Abstract
Oral Submucous Fibrosis (OSF) is a chronic disorder characterized by fibrosis of the mucosa lining the upper digestive tract involving the oral cavity, oro- and hypopharynx and the upper third of the oesophagus. The alkaloids from areca nut are the most important chemical constituents biologically, in producing this lesion. These chemicals appear to interfere with the molecular processes of deposition and/or degradation of extracellular matrix molecules such as collagen. Increased collagen synthesis or reduced collagen degradation have been considered as a possible mechanism in the development of the disease. Increased and continuous deposition of extracellular matrix may also take place as a result of disruption of the equilibrium between matrix metalloproteinases (MMPs) and tissue inhibitors of matrix metalloproteinases (TIMP). Arecoline a product of areca nut was found to elevate Cystatin C mRNA (CST3) and protein expression in a dose-dependent manner. Cystatin C expression was significantly higher in OSF specimens and expressed mainly by fibroblasts, endothelial cells, and inflammatory cells. Cross-links between the molecules are essential for the tensile strength of collagen fibres. These areas are resistant to attack by collagenases but can be attacked by a number of other serine and cysteine proteinases. CST3 encoding a cysteine proteinase inhibitor might contribute to the stabilization of collagen fibrils in OSMF. Treatment directed against Cystatin C may serve as a novel treatment for submucous fibrosis and also in preventing its transformation into malignancy.
Keywords: Oral submucous fibrosis, Arecoline in areca nut, TGF β, Cystatin C
1. Introduction
Oral submucous fibrosis was first described by Schwartz in 1952 as a fibrosing condition that occurred in five Indian women in Kenya and he called it as Atrophica idiopathic (tropica) mucosa oris'. Oral submucous fibrosis is well established in medical literature since the time of Sushrutha, a renowned Indian physician, who lived in 2500–3000 BC and described a condition resembling OSF which he referred to as ‘Vidari’. Similar conditions were found in betel nut (BN) chewers in early texts dating back to 1908. The submucous fibrosis is characterized by juxta-epithelial inflammatory reaction followed by chronic change in the fibro-elasticity of the lamina propria and epithelial atrophy. This resulted in burning sensation in the oral cavity, blanching, and stiffening of oral mucosa and oropharynx, resulting in restricted mouth opening, limited food consumption which further resulted in difficulty in maintaining oral health, and impairing the ability to speak. The signs and symptoms depend on the evolution of lesions and number of affected sites. The malignant transformation rate of OSF has been reported to be around 7.6% over a 17-year period.1
2. Areca nut or betel nut
The constituents of BN include crude fiber, carbohydrates, fats, polyphenols, alkaloids, tannins, proteins and water. Trace amounts of fluorine, sapogenins (glycosidic derivatives of steroids and triterpenoids) and free amino acids have also been reported in some forms. The active components of dry and wet forms of BN, which produce BN associated effects, are primarily the alkaloids, polyphenols, and tannins. Prolonged as well as excessive usage of BN has been reported to exert significantly adverse effects on human health. There is enough evidence to suggest that BN products, even without tobacco, are associated with increased risk for the development of oral malignancy, such as oral squamous cell carcinoma (OSCC). A vast majority of BN users show pre-cancerous clinical conditions, such as oral leukoplakia (OL) as well as its variant, oral erythroplakia or oral submucous fibrosis (OSF) among others. The risk is reported to be higher for paan masala chewers. The popularity of BN mixtures like paan masala, gutkha and mawa has resulted in an epidemic of OSF, particularly among young individuals in India and South east Asia.1
Paan masala is basically a preparation of areca nut, catechu, cardamom, lime and a number of natural and artificial perfuming and flavoring materials. Gutkha is a variant of paan masala, in which in addition to these ingredients flavored chewing tobacco is added. Both products are often sweetened to enhance the taste. Paan masala chewing was found to have the highest risk for developing OSF.2
Among the chemical constituents, alkaloids from areca nut are the most important, biologically, whilst tannin may have a synergistic role. These chemicals appear to interfere with the molecular processes of deposition and/or degradation of extracellular matrix (ECM) molecules such as collagen. In vitro studies on human fibroblasts using areca extracts or chemically purified arecoline support the theory of fibroblastic proliferation and increased collagen formation that is also demonstrable histologically in human OSF tissues.3
Etiology of submucous fibrosis was found to be multifactorial. Consumption of chillies, nutritional deficiency, tobacco, genetic susceptibility, immunologic basis, and areca nut chewing were all said to play a role in formation of OSF. It is apparent that fibrosis and hyalinization of connective tissue account for most of the clinical features encountered in this condition. Moreover, substantial amount of research on elucidating the etiology and pathogenesis appear to have been focused on changes in the extracellular matrix. It is logical to hypothesize that the increased collagen synthesis or reduced collagen degradation as possible mechanisms in the development of the disease. There are numerous biological pathways involved in the above processes and, it is likely that the normal regulatory mechanisms are either down-regulated or upregulated at different stages of the disease. Not a single case of OSF was found without any chewing habits in a study conducted by Shah et al1 The involvement of HLA and genetic predisposition has been reported, but specific haplotypes have not been determined. Nutritional deficiencies may not play a primary role but it could synergize the symptomatology by contributing to epithelial atrophy.4
3. Molecular basis for the changes caused by areca nut
From a clinico-pathologic point of view, fibrosis may be considered as a somewhat irreversible state of tissue alteration, during which resolution of the healing process fails to occur. Increasingly, it has become appreciated that certain of these actions of ECM derive from its ability to sequester and modulate the activity of specific growth factors (Nathan and Sporn, 1991). Of all of the growth factors, none has been found to have the diversity of effects on ECM ascribed to transforming growth factor-β (TGF-β). This peptide plays a critical role not only in synthesis and degradation of ECM but also in response of cells to ECM; moreover, specific components of the ECM, in turn, can both deliver TGF-β and regulate its activity. TGF-β was also produced during wound healing.2 During tumorigenesis, however, the prevailing model suggests a process whereby pre-cancerous epithelial cells acquire multiple genetic mutations and the associated stroma becomes “activated” commonly expressing myofibroblastic markers and hence the reduced extensibility of the oral mucosa. The characteristics of an activated carcinoma-associated fibroblast are not completely understood. Such cells are presumed to express α smooth muscle actin, ECM proteins, and growth factors that act in an autocrine and paracrine fashion to potentiate and support the survival of a tumor.5 In addition to anti-inflammatory properties, TGF-β promotes fibrosis as part of altered tissue repair. For example, in pulmonary fibrosis, TGF-β is found in the lung and the elevation in levels of TGF-β correlates with the extent of fibrosis. Thus, defining the specific mechanisms regulating the production and activation of TGF-β may have therapeutic opportunities to help patients with fibrotic diseases. It is held that the hypersensitivity to local irritants results in persistent mucosal inflammation which acts as the initiating factor for a protracted and defective inflammatory-reparative response, culminating in fibrotic healing. Thus understanding the molecular regulation of TGF-β activation and recognition may provide opportunity to intercede on this process, being a significant mediator of tissue repair. TGF-β signaling pathway has been considered both as a tumor suppressor pathway and a promoter of tumor progression and invasion.5
Growth factors like TGF-α, INF-α are also stimulated and over a period of time, due to persistent areca nut chewing habit, chronic inflammation sets in at the site. Initial irritation leads to further atrophy and ulceration of the mucosa. Cytokines like interlukin-6 (IL-6), tumor necrosis factor (TNF), interferon α– increases the collagen production and decreases the collagen degradation.6
4. Collagen architecture
Collagen is composed of a triple helix, which generally consists of two identical chains (α1) and an additional chain that differs slightly in its chemical composition (α2).7 A reduced degradation of the α1 (1) collagen trimer synthesized by OSF fibroblasts may induce the alteration of the ratio of α1 (1):α2 (1) chains due to reduced degradation of the α1 (1) collagen trimer synthesized by OSF fibroblasts.4
Collagen molecules assemble into fibres or fibrils with a relatively disordered side-to-side packing of molecules but a regular axial structure. Molecules are staggered length-wise such that there is a characteristic axial repeat every 67 nm. In some tissues these fibres can grow up to nearly 1 mm wide and of unknown length, although commonly they are 10–100 um across. In conjunction with other matrix components they form all the body's supporting tissues. Like ropes, collagen fibres are strong in tension but cannot sustain compression or bending. These tissues can be considered as fibre-composite materials in which the collagen fibres provide reinforcing to a weak gel-like matrix. Cross-links between the molecules are essential for the tensile strength of collagen fibres. Initial formation of reducible cross-links, largely based on lysine, is followed by maturation of non-reducible pyridinium and pyrrolic cross-links. In bone, the addition of a mineral phase, a form of hydroxyapatite, further stiffens the tissue. Because these tissues are fibre-composites it is no longer possible to attribute tensile or compressive properties to a specific component, it is the combination and interactions between them that determine the properties.8 COLase (MMP-1 or fibroblast-type collagenase) is a member of the MMP family and the principal human enzyme that cleaves native fibrillar collagen. In addition to the regulation of collagen biosynthesis and degradation, collagen cross-linking genes also need to be precisely regulated to maintain tissue integrity. The activity of LYOXase, an extracellular enzyme stabilizing the collagen fibrillar array by covalent cross-links, was found to increase in fibroblasts cultured from OSF patient. Collagen fibers form three-dimensional scaffolding by combining cross-linked collagen molecules with other extracellular matrix components. The terminal regions of each collagen molecule consist of terminal peptides, which contain the sites of intra- and intermolecular cross-links. These areas are resistant to attack by collagenases but can be attacked by a number of other serine and cysteine proteinases. CST3 (Cystatin C) encoding a cysteine proteinase inhibitor might contribute to the stabilization of collagen fibrils. Because COLase, CST3, and LYOXase have been well documented to be involved in the collagen biosynthesis and degradation, they are hypothesized to contribute to the pathogenesis of OSF by increasing collagen synthesis and reducing collagen degradation.9 Cystatin C, in turn, inhibited the lysosomal cysteine proteases like Cathepsin B and H, resulting in decreased degradation of collagen. Polyphenols of BN, such as flavonoid, catechin and tannins cause collagen fibers to crosslink, (Figs. 1 and 2) making them less susceptible to collagenase degradation. This results in increased fibrosis due to decreased collagen breakdown.1
Fig. 1.
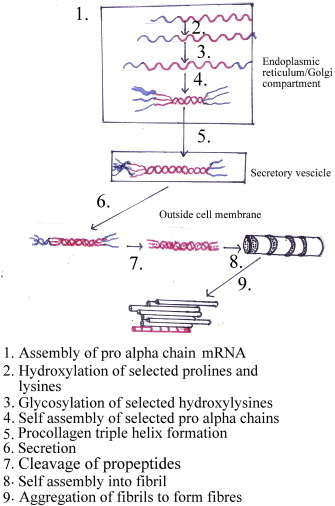
Intracellular and extracellular events in the formation of a collagen fibril. Collagen fiber formation.10
Fig. 2.

Showing the collagen fiber cross-links.11
5. Cystatin C
CST3 or Cystatin C is a 13 kDa non-glycosylated basic protein belonging to cystatin family. It is consistently and dramatically upregulated in a variety of fibrotic diseases12 including the gingival hyperplasia following cyclosporine A therapy.13
In humans, all cells with a nucleus cell core containing the DNA produce Cystatin C as a chain of 120 amino acids,14 and has a molecular mass of 13,343 Da.15 It is found in virtually all tissues and body fluids. It is a potent inhibitor of lysosomal proteinases (enzymes from a special subunit of the cell that breakdown proteins) and probably one of the most important extracellular inhibitors of cysteine proteases. Cystatin C belongs to the type 2 Cystatin gene family. The cystatin superfamily encompasses proteins that contain multiple Cystatin-like sequences. Some of the members are active cysteine protease inhibitors, while others have lost or perhaps never acquired this inhibitory activity. There are three inhibitory families in the superfamily, including the type 1 Cystatins (stefins A, B15), type 2 Cystatins (Cystatins C, F and E/M15) and the kininogens. The type 2 Cystatin proteins are a class of cysteine proteinase inhibitors found in a variety of human fluids and secretions, where they appear to provide protective functions. The Cystatin locus on the short arm of chromosome 20 contains the majority of the type 2 Cystatin genes and pseudogenes. It encodes the most abundant extracellular inhibitor of cysteine proteases. It is found in high concentrations in biological fluids and is expressed in virtually all organs of the body (CST3 is a housekeeping gene). The highest levels are found in semen, followed by breast milk, tears and saliva.16
Among type II Cystatins, the most prominent in immune cells are Cystatins C and F, the former being the most abundant human cystatin. Cystatin C was shown to be implicated in Alzheimer's disease (AD). It co-deposits with amyloid-beta (Aβ) in amyloid plaques of AD patients. Thus, Cystatin C could protect the brain from amyloid-induced toxicity and may have therapeutic implications for AD but reducing cystatin production can enhance collagen degradation in oral submucous fibrosis. Their concentrations in saliva of subjects with periodontitis also were found to be significantly increased. Potent inhibition of cysteine proteases by subtypes from parasitic organisms clearly indicates that salivary Cystatins help to control the oral microorganisms rather than prevent periodontal diseases. Given the high concentrations of Cystatins in saliva and tears it is likely to have a more specialized role. Recently, it has been studied for its role in predicting new-onset or deteriorating cardiovascular disease. It has been reported that transforming growth factor β is a potent inducer of Cystatin C in human vascular smooth muscle cells.
Type II Cystatins, at least Cystatins C and E/M are generally down-regulated in tumors. Although their role remains protective, their lower levels could allow a surplus of harmful tumor associated proteolytic activity. Outside the cells, higher levels of type II Cystatins may impair extracellular activity of cysteine proteases, associated directly or indirectly with the degradation of ECM and resulting in tumor cell invasion and metastasis. However, higher levels of Cystatins in body fluids have been associated with poor prognosis in cancer patients supporting their role in regulation of proteases involved in the regression of tumors. In melanoma and colorectal cancer, increased extracellular levels of Cystatin C as well as stefins correlated significantly with high risk of adverse outcome in cancer patients. Cathepsin B/cystatin C complex was also found to be less abundant in sera of patients with tumors suggesting an imbalance between the enzyme and its inhibitor in cancer patients.
Type II Cystatins are involved also in processes resulting in tumor regression such as anti-tumor immune response, apoptosis, and cell migration and seeding. In particular, the role of cysteine proteases is very important for proper maturation of antigen presenting cells, antigen processing and the presentation to T cells; therefore, the enhanced inhibition may affect the activation of naive T cells by tumor associated antigens and impair T cell dependent anti-tumor immune response, known to be effective for eradication of tumor cells. Higher levels of Cystatins may affect also the innate immunity; increased levels of Cystatin F inactivate cathepsin C and thus impair activation of granzymes and cytotoxicity of NK cells. Thus, besides the concentration, cell and tissue localization of Cystatins could make a critical switch between harmless and harmful and their application as anti-cancer agent has to be considered with caution and should be directed specifically to cysteine proteases which promote specific stage of tumor progression. It was found that CST C physically interacts with TGF-β receptor II, which antagonizes TGF-β binding to the cell surface receptors of normal and cancer cells thereby abrogating the binding of TGF-β.15
As tumors progress towards the metastatic end stage, the levels of the Cystatins in both the cytosol and extracellular spaces are drastically reduced. In addition, studies have demonstrated a direct correlation between high Cystatin C levels and improved tumor prognosis. Studies by Sokol et al identified Cystatin C as a novel antagonist of TGF-β signaling. It is known that TGF-β has growth suppressing properties, particularly in normal epithelial cells, but that during tumor progression the tumor suppressing properties of TGF-β is frequently subverted, and it becomes a powerful progression factor for the transformed cells. Therefore, depending on the particular circumstances, Cystatin family members may provide both favorable and unfavorable microenvironments for tumor growth. Once activated, several proteinases including matrix metalloproteinases have the capacity for autolytic inactivation when they are in their active state. Purified MMP-2 and MMP-9 in their active state are rapidly inactivated within hours unless they are in the presence of any of the Cystatin family members or TIMPs.17 Cystatin C or Cystatin 3 (formerly gamma trace, post-gamma-globulin or neuroendocrine basic polypeptide), a protein encoded by the CST3 gene, is mainly used as a biomarker of kidney function.18
6. Conclusion
In summary, the available literature indicates that the main aetiological factors for OSF are the constituents of areca nut, mainly arecoline, whilst tannin may have a synergistic role. These chemicals appear to interfere with the molecular processes of deposition and/or degradation of extracellular matrix molecules such as collagen, causing imbalance in the normal process. The most likely events that take place with regards to the above imbalance may be reduced phagocytosis of collagen by fibroblasts, up or down regulation of key enzymes such as lysyl oxidase, matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases. The process may also be influenced by increased secretion of inflammatory cytokines, like TGF β and hence Cystatin C, growth factors and decreased production of anti-fibrotic cytokines. Although the above mechanisms may explain the induction, maintenance and progression of fibrosis in OSF, further research is required in order to identify the mechanism leading to carcinogenesis in this fibrotic oral mucosa. There appears to be increased levels of Cystatin C in OSMF and reduced levels as it transforms into malignancy. The individual mechanisms operating at various stages of the disease – initial, intermediate and advanced – need further study in order to propose appropriate therapeutic interventions. It is our hope that in future more proteins with Cystatin-like domains in OSF will be identified and their physiological roles described so that new drugs can be instituted against them.
Conflicts of interest
There is no conflict of interest with regard to the above written article.
Footnotes
Cystatin C expression was significantly higher in oral submucous fibrosis specimens and expressed mainly by fibroblasts, endothelial cells, and inflammatory cells.
References
- 1.Sharan R.N., Mehrotra R., Choudhury Y., Asotra K. Association of betel nut with carcinogenesis: revisit with a clinical perspective. PLoS ONE. 2012;7(8):e42759. doi: 10.1371/journal.pone.0042759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair Urmila, Bartsch Helmut, Nair Jagadeesan. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and paan masala: a review of agents and causative mechanisms. Mutagenesis. 2004;19(4):251–262. doi: 10.1093/mutage/geh036. [DOI] [PubMed] [Google Scholar]
- 3.Tilakaratne W.M., Klinikowski M.F., Saku T., Peters T.J., Warnakulasuriya S. Oral submucous fibrosis: review on aetiology and pathogenesis. Oral Oncol. 2006 Jul;42(6):561–568. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Dyavanagoudar S.N. Oral submucouis fibrosis: review on etiopathogenesis. J Cancer Sci Ther. 2009;1:72–77. [Google Scholar]
- 5.Rajendran R., Harish R.K., Sukumaran Anil. Transforming growth factor-β-1 polymorphisms are infrequent but exist at selected loci in oral submucous fibrosis. Indian J Dent Res. 2010;21(3):413–419. doi: 10.4103/0970-9290.70815. [DOI] [PubMed] [Google Scholar]
- 6.Yadav S., Verma A., Sachdeva A., Virdi M. Etiopathogenesis and management of oral submucous fibrosis. Internet J Bioeng. 2010;5(1) [Google Scholar]
- 7.Szpak Paul. Fish bone chemistry and ultrastructure: implications for taphonomy and stable isotope analysis. J Archaeol Sci. 2011 July;38(12):3358–3372. [Google Scholar]
- 8.Nikolaeva T.I., Tiktopulo E.I., Il’yasova E.N., Kuznetsova S.M. Collagen type 1 fibril packing in vivo and in vitro. Biophysics. 2007;52(5):489–497. [Google Scholar]
- 9.Chung-Jung Chiu, Min-Lee Chang, Chun-Pin Chiang. Interaction of collagen-related genes and susceptibility to betel quid-induced oral submucous fibrosis. Cancer Epidemiol Biomarkers Prev. July 2002;11:646. [PubMed] [Google Scholar]
- 10.Alberts Bruce, Johnson Alexander. 4th ed. Garland Science; New York: 2002. Molecular Biology of the Cell. [Google Scholar]
- 11.Li J., Liao X.P., Zhang Q.X., Shi B. Adsorption and separation of proteins by collagen fiber adsorbent. J Chromatogr B. June 2013;928(1):131–138. doi: 10.1016/j.jchromb.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Chung-Hung T., Shun-Fa Y., Yu-Chao C. The upregulation of cystatin C in oral submucous fibrosis. Oral Oncol. 2007 Aug;43(7):680–685. doi: 10.1016/j.oraloncology.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Tsai C.H., Yang S.F. The upregulation of cystatin C in human gingival fibroblasts stimulated with cyclosporine A. J Periodontal Res. 2009 Aug;44(4):459–464. doi: 10.1111/j.1600-0765.2008.01147.x. [DOI] [PubMed] [Google Scholar]
- 14.Grzonka Zbigniew, Jankowska Elżbieta, Franciszek Kasprzykowski. Structural studies of cysteine proteases and their inhibitors. Acta Biochim Pol. 2001;48(1):1–20. [PubMed] [Google Scholar]
- 15.Magister Špela, Kos Janko. Cystatins in immune system. J Cancer. 2013;4(1):45–56. doi: 10.7150/jca.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szymańska Aneta, Jankowska Elżbieta. Influence of point mutations on the stability, dimerization, and oligomerization of human cystatin C and its L68Q variant. Front Mol Neurosci. 2012;5:82. doi: 10.3389/fnmol.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ochieng Josiah, Chaudhuri Gautam. Cystatin superfamily. J Health Care Poor Undeserved. 2010 Feb;21(1):51–70. doi: 10.1353/hpu.0.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dharnidharka V.R., Kwon C., Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002 Aug;40(2):221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]


