (Figure 1A). For each test mouse, interest in social interaction with the stranger mouse was corrected for differences in general activity by dividing the time sniffing the stranger by the total time sniffing either the stranger or the empty enclosure. The resulting values were normally distributed with a mean of 58.2% and a standard deviation of 10.4% (Figure 1B). Overall, GR mice tended to spend less time interacting with the stranger mouse than controls (GR: 55+/−2%, control: 60+/−12%, P=0.08). Beyond this group-wide effect, the weanling weight of individual mice was directly related to social interaction (R=0.3, P<0.05). Finally, when mice are categorized based on social interaction times, a significantly greater proportion of GR-saline mice had values more than 1 SD below the mean (30% versus 7% of control-saline mice, P<0.05) (Figure 1C). This relative avoidance of the stranger mouse in preference for the inanimate object was partially normalized by neonatal leptin (Figure 1D).
Figure 1.
Control (black symbols, N=29), growth restricted (GR, white symbols, N=30) and GR-leptin mice (gray symbols, N=38) were placed in a tripartite chamber with open access to lateral chambers with or without a stranger mouse. There was no difference between groups in average time spent in either chamber, or in the amount of time spent sniffing the stranger mouse or the empty enclosure (A). Social interaction was quantified as the percent of time sniffing the stranger mouse out of the overall total time spent sniffing. The results were normally distributed with values from 47.8% to 68.6% falling within one standard deviation of the colony mean (B). While a majority of the control and GR-leptin mice fell within this range (delimited by the solid lines in panel C at 0.5 and 0.7), GR mice were more likely to have values more than one standard deviation below the mean (D, *p<0.05).
3.3. Spatial Learning
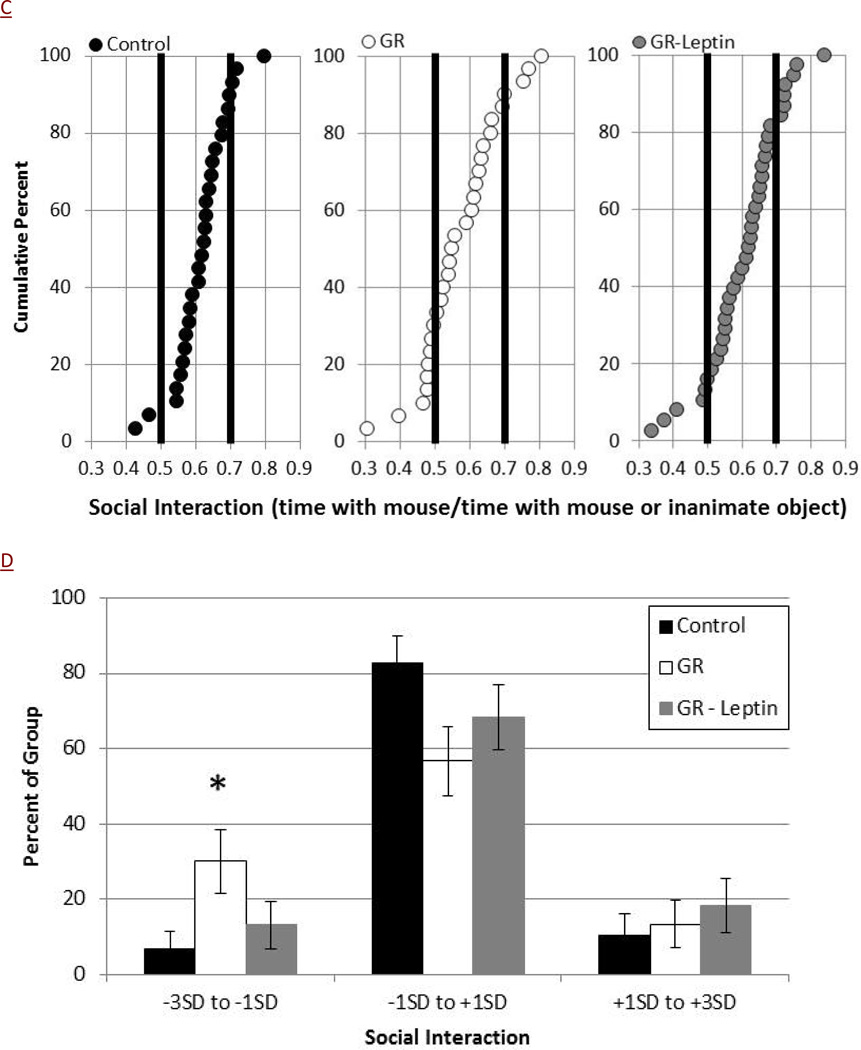
Consistent with heightened activity or anxiety, GR-saline mice had shortened baseline escape times from the Barnes maze on training day 1 compared to average growth controls (Figure 2A, 65+/−6 vs 87+/−7 sec, p<0.05). Compared to these baseline values, GR-saline mice had a regression in their escape times on days 2 and 3 with no improvement seen until training day 4, consistent with impaired spatial learning (Figure 2B). Among the GR-saline mice, 56% escaped slower on day 2 then on day 1, while only 22% of control-saline mice showed regression in their performance within the Barnes maze (P<0.05). Neonatal leptin supplementation significantly improved the escape times of GR mice on both days 2 and 3, and only 36% of GR-leptin mice had regression in their performance from days 1 to 2 (Figure 2B).
Figure 2.
Barnes maze was used to assess escape times for control (black symbols, N=32), GR (white symbols, N=25) and GR-leptin mice (gray symbol, N=25). GR mice found the escape hole faster on Day 1 (A, *p=0.02). Relative to baseline, GR mice did not improve their escape times on days 2 and 3 (#p<0.05 versus control) (B). Leptin supplementation normalized the abnormalities seen with GR mice.
3.4. Anxiety/Fear
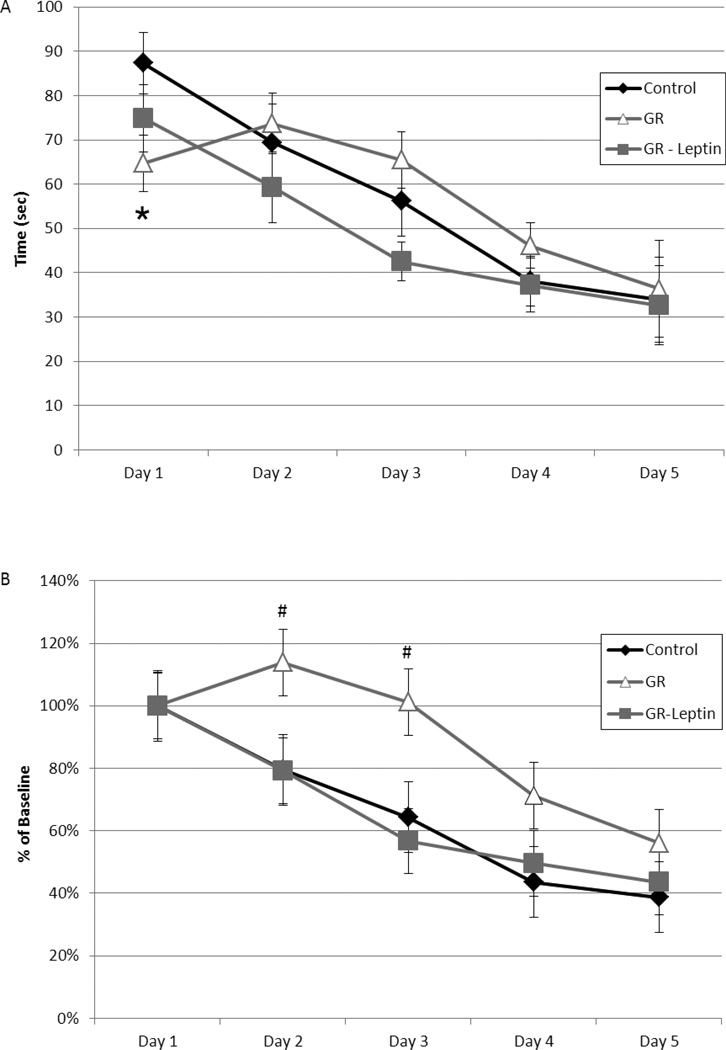
Compared to control mice, GR-saline mice had more entries of the open arms of the elevated plus maze (11.8 vs 7.9, p<0.01) and spent twice as much time there (Figure 3, 72+/−10 vs 36+/−5 sec, p<0.01). Compared to GR-saline mice, GR-leptin mice had a decrease in time spent exploring the open arms of the maze, closely mirroring the control mice. The amount of time spent in the closed arms was less for GR-saline compared to controls (138 sec vs 202 sec, p <0.01). This effect was also partially corrected by neonatal leptin supplementation (163 sec, p<0.05 vs GR-saline).
Figure 3.
Control (black bars, N=26), GR (white bars, N=27) and GR-leptin mice (gray bars, N=25) were placed in an elevated plus maze with alternating arms open or closed to ambient light. GR mice spent more time in the open arms and less time in the closed arms compared to control mice (*p<0.01). Leptin supplementation partially normalized these alterations (#p<0.05 vs GR).
4. Discussion
Prematurity carries an increased risk of mortality and lifelong morbidity. The increased risks of neurobehavioral complications of prematurity are well described [19]. Postnatal growth restriction has also been shown to have significant effects on neurodevelopmental outcomes [13], [6], and [20]. The additive effects of postnatal growth restriction on the developing premature brain can lead to significant neurologic changes that increase the risk for diagnoses including ADHD and Autism Spectrum Disorder. While acknowledging the inherent limitations of non-human animal models, we attempted to model the effects of prematurity-related neonatal growth restriction in mice, a species whose first two weeks of postnatal neurodevelopment approximate the third trimester of human neurodevelopment. Our study is the first to show, in a naturalistic murine model that takes advantage of the decrement in growth that naturally occurs as a consequence of increased litter size, neonatal GR elicits adult behavioral disturbances that can be readily and nearly completely ameliorated by neonatal leptin supplementation.
The propensity of GR mice towards decreased social interaction is consistent with clinical data showing premature infants are less likely to be involved in intimate peer relationships [21] and [22]. Notably, there was a general decrease in social interaction in GR mice, but there were also substantial within group differences with some GR mice meeting our criteria for impaired social interaction while others had above average social interactions. Because we utilized an isogenic strain, these within group differences are likely mediated either epigenetically or environmentally. All of our adult mice are co-housed with same-sex littermates, and it is possible the natural evolution of dominant and submissive relationships contributed to this finding; particularly if these relationships are influenced by neonatal GR. Future studies could utilize single-housed mice, although solitary confinement typically elicits significant behavioral disturbances, even in mice. It is scientifically reassuring that the phenotype of GR-saline and GR-leptin mice differed despite the matching of their environments in all ways other than the randomization to either saline or leptin administration.
The impairment in learning of GR mice, both collectively and individually, mirrors the increased incidence of learning difficulties seen in premature infants [23] and [24]. This learning impairment can have a significant impact on the ability of ex-preterm infants to attain lifetime career and personal goals [21]. The faster escape times on Day 1 of GR mice indicates they may have increased stress responses to their environment, and heightened stress may play a role in their subsequent learning impairment [13] and [25]. With all mice in our colony housed in the same living environment, the effects of different experiences during development on the ability to learn are not a factor in our results. Future studies could examine the effects of different living environments on learning in GR mice to see if placing the mice in a more stimulating environment may decrease the learning impairment they exhibit as adults.
GR mice demonstrated increased exploration of the open arms of the elevated plus maze, consistent with a lack of fear or impulse control. Interestingly, a similar phenotype has been reported in the offspring of rats with experimental hyperleptinemia during the first 10 days of lactation [26]. It is plausible those offspring also suffered the consequence of neonatal malnutrition given the diametrically opposed results reported when the leptin administration was provided to the pup rather than the lactating dam [27]. Inappropriate control of impulsive behavior, as seen in ADHD, can have a significant impact on an individual’s life, from both a social and safety aspect [28]. Further investigation into this abnormal behavior could examine if there is abnormal play behaviors in GR mice, or if GR mice would modulate their impulsive behavior with repeated exposures to fear inducing environments.
Across the three classic behavioral assays we utilized, neonatal leptin supplementation normalized the phenotypes of GR mice. Leptin has been shown to be an important mediator of appetite/satiety, and also plays an important neurotrophic role during development [13]. Deficiency of leptin during periods of fasting or undernutrition has been demonstrated, but the full effects of this deficiency on the developing brain have not been well described. Investigations by Erkonen [13] and others have suggested that there are significant morphologic differences in the brains of leptin deficient neonatal mice, and that supplementation of leptin in the neonatal period normalizes these changes. This work demonstrates that neonatal GR exerts significant effects on the developing mouse brain that leads to abnormal behavioral phenotypes as adults. It also shows that neonatal leptin supplementation normalizes abnormal learning and fear response in these GR mice suggesting that it may have a protective effect against the behavioral changes caused by neonatal GR.
While neonatal leptin replacement may have neuroprotective effects, several studies have shown that treatment of average growth pups with neonatal leptin can increase the incidence of obesity and lead to central leptin resistance in adulthood [29], [30], [31], and [32]. This phenomenon may be due to exposure to supraphysiologic levels of leptin during an important period of neurodevelopment. Due to this effect, we chose to focus our studies on the effects of leptin supplementation on leptin deficient mice to target the population with the most potential gain while minimizing the exposure to those with little or no expected benefit.
5. Conclusions
Postnatal GR leads to behavioral changes in adult mice with characteristics reminiscent of some of the features of ADHD and Autism, including lack of impulse control, poor learning and restricted social interaction. Supplementation of GR mice with leptin during the neonatal period helps ameliorate some of the behavioral changes seen in this population. While leptin supplementation cannot be seen as the “magic bullet” that will prevent the increased risk of behavioral problems in premature neonates, further investigations are needed to better elucidate the mechanistic link between leptin deficiency and the increased incidence of behavioral changes seen in GR neonates. Clearly, further research is needed to identify environmental enrichments, including nutritional supplements, which could be added to our armamentarium as we seek to optimize the long-term health of premature and otherwise GR individuals.
Acknowledgements
This study was funded the National Institutes of Health (HD050359) and the Children’s Miracle Network
Abbreviations
- GR
growth restriction
- ADHD
attention deficit hyperactivity disorder
Footnotes
Competing Interests
The authors have no conflicts of interest.
References
- 1.Valuniene M, Verkauskiene R, Boguszewski M, Dahlgren J, Lasiene D, Lasas L, et al. Leptin levels at birth and in early postnatal life in small- and appropriate-for-gestational-age infants. Medicina (Kaunas) 2007;43:784–791. [PubMed] [Google Scholar]
- 2.Udagawa J, Hatta T, Hashimoto R, Otani H. Roles of leptin in prenatal and perinatal brain development. Congenit Anom. 2007;47:77–83. doi: 10.1111/j.1741-4520.2007.00150.x. [DOI] [PubMed] [Google Scholar]
- 3.Bouret S. Neurodevelopmental actions of leptin. Brain Res. 2010;1351:2–9. doi: 10.1016/j.brainres.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle LW, Roberts G, Anderson PJ. Changing long-term outcomes for infants 500–999 g birth weight in Victoria 1979–2005. Arch Dis Child Fetal Neonatal Ed. 2011;96:F443–F447. doi: 10.1136/adc.2010.200576. [DOI] [PubMed] [Google Scholar]
- 5.Kuzniewicz M, Wi S, Qian Y, Walsh E, Armstrong MA, Croen L. Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J Pediatr. 2014;164:20–25. doi: 10.1016/j.jpeds.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 6.Wong H, Huertas-Ceballos A, Cowan F, Modi N. Evaluation of early childhood social-communication difficulties in children born preterm using the quantitative checklist for autism in toddlers. J Pediatr. 2014;164:26–33. doi: 10.1016/j.jpeds.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Keir A, McPhee A, Wilkinson D. Beyond the borderline: outcomes for inborn infants born at ≤ 500 grams. J Paediatr Child Health. 2014;50:146–152. doi: 10.1111/jpc.12414. [DOI] [PubMed] [Google Scholar]
- 8.Belfort M, Rifas-Shipman S, Sullivan T, Collins C, McPhee A, Ryan P, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011;128:e899–e906. doi: 10.1542/peds.2011-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenkranz R, Dusick A, Vohr B, Wright L, Wrage L, Poole W. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 10.Jou M, Lonnerdal B, Griffin I. Effects of early postnatal growth restriction and subsequent catch-up growth on body composition, insulin sensitivity, and behavior in neonatal rats. Pediatr Res. 2013;73(5):596–601. doi: 10.1038/pr.2013.27. [DOI] [PubMed] [Google Scholar]
- 11.Latal-Hajnal B, von Siebenthal K, Kovari H, Bucher H, Largo R. Postnatal growth in VLBW infants: significant association with neurodevelopmental outcome. J Pediatr. 2003;143:163–170. doi: 10.1067/S0022-3476(03)00243-9. [DOI] [PubMed] [Google Scholar]
- 12.Lampi KM, Lehtonen L, Tran PL, Suominen A, Lehti V, Banerjee PN, et al. Risk of autism spectrum disorders in low birth weight and small for gestational age infants. J Pediatr. 2012;161:830–836. doi: 10.1016/j.jpeds.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erkonen G, Hermann G, Miller R, Thedens D, Nopoulos P, Wemmie J, et al. Neonatal leptin administration alters regional brain volumes and blocks neonatal growth restriction-induced behavioral and cardiovascular dysfunction in male mice. Pediatr Res. 2011;69:406–412. doi: 10.1203/PDR.0b013e3182110c7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawley J. Designing mouse behavioral tasks relevant to autistic-like behaviors. MRDD Res Rev. 2004;10:248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 15.Crawley J. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverman J, Yang M, Lord C, Crawley J. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrison F, Reiserer R, Tomarken A, McDonald M. Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem. 2006;13:809–819. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brielmaier J, Matteson P, Silverman J, Senereth J, Kelly S, Genestine M, et al. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS ONE. 7(7):e40914:1–e40914:27. doi: 10.1371/journal.pone.0040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saigal S, Doyle L. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 20.Heinonen K, Rajkkonen K, Pesonen AK, Andersson S, Kajantie E, Eriksson JG, et al. Trajectories of growth and symptoms of attention-deficit/hyperactivity disorder in children: a longitudinal study. BMC Pediatr. 2011;11:84. doi: 10.1186/1471-2431-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darlow B, Horwood J, Pere-Bracken H, Woodward L. Psychosocial outcomes of young adults born very low birth weight. Pediatrics. 2013;132:e1521–e1528. doi: 10.1542/peds.2013-2024. [DOI] [PubMed] [Google Scholar]
- 22.Lund L, Vik T, Lydersen S, Lohaugen G, Skranes J, Brubakk A, et al. Mental health, quality of life and social relations in young adults born with low birth weight. Health Qual Life Outcomes. 2012;10:146. doi: 10.1186/1477-7525-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aarnoudse-Moens C, Weisglas-Kuperus N, van Goudoever J, Oosterlaan J. Meta-Analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- 24.Ni T, Huang C, Guo N. Executive function deficit in preschool children born very low birth weight with normal early development. Early Hum Dev. 2011;87:137–141. doi: 10.1016/j.earlhumdev.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Dexter B, Rahmouni K, Cushman T, Hermann G, Ni C, Nopoulos P, et al. Neonatal leptin deficiency reduces frontal cortex volumes and programs adult hyperactivity in mice. Behav Brain Res. 2014;263:115–121. doi: 10.1016/j.bbr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fraga-Marquez MC, Moura EG, Silva JO, Claudio-Neto S, Pereira-Toste F, Passos MC, et al. Effects of maternal hyperleptinaemia during lactation on short-term memory/learning, anxiety-like and novelty-seeking behavioral traits of adult male rats. Behav Brain Res. 2010;206:147–150. doi: 10.1016/j.bbr.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 27.Fraga-Marquez MC, Moura EG, Claudio-Neto S, Trevenzoli IH, Toste FP, Passos MC, et al. Neonatal hyperleptinaemia programmes anxiety-like and novelty seeking behaviours but not memory/learning in adult rats. Horm Behav. 2009;55:272–279. doi: 10.1016/j.yhbeh.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Bhutta A, Cleves M, Casey P, Cradock M, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm. JAMA. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 29.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, et al. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 30.Vickers MH, Gluckman PD, Coveny AH, Hofman P, Cutfield WS, Gertler A, et al. The effect of neonatal leptin treatment on postnatal weight gain in male rats is dependent on maternal nutritional status during pregnancy. Endocrinology. 2008;149:1906–1913. doi: 10.1210/en.2007-0981. [DOI] [PubMed] [Google Scholar]
- 31.Yura S, Itoh H, Sagawa N, Yamamoto H, Masuzaki H, Nakao K, et al. Role of premature leptin surge in obesity resulting from intrauterine undernutrition. Cell Metab. 2005;1:371–378. doi: 10.1016/j.cmet.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Toste FP, de Moura EG, Lisboa PC, Fagundes AT, de Oliveira E, Passos MC. Neonatal leptin treatment programmes leptin hypothalamic resistance and intermediary metabolic parameters in adult rats. Br J Nutr. 2006;95:830–837. doi: 10.1079/bjn20061726. [DOI] [PubMed] [Google Scholar]