Abstract
In the United States, the economically disadvantaged and some ethnic minorities are often exposed to chronic psychosocial stressors and disproportionately affected by asthma. Current evidence suggests a causal association between chronic psychosocial stress and asthma or asthma morbidity. Recent findings suggest potential mechanisms underlying this association, including changes in the methylation and expression of genes that regulate behavioral, autonomic, neuroendocrine, and immunologic responses to stress. There is also evidence suggesting the existence of susceptibility genes that predispose chronically stressed youth to both post-traumatic stress disorder and asthma. In this review, we critically examine published evidence and suggest future directions for research in this field.
Keywords: Asthma, psychosocial stress, immune system, neuroendocrine system, genetics
Introduction
Asthma is a major public health problem in the United States (U.S.), where ~25.7 million children and adults are currently living with asthma1. In this country, members of certain ethnic minority groups (e.g. Puerto Ricans and African Americans) and the economically disadvantaged share a disproportionate burden of the “asthma epidemic”2.
In the U.S., ethnic minorities and the economically disadvantaged are disproportionately exposed to chronic psychosocial stressors (e.g. poverty, discrimination and violence)3. A growing body of literature supports a causal link between exposure to these stressors at the individual or community level and asthma or morbidity from asthma in children and adults (recently reviewed by Yonas et al)4. For example, physical or sexual abuse during childhood, a major stressor, has been associated with asthma or asthma morbidity in Puerto Rican school-aged children5, as well as with adult-onset asthma in African American women6. Moreover, a birth cohort study of 145 children with maternal history of asthma found that parental difficulties in early post-natal life (at age 3 months) were associated with asthma at ages 6 to 8 years7. In another birth cohort study including 708 children in Boston, prenatal exposure to community violence was associated with recurrent wheeze at age 2 years (a risk marker for asthma)8. Current evidence also suggests that the relation between stress and asthma is complex and partially mediated and modified by environmental exposures (e.g. outdoor air pollution8, cigarette smoking9), adherence with treatment, and coping mechanisms (e.g. shift-and-persist strategies10, family support).
Yet on top of these factors, stress is likely to affect the onset and course of asthma by directly acting on pathogenic mechanisms in the airways11,12. Although these pathways have yet to be fully elucidated, preliminary evidence suggests a role for stress in modulating lung development, neuroendocrine and autonomic nervous system responses, and the immune system4,13. Decades of research show that stressors, when perceived as threatening and unmanageable, modify the activity of the hypothalamic-pituitary-adrenocortical (HPA) axis and the autonomic nervous system (ANS). HPA activation occurs when neurons in the paraventricular nucleus of the hypothalamus secrete corticotropin releasing hormone (CRH). This molecule travels through the hypophyseal portal circulation to the anterior pituitary gland, which responds to its presence by secreting a pulse of adrenocorticotropin hormone (ACTH). The ACTH signal is carried through the peripheral circulation to the adrenal glands, which synthesize and release cortisol in the zona fasciculata. The ANS consists of sympathetic and parasympathetic branches whose effector molecules include epinephrine, and norepinephrine, and acetylcholine. By changing the outflow of these systems, stress alters the systemic balance of glucocorticoids and catecholamines, as well as concentrations of these (and other) hormones in primary and secondary lymphoid organs 14. Macrophages and lymphocytes have functional receptors for these hormones (glucocorticoid receptors for cortisol, alpha and beta adrenergic receptors for catecholamines), and ligation of those receptors alters these cells’ repertoires of gene expression, with downstream implications for trafficking, signaling, proliferation and differentiation, and effector functions15. Via these modulatory influences, chronic stressors potentiate reactivity to asthma triggers, e.g., allergens, infections, and in doing so may exacerbate airway inflammation and airflow obstruction16,17.
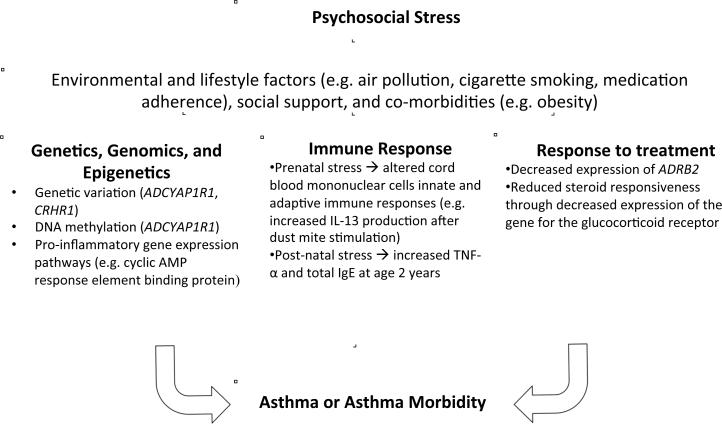
More recently, mechanistic research in this area has begun to focus on the role of allelic variation in genes that regulate stress responses, as well as stress-induced changes in DNA methylation patterns and gene expression. In this report, we first review recent findings on potential biologic mechanisms for stress-related asthma (summarized in Table 1), which may be modified by environmental and lifestyle factors, social support or co-morbidities, as shown in Figure 1. We then discuss future directions for research in this field.
Table 1.
Selected human studies of potential genetic, epigenetic and immunologic mechanisms for stress-related asthma or asthma morbidity
| Study (first author, year, reference number) |
Study population | Major findings |
|---|---|---|
| Chen, 2013,43 | 516 Puerto Rican children with and without asthma |
DNA methylation and a SNP in ADCYAP1R1 were associated with asthma risk |
| Tsartsali, 2012,48 | 62 Greek children with asthma, on ICS |
SNPs in CRHR1 were associated with baseline Cortisol levels and Cortisol response |
| Tantisira, 2004,49 | 1,117 North American children and adults with asthma |
SNPs in CRHR1 were associated with ICS response in multiple populations |
| Rogers, 2009,50 | 311 North American children with asthma |
SNPs in CRHR1 were associated with poor long- term response to ICS |
| Kim, 2009,70 | 87 Korean adults with COPD |
A SNP in CRHR1 was associated with reduced ICS response |
| Dijkstra,2008,52 | 281 Dutch adults with asthma |
No association between CRHR1 variants and ICS response |
| Chen, 2009, 53 | 31 Canadian children with asthma |
Children from low SES households had increased expression of pro- inflammatory cytokines |
| Miller, 2009,54 | 103 healthy Canadian adults |
Low SES in early life was associated with up- regulation of adrenergic signaling and down- regulation of genes with glucocorticoid response elements |
| Wright, 2010, 62 | Birth cohort of 557 American inner-city children |
Cumulative prenatal maternal stress was associated with increased inflammatory cytokine responses in cord blood |
| Wright, 2004, 63 | 499 infants from Boston, MA with family history of atopy or asthma |
Higher post-natal stress in caregivers was associated with increased total IgE at age 2 years |
| Sternthal, 2011, 64 | Birth cohortstudy of 510 urban children from Boston, MA |
Children from low SES households had higher cord blood IgE and increased risk of wheeze |
| Azad, 2012, 65 | Cross-sectional study of 267 Canadian children |
No difference in IL-6 production by ex vivo PBMCs between children with high SES and children who experienced upward social mobility, but PBMC’s from children with persistently low SES had decreased IL-6 production |
| Miller, 2006, 67 | 77 children with and without asthma from Vancouver, BC |
Acute and chronic stress were associated with reduced expression of glucocorticoid receptor and beta-adrenergic genes in children with asthma, but with increased expression of these genes in children without asthma |
| Miller, 2009, 68 | 143 children with and without asthma from Vancouver, BC |
Low perceived parental support was associated with higher levels of eosinophil cationic protein and increased resistance to corticosteroids in ex vivo PBMCs |
| Marin, 2009, 66 | Prospective study of 147 children with and without asthma from Vancouver, BC |
Only asthmatic children with higher levels of chronic stress had increased pro- inflammatory cytokine production when exposed to acute stressors |
Figure 1.
Potential causal mechanisms for stress-related asthma or asthma morbidity.
Genetics, Genomics and Epigenetics of Stress and Asthma
As for other complex diseases, genome-wide association studies have identified common genetic variants that confer susceptibility to asthma but do not account for a large proportion of its heritability (phenotypic variation explained by genetic factors)18. This “missing heritability” of asthma may be explained by unaccounted phenotypic heterogeneity19, structural variation (e.g. copy number variants)20, rare genetic variants with strong effects21, gene-by-gene interactions (epistasis) 21 gene-by-environment interactions22,23 or epigenetic mechanisms such as DNA methylation24 or microRNAs25. Few studies have examined the role of genetic or epigenetic mechanisms on stress-related asthma.
In a study of over 1,200 (predominantly African American) adults exposed to traumatic events, Ressler and colleagues implicated the pituitary adenylate cyclase activating peptide (PACAP)-PAC1 receptor pathway on the pathogenesis of PTSD26. In this study, both PACAP38 (PACAP peptide containing 38 residues) blood levels and the C allele of a functional single nucleotide polymorphism (SNP, rs2267735) in an estrogen-receptor element of the gene for the PAC1 receptor (ADCYAP1R1) were significantly associated with PTSD or more PTSD symptoms in females but not in males. For example, the correlation coefficient (r) for PACAP38 blood level and PTSD symptoms was 0.497 (P <0.005) in females but non-significant in males (P >0.5). In contrast to these sex-specific associations, methylation of a CpG site in the promoter of ADCYAP1R1 (assessed in DNA from white blood cells) was shown to be associated with PTSD or more PTSD symptoms (r for symptoms= 0.35, P <0.0005). To further support the plausibility of the human findings, ADCYAP1R1 mRNA was shown to be inducible after fear conditioning in rodents26. A female-specific association between the C allele of rs2267735 and PTSD or PTSD symptoms has been replicated in studies of highly traumatized Chinese27 and African American28 adults, but not in a study of adults of European or African American descent who were not selected on the basis of traumatic exposures29. Using magnetic resonance imaging, the C allele of rs2267735 was recently shown to impact fear responses in the amygdala and hippocampus of women with lifetime history of exposure to traumatic events30. Of interest, the C allele of rs2267735 was associated with anxiety in school-aged boys and girls, suggesting that any sex-specific effects of this SNP are not present before puberty31. In contrast to the published work replicating an association between SNP rs2267735 and PTSD, findings for ADCYAP1R1 methylation have yet to be replicated for PTSD. Given that PTSD has been associated with asthma or asthma symptoms32,33, there has been recent interest in studying both methylation and genetic variants in ADCYAP1R1 and asthma.
Puerto Ricans are disproportionately affected by asthma in the U.S 34-37 and often exposed to violence, both in the household and in the community 38-40. Our group has shown that physical/sexual abuse and parental stress are associated with asthma in Puerto Rican children5,41. Given these findings, known increased susceptibility of Puerto Rican adults to developing PTSD after exposure to traumatic events, and experimental evidence suggesting a potential role of ADCYAP1R1 on regulating expression of the glucocorticoid receptor gene42, we examined exposure to violence (assessed using a validated scale), ADCYAP1R1 and asthma in 516 Puerto Rican children ages 6 to 14 years43. In this study, we demonstrated that exposure to violence is associated with methylation of a CpG site in the promoter of ADCYAP1R1 (adjusted β per each 10-point increment in the ETV scale, obtained from a linear regression model= 0.5%, 95% confidence interval [CI]=0.1% to 0.9%, P=0.02) and that such methylation is associated with asthma in Puerto Rican children (adjusted odds ratio [aOR] per each 1% increment in methylation, obtained from a logistic regression model=1.3, 95% CI=1.0-1.6, P=0.03). Moreover, we showed that the C allele of SNP rs2267735 (previously implicated in PTSD and anxiety) is associated with 30% increased odds of asthma (95% CI for aOR=1.0-1.7, P=0.03) in these children.
Our findings for ADCYAP1R1 have yet to be replicated, and we cannot exclude “reverse causation” for the methylation findings (e.g. asthma leading to increased DNA methylation) in a cross-sectional study. However, the biological plausibility of our results is supported by experimental models showing that PACAP acts as endogenous bronchodilator, relaxing airway smooth muscle44,45. Moreover, PACAP protects against endotoxin-induced allergic airway inflammation (AAI) in rodents46, in which the PAC1 receptor mediates anti-inflammatory effects in AAI47. Together with these experimental findings, our results suggest that genetic and epigenetic variation in a susceptibility gene for PTSD and childhood anxiety (ADCYAP1R1) is implicated in the pathogenesis of asthma in children disproportionately exposed to violence or traumatic events, such as Puerto Ricans.
CRH, along with signaling of PACAP, regulates anxiety-related behavior26 and is thus in a candidate pathway for stress-related asthma. SNPs in the gene encoding the main receptor for CRH (CRHR1) have been associated with change in lung function in response to inhaled corticosteroids (ICS) in subjects with asthma or chronic obstructive pulmonary disease (COPD) in some studies48-51 but not in others52.
Few studies have examined the effects of psychosocial stress on genome-wide expression in tissues relevant to asthma. In a genome-wide study of transcriptional profiles from CD2+ T lymphocytes of 31 school-aged children, low socioeconomic status (a stressor correlated with other stressors such as exposure to violence) was associated with overexpression of genes regulating inflammation, including chemokine activity and cytokine production 53. In this small study, results from a bioinformatics analysis offered preliminary support for a mediating role of cyclic AMP response element binding protein, nuclear factor Y, and nuclear factor κβ on the observed effects 53.
Social adversity in early life may program biological systems in a manner predisposing to chronic diseases such as asthma. In a study of genome-wide transcriptional profiles in peripheral blood mononuclear cells (PBMCs) from 103 healthy adults aged 25 to 40 years, low socioeconomic status in early life was associated with up-regulation of genes bearing responses for the CREB/ATF family of transcription factors conveying adrenergic signals to white blood cells, as well as with down-regulation of genes with response elements for the glucocorticoid receptor (which, as noted above, transduces cortisol’s anti-inflammatory effects in macrophages and lymphocytes)54. In this study, low socioeconomic status was also associated with over-expression of transcripts with response elements for NF-kB, and increased stimulated production of interleukin 6 (e.g. PBMCs from subjects with low early-life SES produced 51% more IL6 in response to TLR3 stimulation with the ligand poly(I:C) than did those with high early-life SES, P = 0.03). Taken together with those from other studies, these results suggest that low socioeconomic status in early life programs sustained resistance to glucocorticoid signaling, ultimately leading to increased adrenocortical and inflammatory responses in adulthood. Among those who go on to develop asthma, this programmed resistance may also undermine the efficacy of steroid therapy. Large longitudinal studies are needed to validate and expand on these findings.
Stress, immune responses and asthma
Findings from recent experimental studies suggest that stress may predispose to asthma or asthma morbidity through effects on the immune system55,56. In an experimental model, Fischer 344 rats were subjected to repeated handling stimulation (HA), maternal separation (MS), or no intervention at age 4 weeks. At age 5 months, HA rats had increased ex vivo natural killer cell cytotoxicity but no other alterations in immune responses. After induction of experimental asthma, MS rats had greater number of eosinophils in broncho-alveolar lavage than controls or HA rats (P =0.002). In HA rats, induction of experimental asthma was associated with markedly increased levels of adreno-corticotropin compared with the MS or control groups (P for one-way analysis of variance=0.02)47. Taken together, these results suggest that early post-natal stressors have effects on the “neuroendocrine immune system” lasting into adult life.
Consistent with results from human studies8,57,58, findings in rodents suggest that stress enhances the detrimental effects of environmental exposures on asthma59. In particular, rats exposed to both concentrated ambient particles and stress had higher blood levels of C-reactive protein and tumor necrosis factor alpha, but lower lung function, than those exposed only to either concentrated ambient articles or stress59. For example, exposure to particulate matter 2.5 (PM2.5) was significantly associated with a lower peak expiratory flow in stressed animals (adjusted β per each μg/m3 increment= −3.9 × 10−3, P=0.003) but not in non-stressed animals (P=0.92).
Because pre- or early-post natal exposures are likely to have major effects on immune system development60, there is considerable interest in studying whether stressors may lead to asthma development through alteration of immune responses in early life. However, the relationship between cytokine profiles in early life and asthma is insufficiently understood61, and thus children participating in birth cohort studies must be followed up to age six years or greater, when asthma can be confidently diagnosed. To date, published studies of pre- and early post-natal stress and childhood asthma are lacking sufficient data on stress or follow up of participants into school age. Nonetheless, emerging evidence suggests that maternal stress influences immune responses and asthma symptoms in infancy. For example, a birth cohort study of 557 inner-city children found that cumulative prenatal maternal stress was associated with cord blood mononuclear cell innate and adaptive cytokine responses62. In this study, prenatal maternal stress was associated with increased production of IL-8 and TNF-α after microbial stimuli, as well as with increased IL-13 production after dust mite stimulation and reduced phytohemagglutinin-induced IFN-γ (P <0.05 in all instances). Post-natal family stress also seems to influence patterns of early-life immune responding relevant to asthma. One birth cohort tracked parents’ stress levels every two months for the initial 24 months years of their child’s life. In this study, greater parental stress between ages 6 and 18 months was associated with a total IgE ≥100 IU/ml (aOR=2.0, 95% CI=1.1-3.6, P<0.05) and larger TNF-α production in offspring at age 2 years63.
Early-life stress may lead to programming of neuroendocrine and immune responses in girls that is carried over to adulthood and to pregnancy, and ultimately to child’s development54. A recent birth cohort study of 510 urban children showed that maternal low socioeconomic status during childhood was directly associated with cord blood IgE level (adjusted β from a structural equation model=0.21, P=0.003) and indirectly (through prenatal cumulative stress, low socioeconomic status in adulthood and air pollution) to recurrent wheeze in their children64. In contrast to these findings, a cross-sectional study of 267 Canadian children found no difference in IL-6 production by PBMCs stimulated with lipopolysaccharide between children with persistently high socioeconomic status since birth and those who experienced upward mobility (going from lower-middle to higher-middle status), suggesting that some programming effects of early-life stressors can be reversed 65. In contrast to children with an “upward SES trajectory”, those with persistently low socioeconomic status had increased IL-6 production by stimulated PBMCs, particularly if they were overweight. In addition, atopic asthma was associated with a 54% increment in IL-6 level in urban children (P=0.03) but was not significantly associated with IL-6 in rural children.
A two-year prospective study of 147 children aged 9 to 18 years examined whether acute or chronic stress is associated with cytokine responses or asthma symptoms at school age66. In this study, acute stress was associated with increased cytokine (IL-4, IL-5 and IFN-γ) production by PBMCs after mitogenic stimulation, but only in children with asthma and high levels of chronic stress. In this study, chronic stress (particularly in the presence of acute stress) and IL-5 levels were associated with increased symptoms in a subset of 32 children with moderate to severe asthma (P <0.05 in both instances). The main limitations of this study are limited statistical power to assess stress effects on multiple cytokines, the fact that acute stress could have occurred as early as six months before the study visits (since they were scheduled twice per year), and lack of assessment of corticosteroid responses.
Stress and Response to Treatment
Stress may increase asthma morbidity by reducing response to inhaled corticosteroids and inhaled beta2 agonists. Acute stress and chronic stress have been associated with reduced expression of the genes encoding the glucocorticoid receptor (by 5.5 fold) and the beta-2 adrenergic receptor (by 9.5 fold) in leukocytes of children with asthma (adjusted P <0.05 in both instances)67. In another study including 143 school-aged children, perception of low parental support was associated with reduced response to corticosteroids in vitro and higher circulating levels of eosinophil cationic protein in children with asthma68. In this study, response to corticosteroids was assessed by measuring production of IL-5, IL-13 and IFN-γ by peripheral blood mononuclear cells (incubated with a mitogen cocktail) after adding physiologic doses of hydrocortisone68. Although limited by a cross-sectional design, findings from the two studies referenced above67,68 support the hypothesis that chronic stress leads to down-regulation of glucocorticoid receptor expression and function. Nevertheless, these findings must be substantiated with in vivo measurements of glucocorticoid sensitivity.
Future Directions
Development of novel indicators or biomarkers of chronic stress is imperative, given that currently used indicators of stress cannot be used in young children (e.g. questionnaires) or are difficult to implement in large studies (e.g. detailed interviews with parents, multiple measures of salivary cortisol to assess circadian rhythm). Measuring cortisol in hair is one potential strategy, which has the advantage of capturing more chronic HPA activity, as experienced over a several-month timeframe69. Moreover, one could gain novel insights into stress-related asthma by examining the relation between chronic stress and gene expression and epigenetic changes in tissues relevant to asthma (e.g. airway epithelium and lymphocytes).
Accounting for mediators and modifiers of the effect of stress on asthma is key in future longitudinal studies of stress and asthma or asthma morbidity. For example, which proportion of the effect of stress on asthma morbidity or treatment response is explained by reduced adherence with controller medications? To which extent does having coping mechanisms or social support attenuate the effects of stress on asthma? Does exposure to other environmental exposures (e.g. cigarette smoking, air pollution) or co-morbidities (e.g. obesity) modify the effect of stress on asthma, and –if so- to which extent? Does stress lead to epigenetic changes in tissues relevant to the pathogenesis of asthma? Do variants that confer susceptibility to stress-related mental illness or anxiety impact asthma (by themselves or interacting with stress) on populations at risk?
Phenotypic assessment of asthma and immune responses has been often overlooked in studies of stress and asthma. Sufficiently long follow up of ongoing birth cohort studies, assessment of objective markers of disease severity or control (e.g. pulmonary function tests and airway responsiveness), examining sub-phenotypes of the “asthma syndrome” (e.g. atopic vs. non-atopic asthma, eosinophilic vs. non-eosinophilic asthma), and measuring cytokine profiles other than Th1/Th2 (e.g. Th17) will be important in future studies of stress and asthma.
Studying the role of stress on treatment responses in vivo is a high priority. Does stress reduce the efficacy of treatment responses, independently of adherence with medications? If so, is this mediated by down-regulation of the glucocorticoid receptor? Are the effects of stress on treatment response (if any) more marked in populations exposed to heavily traumatic events?
In summary, there is compelling evidence for a link between chronic psychosocial stress and the onset and course of asthma. Over the past decade, there has been substantial progress identifying alterations in the HPA axis and the ANS, as well as immunologic mechanisms likely to underlie these phenomena. More recently, studies have begun to highlight specific signal transduction pathways through which stress modulates epigenetic and transcriptional activity in asthma-relevant cells, and to identify susceptibility genes that may confer risk for stress-related exacerbations of asthma. Further understanding of these mechanisms will improve our capacity to prevent and treat asthma, particularly in vulnerable populations (e.g. ethnic minorities and the economically disadvantaged) who experience disproportionate rates of the asthma burden in this country. Such progress could have a major impact in reducing unacceptable health disparities in asthma in the United States and worldwide.
Acknowledgments
This work was supported by grants HL079966 and HL117191 from the US National Institutes of Health (NIH). Dr. Celedón served as a single-time consultant for Genentech in 2011 on a topic unrelated to this manuscript. Dr. Brehm’s contribution was supported by grant HD052892 from the NIH.
Abbreviations
- PACAP
Pituitary adenylate cyclase-activating polypeptide
- CRH
Corticotropin releasing hormone
- SNP
Single nucleotide polymorphisms
- ICS
Inhaled corticosteroids
- COPD
Chronic obstructive pulmonary disease
- PTSD
Post-traumatic stress disorder
- CBMC
Cord blood mononuclear cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of conflicts of interest
The other authors have no conflicts of interest to declare.
REFERENCES
- 1.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS data brief. 2012:1–8. [PubMed] [Google Scholar]
- 2.Forno E, Celedon JC. Health disparities in asthma. Am J Respir Crit Care Med. 2012;185:1033–5. doi: 10.1164/rccm.201202-0350ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews KA, Gallo LC. Psychological perspectives on pathways linking socioeconomic status and physical health. Annu Rev Psychol. 2011;62:501–30. doi: 10.1146/annurev.psych.031809.130711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yonas MA, Lange NE, Celedon JC. Psychosocial stress and asthma morbidity. Curr Opin Allergy Clin Immunol. 2012;12:202–10. doi: 10.1097/ACI.0b013e32835090c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen RT, Canino GJ, Bird HR, Celedon JC. Violence, abuse, and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2008;178:453–9. doi: 10.1164/rccm.200711-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coogan PF, Wise LA, O'Connor GT, Brown TA, Palmer JR, Rosenberg L. Abuse during childhood and adolescence and risk of adult-onset asthma in African American women. J Allergy Clin Immunol. 2013;131:1058–63. doi: 10.1016/j.jaci.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klinnert MD, Nelson HS, Price MR, Adinoff AD, Leung DY, Mrazek DA. Onset and persistence of childhood asthma: predictors from infancy. Pediatrics. 2001;108:E69. doi: 10.1542/peds.108.4.e69. [DOI] [PubMed] [Google Scholar]
- 8.Chiu YH, Coull BA, Sternthal MJ, Kloog I, Schwartz J, Cohen S, et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen E, Chim LS, Strunk RC, Miller GE. The role of the social environment in children and adolescents with asthma. Am J Respir Crit Care Med. 2007;176:644–9. doi: 10.1164/rccm.200610-1473OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen E, Strunk RC, Trethewey A, Schreier HM, Maharaj N, Miller GE. Resilience in low-socioeconomic-status children with asthma: adaptations to stress. J Allergy Clin Immunol. 2011;128:970–6. doi: 10.1016/j.jaci.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright RJ. Exploring biopsychosocial influences on asthma expression in both the family and community context. Am J Respir Crit Care Med. 2008;177:129–30. doi: 10.1164/rccm.200710-1526ED. [DOI] [PubMed] [Google Scholar]
- 12.Wright RJ. Perinatal stress and early life programming of lung structure and function. Biol Psychol. 2010;84:46–56. doi: 10.1016/j.biopsycho.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahreinian S, Ball GD, Vander Leek TK, Colman I, McNeil BJ, Becker AB, et al. Allostatic load biomarkers and asthma in adolescents. Am J Respir Crit Care Med. 2013;187:144–52. doi: 10.1164/rccm.201201-0025OC. [DOI] [PubMed] [Google Scholar]
- 14.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–51. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 15.Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625–32. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen E, Miller GE. Stress and inflammation in exacerbations of asthma. Brain Behav Immun. 2007;21:993–9. doi: 10.1016/j.bbi.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright RJ, Cohen RT, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol. 2005;5:23–9. doi: 10.1097/00130832-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43:887–92. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonnelykke K, Sleiman P, Nielsen K, Kreiner-Møller E, Mercader JM, Belgrave D, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46:51–5. doi: 10.1038/ng.2830. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira MA, McRae AF, Medland SE, Nyholt DR, Gordon SD, Wright MJ, et al. Association between ORMDL3, IL1RL1 and a deletion on chromosome 17q21 with asthma risk in Australia. European journal of human genetics : EJHG. 2011;19:458–64. doi: 10.1038/ejhg.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunninghake GM, Soto-Quiros ME, Lasky-Su J, Avila L, Ly NP, Liang C, et al. Dust mite exposure modifies the effect of functional IL10 polymorphisms on allergy and asthma exacerbations. J Allergy Clin Immunol. 2008;122:93–8. doi: 10.1016/j.jaci.2008.03.015. 8 e1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bouzigon E, Corda E, Aschard H, Dizier MH, Boland A, Bousquet J, et al. Effect of 17q21 variants and smoking exposure in early-onset asthma. N Engl J Med. 2008;359:1985–94. doi: 10.1056/NEJMoa0806604. [DOI] [PubMed] [Google Scholar]
- 24.Perera F, Tang WY, Herbstman J, et al. Relation of DNA methylation of 5'-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 2009;4:e4488. doi: 10.1371/journal.pone.0004488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levanen B, Bhakta NR, Torregrosa Paredes P, Tang D, Levin L, Miller R, et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013;131:894–903. doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–7. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L, Cao C, Wang R, Qing Y, Zhang J, Zhang XY. PAC1 receptor (ADCYAP1R1) genotype is associated with PTSD's emotional numbing symptoms in Chinese earthquake survivors. Journal of affective disorders. 2013;150:156–9. doi: 10.1016/j.jad.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Almli LM, Mercer KB, Kerley K, Feng H, Bradley B, Bonneely KN, et al. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2013;162B:262–72. doi: 10.1002/ajmg.b.32145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang SC, Xie P, Anton RF, De Vivo I, Farrer LA, Kranzier HR, et al. No association between ADCYAP1R1 and post-traumatic stress disorder in two independent samples. Molecular psychiatry. 2012;17:239–41. doi: 10.1038/mp.2011.118. [DOI] [PubMed] [Google Scholar]
- 30.Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, et al. PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1318954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jovanovic T, Norrholm SD, Davis J, Mercer KB, Almli L, Nelson A, et al. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spitzer C, Koch B, Grabe HJ, Ewert R, Barnow S, Felix SB, et al. Association of airflow limitation with trauma exposure and post-traumatic stress disorder. Eur Respir J. 2011;37:1068–75. doi: 10.1183/09031936.00028010. [DOI] [PubMed] [Google Scholar]
- 33.Brackbill RM, Hadler JL, DiGrande L, Ekenga C, Farfel F, Friedman S, et al. Asthma and posttraumatic stress symptoms 5 to 6 years following exposure to the World Trade Center terrorist attack. JAMA : the journal of the American Medical Association. 2009;302:502–16. doi: 10.1001/jama.2009.1121. [DOI] [PubMed] [Google Scholar]
- 34.Hunninghake GM, Weiss ST, Celedon JC. Asthma in Hispanics. Am J Respir Crit Care Med. 2006;173:143–63. doi: 10.1164/rccm.200508-1232SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter-Pokras OD, Gergen PJ. Reported asthma among Puerto Rican, Mexican-American, and Cuban children, 1982 through 1984. American journal of public health. 1993;83:580–2. doi: 10.2105/ajph.83.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen RT, Canino GJ, Bird HR, Shen S, Rosner BA, Celedon JC. Area of residence, birthplace, and asthma in Puerto Rican children. Chest. 2007;131:1331–8. doi: 10.1378/chest.06-1917. [DOI] [PubMed] [Google Scholar]
- 37.David MMA, Hanrahan JP, Carey V, Speizer FE, Tager IB. Respiratory symptoms in urban hispanic and non-hispanic white women. Am J Respir Crit Care Med. 1996;153:1285–91. doi: 10.1164/ajrccm.153.4.8616555. [DOI] [PubMed] [Google Scholar]
- 38.Martinez-Taboas A, Canino G, Wang MQ, Garcia P, Bravo M. Prevalence and victimization correlates of pathological dissociation in a community sample of youths. Journal of traumatic stress. 2006;19:439–48. doi: 10.1002/jts.20144. [DOI] [PubMed] [Google Scholar]
- 39.Purugganan OH, Stein RE, Silver EJ, Benenson BS. Exposure to violence among urban school-aged children: is it only on television? Pediatrics. 2000;106:949–53. [PubMed] [Google Scholar]
- 40.Vermeiren R, Schwab-Stone M, Deboutte D, Leckman PE, Ruchkin V. Violence Exposure and Substance Use in Adolescents: Findings From Three Countries. Pediatrics. 2003;111:535–40. doi: 10.1542/peds.111.3.535. [DOI] [PubMed] [Google Scholar]
- 41.Lange NE, Bunyavanich S, Silberg JL, Canino G, Rosner BA, Celedon JC. Parental psychosocial stress and asthma morbidity in Puerto Rican twins. J Allergy Clin Immunol. 2010 doi: 10.1016/j.jaci.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hashimoto H, Hashimoto R, Shintani N, Tanaka K, Yamamoto A, Hatanaka M, et al. Depression-like behavior in the forced swimming test in PACAP-deficient mice: amelioration by the atypical antipsychotic risperidone. Journal of neurochemistry. 2009;110:595–602. doi: 10.1111/j.1471-4159.2009.06168.x. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Boutaoui N, Brehm JM, Han YY, Schmitz C, Cressley A, et al. ADCYAP1R1 and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2013;187:584–8. doi: 10.1164/rccm.201210-1789OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinhult J, Andersson JA, Uddman R, Stjarne P, Cardell LO. Pituitary adenylate cyclase-activating peptide 38 a potent endogenously produced dilator of human airways. Eur Respir J. 2000;15:243–7. doi: 10.1034/j.1399-3003.2000.15b04.x. [DOI] [PubMed] [Google Scholar]
- 45.Foda HD, Sharaf HH, Absood A, Said SI. Pituitary adenylate cyclase-activating peptide (PACAP), a VIP-like peptide, has prolonged airway smooth muscle relaxant activity. Peptides. 1995;16:1057–61. doi: 10.1016/0196-9781(95)00087-z. [DOI] [PubMed] [Google Scholar]
- 46.Elekes K, Sandor K, Moricz A, Kereskai L, Kemeny A, Szoke E, et al. Pituitary adenylate cyclase-activating polypeptide plays an anti-inflammatory role in endotoxin-induced airway inflammation: in vivo study with gene-deleted mice. Peptides. 2011;32:1439–46. doi: 10.1016/j.peptides.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 47.Lauenstein HD, Quarcoo D, Plappert L, Schleh C, Nassimi M, Pilzner C, et al. Pituitary adenylate cyclase-activating peptide receptor 1 mediates anti-inflammatory effects in allergic airway inflammation in mice. Clin Exp Allergy. 2011;41:592–601. doi: 10.1111/j.1365-2222.2010.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsartsali L, Papadopoulos M, Lagona E, Papadimitriou A, Kanaka-Gantenbein C, Louizou E, et al. Association of hypothalamic-pituitary-adrenal axis-related polymorphisms with stress in asthmatic children on inhaled corticosteroids. Neuroimmunomodulation. 2012;19:88–95. doi: 10.1159/000329592. [DOI] [PubMed] [Google Scholar]
- 49.Tantisira KG, Lake S, Silverman ES, Palmer LF, Lazarus R, Silverman EK, et al. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004;13:1353–9. doi: 10.1093/hmg/ddh149. [DOI] [PubMed] [Google Scholar]
- 50.Rogers AJ, Tantisira KG, Fuhlbrigge AL, Litonjua AA, Lasky-Su JA, Szefler SJ, et al. Predictors of poor response during asthma therapy differ with definition of outcome. Pharmacogenomics. 2009;10:1231–42. doi: 10.2217/PGS.09.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim WJ, Sheen SS, Kim TH, Huh JW, Lee JH, Kim EK, et al. Association between CRHR1 polymorphism and improved lung function in response to inhaled corticosteroid in patients with COPD. Respirology. 2009;14:260–3. doi: 10.1111/j.1440-1843.2008.01425.x. [DOI] [PubMed] [Google Scholar]
- 52.Dijkstra A, Koppelman GH, Vonk JM, Bruinenberg M, Schouten JP, Postma DS. Pharmacogenomics and outcome of asthma: no clinical application for long-term steroid effects by CRHR1 polymorphisms. J Allergy Clin Immunol. 2008;121:1510–3. doi: 10.1016/j.jaci.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 53.Chen E, Miller GE, Walker HA, Arevalo JM, Sung CY, Cole SW. Genome-wide transcriptional profiling linked to social class in asthma. Thorax. 2009;64:38–43. doi: 10.1136/thx.2007.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–21. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang DH, Weaver MT. Airway cytokine responses to acute and repeated stress in a murine model of allergic asthma. Biol Psychol. 2010;84:66–73. doi: 10.1016/j.biopsycho.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Kruschinski C, Skripuletz T, Bedoui S, Raber K, Straub RH, Hoffmann T, et al. Postnatal life events affect the severity of asthmatic airway inflammation in the adult rat. J Immunol. 2008;180:3919–25. doi: 10.4049/jimmunol.180.6.3919. [DOI] [PubMed] [Google Scholar]
- 57.Islam T, Urman R, Gauderman WJ, Milam J, Lurmann F, Shankardass K, et al. Parental stress increases the detrimental effect of traffic exposure on children's lung function. Am J Respir Crit Care Med. 2011;184:822–7. doi: 10.1164/rccm.201104-0720OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters JL, Cohen S, Staudenmayer J, Hosen J, Platts-Mills TA, Wright RJ. Prenatal negative life events increases cord blood IgE: interactions with dust mite allergen and maternal atopy. Allergy. 2012;67:545–51. doi: 10.1111/j.1398-9995.2012.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clougherty JE, Rossi CA, Lawrence J, Long MS, Diaz EA, Lim RH, et al. Chronic social stress and susceptibility to concentrated ambient fine particles in rats. Environ Health Perspect. 2010;118:769–75. doi: 10.1289/ehp.0901631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ly NP, Litonjua A, Gold DR, Celedon JC. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J Allergy Clin Immunol. 2011;127:1087–94. doi: 10.1016/j.jaci.2011.02.015. quiz 95-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005;5:161–6. doi: 10.1097/01.all.0000162309.97480.45. [DOI] [PubMed] [Google Scholar]
- 62.Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. 2010;182:25–33. doi: 10.1164/rccm.200904-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wright RJ, Finn P, Contreras JP, Cohen S, Wright RO, Staudenmayer J, et al. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. J Allergy Clin Immunol. 2004;113:1051–7. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 64.Sternthal MJ, Coull BA, Chiu YH, Cohen S, Wright RJ. Associations among maternal childhood socioeconomic status, cord blood IgE levels, and repeated wheeze in urban children. J Allergy Clin Immunol. 2011;128:337–45. doi: 10.1016/j.jaci.2011.05.008. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Azad MB, Lissitsyn Y, Miller GE, Becker AB, HayGlass KT, Kozyrskyj AL. Influence of socioeconomic status trajectories on innate immune responsiveness in children. PLoS One. 2012;7:e38669. doi: 10.1371/journal.pone.0038669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marin TJ, Chen E, Munch JA, Miller GE. Double-exposure to acute stress and chronic family stress is associated with immune changes in children with asthma. Psychosomatic medicine. 2009;71:378–84. doi: 10.1097/PSY.0b013e318199dbc3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller GE, Chen E. Life stress and diminished expression of genes encoding glucocorticoid receptor and beta2-adrenergic receptor in children with asthma. Proc Natl Acad Sci U S A. 2006;103:5496–501. doi: 10.1073/pnas.0506312103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miller GE, Gaudin A, Zysk E, Chen E. Parental support and cytokine activity in childhood asthma: the role of glucocorticoid sensitivity. J Allergy Clin Immunol. 2009;123:824–30. doi: 10.1016/j.jaci.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 69.Kirschbaum C, Tietze A, Skoluda N, Dettenborn L. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–7. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]