Abstract
Winged bean [Psophocarpus tetragonolobus (L.) DC.] seed is a potential underexploited source of vegetable protein due to its high protein content. In the present work, undefatted and defatted winged bean seed hydrolysates, designated as UWBSH and DWBSH, respectively were produced separately by four proteolytic enzymes namely Flavourzyme, Alcalase, Bromelain, and Papain using pH-stat method in a batch reactor. Enzymatic hydrolysis was carried out over a period of 0.5 to 5 h. UWBSH and DWBSH produced were tested for their ACE inhibitory activity in relation to the hydrolysis time and degree of hydrolysis (DH). Maximum ACE inhibitory activity, both for UWBSH and DWBSH, were observed during 3 to 5 h of hydrolysis. Both, UWBSH (DH 91.84 %), and DWSBH (DH 18.72 %), produced by Papain at 5 h hydrolysis, exhibited exceptionally high ACE inhibitory activity with IC50 value 0.064 and 0.249 mg mL−1, respectively. Besides, papain-produced UWBSH and DWBSH were further fractionated into three fractions based on molecular weight (UWBSH-I, <10 kDa; UWBSH-II, <5 kDa; UWBSH-III, <2 kDa) and (DWBSH-I, <10 kDa; DWBSH-II, <5 kDa; DWBSH-III, <2 kDa). UWBSH-III revealed the highest ACE inhibitory activity (IC50 0.003 mg mL−1) compared with DWBSH-III (IC50 0.130 mg mL−1). The results of the present investigation revealed that winged bean seed hydrolysates can be explored as a potential source of ACE inhibitory peptides suggesting their uses for physiological benefits as well as for other functional food applications.
Keywords: Undefatted and defatted winged bean seed, Proximate analysis, ACE inhibitory activity, IC50 value, Degree of hydrolysis, pH-stat titration
Introduction
The search for functional components in foods has become a major research area due to their physiological and health functions. Certain plant-derived compounds such as gluconsinolate, phenolic acids, flavonoids, terpenes and organosulfur have been found to have bioactive properties (Li et al. 2004a; Marczak et al. 2003; Menotti et al. 1999; Yust et al. 2003). Similarly, bioactive peptides constitute another group of functional compounds which are present in foodstuff as such, or may be released by hydrolysis of proteins. Recent studies on the potential health benefits of bioactive peptides has urged the need to explore their possible uses against several chronic diseases, in particular the hypertension (Lim 2012).
The intrinsic bioactivities of the peptides encoded in food proteins are latent until they are released and activated by enzymatic hydrolysis during gastrointestinal digestion and food processing (Yust et al. 2003). Bioactive peptides are mainly produced during protein hydrolysis by the action of cysteine proteases and serine proteases such as Alcalase, Flavourzyme, Papain and Bromelain. In addition to their production by protein hydrolysis in foodstuffs during processing and during digestion in the digestive tract, bioactive peptides may also be produced by controlled enzymatic hydrolysis. With this purpose, protein concentrates or isolates are treated with exogenous proteases in reactors such as batch reactor system.
Protein hydrolysates from certain animal and plant sources such as casein, whey, tuna muscle, and corn gluten can be used to produce peptides with potential ACE inhibitory properties (Barbana and Boye 2011; Bedroche et al. 2002; Wijesekara et al. 2011). Several studies have been conducted to produce ACE inhibitory peptides by enzymatic hydrolysis of plant–derived protein rich materials such as rapeseed (Marczak et al. 2003), alfalfa (Kapel et al. 2006), chickpea (Yust et al. 2003), red lentil (Boye et al. 2010), green lentil (Akıllıoğlu and Karakaya 2009), corn gluten (Yang et al. 2007) and soybean (Shin et al. 2001).
Generally, antihypertensive activity of certain bio-peptides is due to inhibition of angiotensin I-converting enzyme (ACE) which it hydrolyzes decapeptides angiotensin I, yielding potent vasoconstrictor octapeptide angiotensin II. In addition, ACE hydrolyzes the peptide bradyquinin, which is a potent vasodilator. ACE inhibitors are applicable in the prevention of hypertension as mentioned by Mark and Davis (2000), which proposed for the treatment of cancer (Lever et al. 1999). Consequently, the urgency for natural food-born ACE inhibitors compared to the adverse effects (hypotension, cough, hyperkalemia, headache, dizziness, fatigue, nausea, and renal impairment) of clinically used synthetic ACE inhibitors such as Captopril, have guided to this research (Chopra et al. 2012).
Legumes are excellent source of dietary protein and oils. Winged bean is one of the important tropical legumes with high protein content (Lim 2012). Winged bean [Psophocarpus tetragonolobus (L.) D.C.], a little-known tropical legume, is grown almost exclusively in Papua New Guinea and Southeast Asia, especially in Malaysia (Lim 2012; Claydon 1975; National Research Council 1981). Winged bean seeds are considered as a good source of dietary protein because of their well-balanced amino acid composition, high protein bioavailability, and relatively low levels of anti-nutritional factors. The seeds have also been reported to contain higher levels of hydrophobic amino acid residues compared to hydrophilic, the former contribute effectively towards ACE inhibitory activity of peptides (National Research Council 1981).
As far as we know there have been no any earlier studies so far reported on the ACE inhibitory potential of winged bean seed proteins. In this study, the undefatted and defatted winged bean seed protein hydrolysates, produced for the first time by hydrolysis with four proteases namely Alcalase, Flavourzyme, Papain and Bromelain were evaluated for their ACE inhibitory activity. The effects of hydrolysis time and degree of hydrolysis (DH), on the ACE inhibitory activity of the hydrolysates produced were also appraised.
Materials and methods
Seeds of Psophocarpus tetragonolobus were obtained from commercial winged bean farm in Selangor, Malaysia. Samples were stored in the cold room (5 °C) until analysis. Flavourzyme 500 MG and Alcalase 2.4L FG with specified activity 2.4 AU/g (Anson Unit/g) purchased from Novozyme were used in this investigation. Papain and Bromelain were purchased from Acros (New Jersey, USA). Hippuryl-his-leu (HHL) powder and Angiotensin converting enzyme (ACE) [from rabbit lung], were from Sigma Chemical Co. (St. Louis, MO, USA). All other reagents chemicals used in this study were of analytical grade.
Proximate composition
Winged bean seeds were ground into fine powder using a grinding machine (Rictec PTE LTD, Singapore). The material that passed through a 60-mesh sieve was used for experimental purposes (Ravindran et al. 1989). The seeds were defatted by extraction with petroleum ether for 24 h at room temperature using a soxhlet apparatus. Proximate composition (moisture, ash, protein and fat contents) of undefatted and defatted winged bean seeds was investigated according to the AOAC methods (AOAC 1984). Total nitrogen content of winged bean seeds, determined by Kjeldahl method, was used to calculate the amount of crude protein by multiplying it with a conversion factor of 6.25 (AOAC 1984). The amount of total carbohydrate was can be quantified by a difference: 100 %- [% moisture + % protein + % fat + % ash]. Crude fiber content was determined according to AOAC method 962.09 (AOAC 1984).
Amino acids composition
Amino acids composition was studied by reversed-phase high performance liquid chromatography (RP-HPLC) system (Rozan et al. 2000). A Waters HPLC (Hitachi Instruments, Tokyo, Japan) system equipped with photodiode array detector (model MD-2010; JASCO, Tokyo, Japan) was used. The samples were hydrolyzed with 6 N HCl for 24, 48 and 72 h at 110 °C and then derivatized by phenylisotiocyanate. A 20-μL volume of the derivatized sample was injected into a C18 reversed phase column (Thermal C18 5 μ, 250 × 4.6 mm) maintained at 43 °C. The mobile phase consisting of buffer A (0.1 M ammonium acetate, pH 6.5) and buffer B (0.1 M ammonium acetate containing acetonitrile) and methanol in a ratio of 44:46:10 (v/v, pH 6.5) was flushed through the column at a flow-rate of 1 mL min−1 using a linear gradient system. The detection of amino acids was done at wavelength of 254 nm. The identification of unknown amino acids was based on the comparison of their retention times with those of pure standards, whereas for quantification standard calibration curves were used. The results were analyzed and computed by the Borwin chromatography software (Version 1.5, Jasco Co. Ltd., Japan).
Enzymatic hydrolysis of winged bean seed
Hydrolysis of protein was performed according to pH-stat method in a 1 L reaction vessel (Sartorius Stedim, Germany) (Adler-Nissen 1986). The jacketed reaction vessel contained known amount of distilled water at optimum operating temperature of the enzyme used. Undefatted and defatted winged bean seeds (20 g) were separately hydrolyzed using four selected proteases under optimum conditions. The protein solution was equilibrated before addition of the enzyme. The pH, and temperature of the mixture were adjusted to the optimum levels for each enzyme (Alcalase, pH 8.0, 60 °C; Bromelain, pH 6.5, 45 °C; Flavourzyme, pH 8.0, 55 °C: Papain, pH 6.5, 70 °C) and the hydrolysis was conducted for 5 h. During the hydrolysis, the pH of the mixture was maintained at the desired level by adding 1 N NaOH or 0.5 N HCl using a pH stat instrument (Metrohm Titrando, Herisau, Switzerland). The solution was agitated with an over-head stirrer (Fisher Scientific, Germany). The volume of NaOH used was measured by an auto-titrator and the data used to calculate the degree of hydrolysis. After 5 h hydrolysis, the process was terminated by raising the temperature of the reaction mixture at 95 °C, for 10 min, to inactivate the enzyme followed by cooling to room temperature. Winged bean seed hydrolysate was then centrifuged at 10,000 × g for 20 min to separate insoluble and soluble fractions. Finally, the soluble phase was recovered and preserved at −20 °C, until used for further analyses.
pH–stat method for calculation of DH
The degree of hydrolysis (DH), is defined as the percent ratio of the number of peptides dissociated (h) to the total number of peptide bonds in the substrate studied (htot). Calculation of DH by the pH-stat method was carried out using data obtained with a 718 Stat Titrino (Metrohm ion analysis, Herisau, Switzerland). The base used for maintaining constant pH was 1.0 N NaOH. In each case, calculation was based on the amount of NaOH or HCI added to maintain the pH constant during the hydrolysis using the following equation (Adler-Nissen 1986):
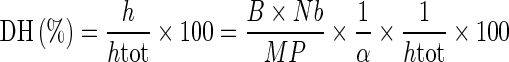 |
Where B is the amount of acid or base consumed (mL) to keep the pH constant during the hydrolysis. Nb is the normality of acid or base, MP is the mass (g) of protein (N × 6.25), and α is the average degree of dissociation of the α-NH2 groups released during hydrolysis expressed as 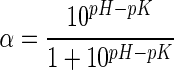
Where pH is the value at which the hydrolysis was conducted. The pK values were calculated using the equation below:
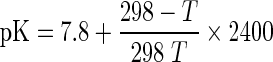 |
Where, T is the hydrolysis temperature in Kelvin. The pK is the average dissociation value for the α-amino groups liberated during hydrolysis and is dependent on temperature, peptide chain length and the nature of the terminal amino acid. The parameter htot is given meqv peptide bonds per gram of protein. This was calculated from amino acid analysis by summing the mmoles of each individual amino acid per gram of protein. The total number of peptide bonds (htot) in winged bean seeds was calculated that is 3.589 meqv g−1 protein.
Protein concentration
Protein concentration was determined following the method of Bradford using protein kit from Merck Chemicals, Germany (Bradford 1976).
Determination of angiotensin I-converting enzyme inhibition activity
Angiotensin I-converting enzyme (ACE) inhibition assay was performed following the method as described by Cheung and Cushman (1973) with slight modifications. A 50 μL volume, containing varying concentration of winged bean seeds protein hydrolysates, was pre-incubated with 20 μL (100 mU mL−1) ACE solutions at 37 °C for 10 min. The mixture was then incubated with 100 μL substrate containing 12.5 mM hippuryl-L-histidyl-L-leucine (HHL), 100 mM borate buffer (pH 8.3), and 300 mM NaCl at 37 °C for 60 min followed by the termination of the reaction by the addition of 125 μL of HCI. The released hippuric acid (HA) was extracted with 850 μL ethyl acetate. After centrifugation at 4,000 × g, for 5 min, 650 μL of ethyl acetate was added into a test tube, the excess solvent was evaporated under vacuum for 2 h. Finally, HA was dissolved in 1 mL of distilled water and the absorbance was noted at 228 nm using a spectrophotometer (U-2800, Hitachi, Tokyo, Japan). The average value for three determinations at each concentration was used to calculate ACE inhibition rate as represented by the following equation:
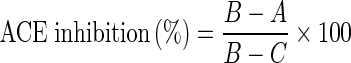 |
Where A is the absorbance of HA produced in the presence of ACE inhibitor component, B is the absorbance of HA produced in the absence of ACE inhibitor component, and C is the absorbance of HA produced in the absence of ACE inhibitor component. IC50 value was defined as the concentration of hydrolysate (mg mL−1) which inhibited ACE activity by 50 %.
Fractionations of papain-produced UWBSH and DWBSH
Both undefatted and defatted winged bean seeds were hydrolyzed with papain at a ratio of protein substrate to enzyme (100:1, w/w) at pH 6.5 and 70 °C for 5 h, and then boiled for 10 min to inactivate the protease. The hydrolysate was centrifuged at 1,000 × g for 20 min to separate the soluble fractions. The resultant undefatted and defatted winged bean seed hydrolysate was fractionated into three fractions (UWBSH-I, < 10 kDa; UWBSH-II, <5 kDa; UWBSH-II, <2 kDa) and (DWBSH-I, < 10 kDa; DWBSH-II, <5 kDa; DWBSH-II, <2 kDa) using a molecular weight cut-off membrane (MWCO, Vivaspin 15 R, Sartorius Stedim, Germany).
Statistical analysis
Results are presented as means ± standard deviation derived from triplicate analysis. Statistical analysis was performed with SAS (Statistical Analysis Software 9.1) using one-way ANOVA. Duncan’s multiple range tests was carried out to compare means. The differences of means were considered to be significant at p < 0.05.
Results and discussion
Proximate composition of undefatted and defatted winged bean seed
Proximate composition of winged bean seeds used in the present study is presented in Table 1. Protein, moisture, fat, ash, fiber and carbohydrate contents of undefatted winged bean seed (UWBS) were found to be 27.81 ± 0.50 %, 10.21 ± 0.80 %, 18.12 ± 0.40 %, 3.52 ± 0.66 %, 11.47 ± 1.03 % and 28.87 ± 0.45 % while for the defatted winged bean seeds (DWBS) 50.22 ± 0.90 %, 8.41 ± 0.80 %, 0.04 ± 0.02 %, 3.42 ± 0.32 %, 10.35 ± 0.56 %, and 27.56 ± 0.23 %, respectively. The protein content of UWBS was 27.81 % which is lower compared to DWBS with the value of 50.22 %. Comparing the present protein content with those of chickpea seed (23 %) (Yust et al. 2003), lentil seed (26 %) (Boye et al. 2010), corn gluten (3.22 %) (Kim et al. 2004) and soybean (36.49 %) (Wu and Ding 2001), it can be concluded that winged bean seed is a good source of protein for production of bioactive peptides. Overall, the defatting process clearly improves DWBS protein content compared to its original state.
Table 1.
Proximate composition (g/100 g sample) of undefatted and defatted winged bean (Psophocarpus tetragonolobus L. DC.) seeds*
| Sample | Moisture | Ash | Protein | Fat | Fiber | Carbohydrate |
|---|---|---|---|---|---|---|
| Undefatted winged bean seeds | 10.21 ± 0.80a | 3.52 ± 0.66a | 27.81 ± 0.50b | 18.12 ± 0.40a | 11.47 ± 1.03a | 28.87 ± 0.45a |
| Defatted winged bean seeds | 8.41 ± 0.80a | 3.42 ± 0.32a | 50.22 ± 0.90a | 0.04 ± 0.02b | 10.35 ± 0.56a | 27.56 ± 0.23b |
*Each value represents the mean of three determinations ± standard deviation. Means denoted with different superscript letters within the same column indicate significant differences (P < 0.05) between the two types of samples
Further analysis on fat for UWBS based on oil extraction using petroleum ether showed that the sample of UWBS is also rich in fat (18.12 %) compared to other legumes such as lentils and chickpea (5.76–6.87 %) (Boye et al. 2010) while the fat content of DWBS is 0.04 %. It can be concluded that the fat of DWBS was successfully reduced to a very low level by the extraction technique. The ash value of UWBS was 3.52 % which is similar with DWBS (3.42 %). These findings were in agreement by previous research on Pigeon pea (Cajanus cajan L.) with the ash value within the range of 3.3 to 4.9 % (Ghadge et al. 2010; Garcia and Palmer 1980). On the other hand, fiber (11.47 %) and carbohydrate (28.87 %) contents of UWBS were nearly similar with DWBS, showing values of 10.35 % and 27.56 %, respectively. Besides, the reported ranges for carbohydrate and fiber are in the range of the present data with the values of 25.0–45.0 % and 3.5–9.4 %, respectively (Claydon 1975; Ekpenyong and Borchers 1982).
From biochemical point of view, proteins are polypeptides with a unique structure to which there is often attached non-peptide moieties (carbohydrate, lipids and inorganic ions) (Gu et al. 2011). High protein content of winged bean seeds could play a significant role in improving the nutritional status of tropical population. Furthermore, the raw material of plant protein sources, especially, winged bean seed is cheaper than that of animal protein and other types of legumes. This is in agreement with a previous study which reveals that winged bean have high seed yield potential compared to soybean, peanut, mung bean, pigeon pea, rice bean, cowpea and bean (Černý et al. 1971; Lim 2012; National Research Council 1981). In this context, therefore, it would be advantageous to produce plant-derived peptides from winged bean seed. In addition, the cost of protein extraction from plant raw material is quite low as against recovery of protein from animal sources. There is also a shift from consumption of meat proteins, which are relatively more expensive to the consumption of less expensive plant proteins. In addition, plants are the primary producers of protein sources and thus, are more economical sources for production of bioactive peptides.
Amino acids profile
The content of different amino acids of winged bean seeds calculated on dry weight basis is given in Table 2. The amino acid profile of winged bean seeds showed that the amounts of hydrophobic (65.02 g/100 g protein) amino acids is higher than hydrophilic amino acids (34.98 g/100 g protein). The presence of appreciable amounts of hydrophobic amino acids in winged bean seed protein is supportive as a previous study on the quantitative structure-activity relationship (QSAR) modeling between ACE inhibitory peptides reveals that hydrophobic amino acid residues and charged side groups promote ACE inhibitory potential (Pripp et al. 2005). For hydrophobic amino acid profile of winged bean seeds, isoleucine dominated among others with content of 24.75 g/100 g. Glycine was the second most abundant component (15.36 g/100 g) followed by Valine (5.51 g/100 g), Leucine (5.05 g/100 g), Tyrosine (3.62 g/100 g), Alanine (3.10 g/100 g), Proline (3.10 g/100 g), Cystine (2.05 g/100 g), Phenylalanine (2.02 g/100 g) and Methionine (0.46 g/100 g). However, hydrophilic amino acid profile of the winged bean seeds showed glutamic acid (14.07 g/100 g) to be the most abundant component followed by aspartic acid (8.01 g/100 g), lysine (4.61 g/100 g), arginine (3.53 g/100 g), serine (2.36 g/100 g), thereonine (1.41 g/100 g) and histidine (0.99 g/100 g).
Table 2.
Amino acid composition of winged bean [Psophocarpus tetragonolobus (L.) DC.] seeds
| Type of amino acid | Amino acid | Concentration (g/100 g protein) |
|---|---|---|
| Hydrophilic | Aspartic acid | 8.01 |
| Glutamic acid | 14.07 | |
| Serine | 2.36 | |
| Histidine | 0.99 | |
| Arginine | 3.53 | |
| Thereonine | 1.41 | |
| Lysine | 4.61 | |
| Total | 34.98 | |
| Hydrophobic | Tyrosine | 3.62 |
| Valine | 5.51 | |
| Methionine | 0.46 | |
| Cystine | 2.05 | |
| Isoleucine | 24.75 | |
| Leucine | 5.05 | |
| Phenylalanine | 2.02 | |
| Glycine | 15.36 | |
| Alanine | 3.10 | |
| Proline | 3.10 | |
| Total | 65.02 | |
Amino acid composition of winged bean seed is quite comparable with that of soybean, which is generally regarded as the legume with the best quality protein (Černý et al. 1971; Pospisil et al. 1971). The superiority of winged bean seed over soybean and casein is in terms of its higher isoleucine content (24.75 g/100 g) (Lever et al. 1999). Isoleucine, a branched-chain aliphatic amino acid is predominant in highly active inhibitors such as ACE inhibitor. On the other hand, lysine content in winged bean seed seems to be lower than that found in the literature for Casein (6.6 g/100 g) and Soybean (4.4 g/100 g) (Černý et al. 1971).
Methionine seems to be the most limiting amino acid in winged bean seed samples, followed by histidine (Ekpenyong and Borchers 1982). The content of lysine (Table 2), 4.61 g/100 g determined in the present work is in agreement with those reported in the literature (Ekpenyong and Borchers 1982). Arginine, although not essential for adults, is essential for children and young people. The appreciable content of this amino acid in winged bean seed can contribute to ACE inhibitory activity. Several ACE-inhibitory peptides have lysine or arginine as the C-terminal residue contributing substantially to the inhibitory potency (Cheung et al. 1980). Proline (3.10 g/100 g) is also important because of its ACE inhibitory role.
Our results of winged bean seed amino acid analysis showed that not only the essential amino acids were present in sufficient amounts, but the non-essential amino acids were also in adequate quantity. This is important because protein synthesis is limited not only by the availability of essential amino acids but also by the speed and efficiency with which the non-essential amino acids are supplied. Under the conditions of the used acid hydrolysis, tryptophan was of course destroyed so we did not detect this component. Literature reports reveal that sensitive amino acids, such as methionine and tryptophan, are detected in smaller amounts after hydrolysis (Shahidi et al. 1995). Studies have shown that peptides with high content of phenylalanine, tyrosine, or proline have potent ACE inhibitory activity at C-terminal, and branced aliphatic amino acid at the N-terminal of peptides contributed to improved ACE inhibition (Li et al. 2004b).
Degree of hydrolysis of undefatted and defatted winged bean seeds
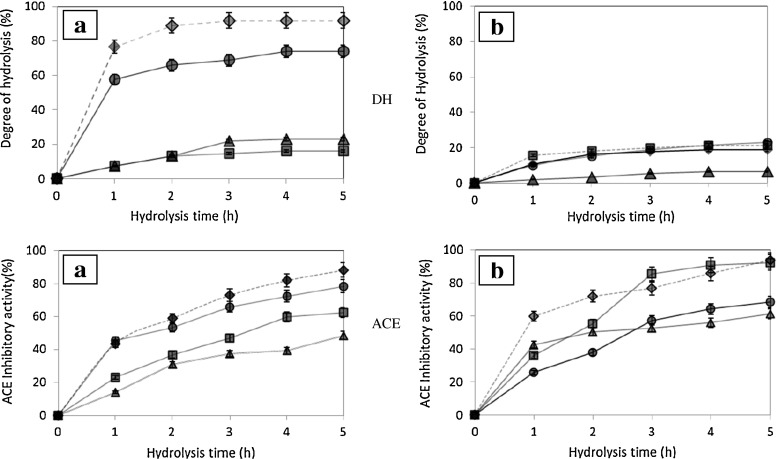
The values of degree of hydrolysis (DH) for the undefatted and defatted winged bean seed hydrolysates produced with four different enzymes, over the period of 0.5 to 5 h hydrolysis are shown in Fig. 1, DH (a) and (b). The DH values obtained for UWBSH (7.48–91.84 %) and DWBSH (2.03–21.34 %) are comparable to those reported in earlier studies for chickpea, sunflower and pea proteins (Humiski and Aluko 2007). Figure 1, DH (a) shows the DH values during hydrolysis of undefatted winged bean seeds using different proteases. The papain-hydrolysate of undefatted winged bean seed was characterized by the initial rapid phase compared to other proteases. In a recently reported work, during the first 10 min of enzymatic hydrolysis using papain, the yield of the hydrolysed protein increased sharply, and then it slowly increased or became essentially constant due to the limited availability of the substrate (Damrongsakkul et al. 2008). Thereafter, a lower rate of increase was generally observed.
Fig. 1.
Changes in the degree of hydrolysis (DH) and (ACE) inhibitory activity over hydrolysis time of UWBSH [a] and DWBSH [b]. ■: Alcalase, ▲: Flavourzyme, ♦: Papain, ● : Bromelain,  DH,
DH,  Highest percentage line. Alcalase, Flavourzyme, Papain and Bromelain were run according to manufacturer’s optimum condition: pH 8.0, Temperature 60 °C, pH 8.0, Temperature, 55 °C, pH 6.5, Temperature 70 °C and pH 6.5, Temperature 45 °C, respectively. Bars represent the standard deviation from triplicate determination (n = 3) and each observation value is mean ± SD. When error bar cannot be seen, the standard deviation is less than the size of the symbol. [a] = Undefatted Winged Bean Seed Hydrolysate and [b] = Defatted Winged Bean Seed Hydrolysate
Highest percentage line. Alcalase, Flavourzyme, Papain and Bromelain were run according to manufacturer’s optimum condition: pH 8.0, Temperature 60 °C, pH 8.0, Temperature, 55 °C, pH 6.5, Temperature 70 °C and pH 6.5, Temperature 45 °C, respectively. Bars represent the standard deviation from triplicate determination (n = 3) and each observation value is mean ± SD. When error bar cannot be seen, the standard deviation is less than the size of the symbol. [a] = Undefatted Winged Bean Seed Hydrolysate and [b] = Defatted Winged Bean Seed Hydrolysate
Papain-produced UWBSH showed the highest DH among the four hydrolysates tested. DH increased rapidly in a time dependent manner during the first 1 h of incubation with DH value of 76.54 % and reached at maximum level of 91.84 % after 3 h and then remained constant thereafter. The high activity of Papain at pH 6.5, 70 °C offered higher hyrolysate production (Damrongsakkul et al. 2008). Previous study by Barbana and Boye (2011) conducted on Papain hydrolysates of red and green lentil showed somewhat lower DH values of 27.21 % and 37.92 %, respectively compared with Papain-derived UWBSH. In the case of Bromelain, DH of UWBSH produced by the enzyme reached the maximum DH value of 74.05 % after 4 h. However, the values obtained after steady state for others (Flavourzyme; 23.06 % and Alcalase; 16.09 %) were significantly lower compared with papain.
Using proteinases such as Flavourzyme and Alcalase to generate hydrolysates with moderate DH values, the relative content of free amino acids and dipeptides is likely to be low (Adler-Nissen 1986). It can be concluded that plant-derived enzymes (Papain and Bromelain) are able to produce better protein hydrolysates from winged bean seed compared with bacteria-derived enzymes (Alcalase and Flavourzyme). Previously, Papain-catalysed hydrolysis of a large number of protein substrates has been reported (Pripp et al. 2005). Papain could interact with up to seven sequential amino acids in a protein, and that interaction at these seven sites attributed to its specificity. Compounds of the type X-Y-R’ (X being hydrophobic in nature, Y are L-amino acids, and R’ is a suitable leaving group), are good substrates (Pripp et al. 2005).
Since winged bean seeds have a high concentration of hydrophobic amino acid (Table 2) such as phenylalanine and isoleucine at position X, thus it would be expected to effectively contribute to higher DH value for Papain-derived hydrolysates compared to Bromelain-derived hydrolysates. Little is known about the mechanism of Bromelain-catalysed hydrolysis other than that a thiol group is required in the reduced form for better activity (Wharton 1974). Hence, Papain can provide a higher catalytic efficiency compared to Bromelain. The Kcat/Km value of papain (Serveau et al. 1994) is 1 × 105 M−1 s−1 while Kcat/Km value for bromelain (Takahashi and Nishibe 1978) is 0.132 × 103 M−1 s−1 indicating higher catalytic efficiency of papain. The Michaelis-Menten kinetic model explains several aspects of the behavior of many enzymes. Each enzyme has its own kinetic characteristic under specified conditions. The measure of efficiency is helpful in determining whether the rate is limited by the creation of product or the amount of substrate in the environment. When the Kcat/Km is low, this means that the rate of product turnover is much lower than the substrate concentration, thus the enzyme and substrate have high affinity for each other.
Figure 1, DH (b) shows DH data for defatted winged bean seed (DWBS) using the same proteases. Generally, all proteases (Bromelain 22.87 %; Alcalase 21.34 % and Papain 18.71 %) offered the same DH range except that lowest DH value was observed for Flavourzyme (6.60 %). The DH of DWBS showed a lower percentage values compared to UWBS, especially with comparison to Flavourzyme [DH (a)]. During the defatting process, the removal of water from protein molecules most likely caused the aggregation of proteins via hydrophobic interaction. The larger aggregate formed was less susceptible to hydrolysis by Flavourzyme. Previous researchers have reported that, DH of proteases hydrolysis is highly affected by not only enzyme specificity, enzyme/substrate ratio, operating conditions and also nature of the sample (Kristinsson and Rasco 2000). Even though, the DH of defatted winged bean seeds was found to be lower than the undefatted winged been seed but the values are higher compared with that reported for soybean using proteases at 22.1 % for 5 h (Junfeng et al. 2003). The low-DH hydrolysates obtained in this study for defatted winged bean seed were in agreement with previous research whereby defatted soya flour hydrolyzed with proteases showing only 6 % DH value (Adler-Nissen 1986).
In the present study, quantification by proton release using a pH-stat method (Adler-Nissen 1986), was used during the process. The experimental setup is being optimized for the process of enzymatic hydrolysis of food protein sources. The auto-titrator and pH-meter controller are used to maintain the required conditions in the system. This is in agreement with the literature investigation revealing that pH-stat titration technique controls the addition of dilute acidic or alkaline solutions to maintain a constant pH in systems where a pH-dependent reaction is taking place (Ficara et al. 2003). The method focuses to assess the protons producing or consuming reactions (Gernaey et al. 1998; Massone et al. 1998)
Under these conditions, the titration rate is proportional to the reaction rate. Generally, this technique is applicable to any biological or physic-chemical reaction affecting the proton concentration using continuous water flows through the jacketed vessel that contained known amount of distilled water to stabilizing temperature inside the system. The degree of hydrolysis is a measure of the extent of hydrolysis or degradation of a protein, and it is the most widely used indicator for comparison among different protein hydrolysates.
Effect of hydrolysis time on ACE inhibitory activity
The results of ACE inhibitor activity against hydrolysis time using Alcalase, Flavourzyme, Bromelain and Papain are presented in Fig. 1, ACE (a) and (b) both for UWSBH and DWBSH, respectively. The non-hydrolysed protein showed no ACE inhibitory activity (result not shown) whereas all hydrolyzed protein displayed ACE inhibitory activity after enzymatic hydrolysis. ACE inhibition increased as the hydrolysis time of different enzymatic hydrolysates generated from both UWBSH and DWBSH were increased. These results showed the effectiveness of enzymatic treatments in generating bioactive peptides from their parent proteins. This result is comparable with previous work conducted on soybean, revealing that the inhibitory activity increased with the hydrolysis time (Junfeng et al. 2003).
Results show that Papain (88.24 %) produces the highest ACE inhibition activity followed by Bromelain (78.42 %) for both UWBSH and DWBSH. However, Alcalase and Flavourzyme showed a lower ACE inhibition both for UWBSH and DWBSH. It can be concluded that Papain is the most suitable enzyme for the degradation of peptide bond for winged bean seed protein compared with Bromelain, Alcalase, and Flavourzyme. The specificity of papain being able to produce a shorter peptide chain compared with others contributes to ACE inhibition potency.
In the case of Flavourzyme and Alcalase, even though Flavourzyme showed a higher degree of hydrolysis than Alcalase, the free amino acid produces during the enzymatic process are better for Alcalase. It should be expected that proteins with a high content of hydrophobic amino acids like winged bean seeds can be more easily hydrolyzed by Alcalase thus producing higher content of hydrophobic amino acids and contributing to ACE inhibitory potency. It can be concluded that the ACE inhibitory activity for Papain-derived (88.23 %) DWBSH was higher than Bromelain (78.42 %). Both of these plant enzymes have broad range specificity and could produce hydrolysate with low molecular weight that helps in ACE inhibitory potency. The substrate specificity of both enzymes is slightly different where Papain hydrolyses peptide bonds contributed by an adjacent amino acid Lysine, Arginine and Phenylalanine while bromelain preferred amino acid next to Lysine, Arginine, Phenylalanine and Tyrosine.
Figure 1, ACE (a) and (b) showed the highest ACE inhibition up to 93.8 % for papain-derived hydrolysates, a higher value than those reported for soybean hydrolysates. This indicates that both proteases could give rise to highly active ACE inhibitory peptides. Although the ACE inhibitory activity cannot be directly compared with data under different measuring conditions, the hydrolysates in this study showed a higher or similar activity than other hydrolysates already reported (Wu and Ding 2001; Suh 2000). Several enzymes have been used to produce seed legume protein hydrolysates having bioactive properties. Previously, there are some works that used sequential treatment of chickpea protein with Alcalase and Flavourzyme to produce a bioactive hydrolysate having ACE inhibitory activity with an IC50 value of 0.190 mg mL−1 (Pedroche et al. 2002). Present study (Table 3) showed a higher IC50 value (0.161 ± 0.03 mg mL−1; 0.091 ± 0.01 mg mL−1), for UWBSH using Flavourzyme and Alcalase, respectively.
Table 3.
Comparison of IC50 value of undefatted winged bean seed hydrolysates and defatted winged bean seed hydrolysates using different enzymes at 5 h of hydrolysis*
| Sample | IC50 value (mg mL−1) | |||
|---|---|---|---|---|
| Papain | Bromelain | Flavourzyme | Alcalase | |
| Undefatted winged bean seed hydrolysates | 0.064 ± 0.004b | 0.073 ± 0.02b | 0.161 ± 0.03b | 0.091 ± 0.01b |
| Defatted winged bean seed hydrolysates | 0.249 ± 0.02a | 0.293 ± 0.01a | 0.377 ± 0.04a | 0.374 ± 0.02a |
*Values are mean ± SD of three independent experiments. Means denoted with different superscript letters within the same column indicate significant differences (P < 0.05) between two types of hydrolysates
Comparison of IC50 value at 5 h of hydrolysis
In order to investigate the effect of hydrolysis on IC50 value of UWBSH and DWBSH, the samples were measured for IC50. Differences in the IC50 values among hydrolysates after 5 h of hydrolysis were found to be significant (P < 0.05) for each sample. These results show a marked difference in the capacity of the different enzymes to produce ACE inhibitory peptides. Overall, all protein hydrolysates produced by all four enzymes showed ACE inhibitory activity (Table 3). A previous work reported that soybean in its native state displayed inhibitory activity to some extent and this inhibitory activity increased with the hydrolysis time (Junfeng et al. 2003). The IC50 value of hydrolysates produced after 5 h with different enzymes, ranged from 0.161 mg mL−1 to 0.064 mg mL−1 for undefatted winged bean seed hydrolysate (UWBSH) while 0.377 mg mL−1 to 0.249 mg mL−1 for defatted winged bean seed hydrolysates (DWBSH).
Among the tested UWBSH, papain hydrolysate exhibited the most potent ACE inhibition activity with IC50 value at 0.064 mg mL−1 followed by (Bromelain, 0.073 mg mL−1; Alcalase, 0.091 mg mL−1; Flavourzyme, 0.161 mg mL−1). All DWBSH showed lower inhibition values (Papain, 0.249 mg mL−1; Bromelain, 0.293 mg mL−1; Flavourzyme, 0.377 mg mL−1; Alcalase, 0.374 mg mL−1) compared to UWBSH after 5 h of hydrolysis. Papain hydrolysate showed the highest IC50 value both for UWBSH and DWBSH compared to Bromelain, Flavourzyme and Alcalase hydrolysates, revealing its high ACE inhibitory potential. Therefore Papain-derived hydrolysate can be further fractionated at different decreasing molecular weight peptides with respect to its IC50 value.
Papain may produce higher short-chain peptides with hydrophobic amino acid residues and charges side groups that influence inhibitory potential as the rate of ACE inhibition increases with increasing hydrophobicity of the amino acids. Papain-derived UWBSH and DWBSH may have a better access to ACE domain activity that seems to be necessary for controlling blood pressure. It can be assessed specifically in vitro by use of the synthetic substrate hippury-histidyl-leucine (HHL) tested in this experiment. The highest ACE inhibition effect (lowest IC50) was found in the case of UWBSH (0.064 mg mL−1) using Papain whereby this result showed a higher value than an earlier study in which an ACE inhibitory activity with IC50 value of 0.070 mg mL−1 was found for pea protein hydrolysate (Vermeirssen et al. 2004).
Consequently, Hyun and Shin (2000) investigated the use of Papain for undefatted bovine plasma hydrolysis where IC50 values were reported to be (17.19 mg mL−1) lower than the present value (0.064 mg mL−1). Likewise, a lower ACE inhibitory activity was obtained for Papain-hydrolysate of lentil (Hyun and Shin 2000). Kapel et al. (2006) have obtained the different inhibitory effects for the peptides hydrolyzed using diverse enzymes. Among of that, it may be due to the discrepancy in the type of ACE-inhibitory peptides liberated, cleavage mechanism of each enzyme and the specific structure of the parent proteins (Hyun and Shin 2000). In other words, conditions used during the hydrolytic process can also greatly influence the release of ACE-inhibitory peptides (Kapel et al. 2006).
The differences among IC50 values of seed legume or pulses samples by various studies are compared with the current research. Winged bean seed hydrolysate with IC50 at 0.064 mg mL−1 showed the highest ACE inhibition activity to date compared with chick pea seed (Yust et al. 2003), red lentil (Boye et al. 2010), green lentil (Akıllıoğlu and Karakaya 2009), Corn gluten (Yang et al. 2007), Korean soybean (Shin et al. 2001), rapeseed (Marczak et al. 2003) (IC50 values at 0.180 mg mL−1, 0.440 mg mL−1, 0.890 mg mL−1, 0.085 mg mL−1, 0.276 mg mL−1, 0.16 mg mL−1, respectively). All the given protein sources were enzymatically hydrolyzed with proteases without further fractionation and purification steps. Winged bean seed hydrolysate showed an exceptionally high value of IC50 that may be attributed to its high protein content and hydrophobic amino acids compared to chickpea, lentils, Korean soybean and rapeseed. Hence, UWBSH and DWBSH from Papain were further fractionated by membrane-based ultrafiltration (UF) to obtain lower molecular weight peptides with enhanced ACE inhibition potency.
ACE inhibitory activity of fractionated papain -produced UWBSH and DWBSH
Undefatted winged bean seed hydrolysate (UWBSH) and defatted winged bean seed hydrolysate (DWBSH) produced using Papain, owing to having highest ACE inhibitory activity, was further fractionated into three different molecular weight fractions. The UWBSH obtained was fractionated by ultrafiltration into UWBSH-I (<10 kDa) (IC50 value, 0.065 ± 0.004 mg mL−1), UWBSH-II (<5 kDa) (IC50 value, 0.046 ± 0.003 mg mL−1) and UWBSH-III (<2 kDa) (IC50 value, 0.003 ± 0.0001 mg mL−1) while DWBSH obtained DWBSH-I (<10 kDa) (IC50 value, 0.248 mg mL−1), DWBSH-II (<5 kDa) (IC50 value, 0.198 mg mL−1) and UWBSH-III (<2 kDa) (IC50 value, 0.130 mg mL−1). Table 4 shows the molecular weight distributions of fractionated samples.
Table 4.
IC50 value (mg mL−1) of different fractions from papain-derived UWBSH and DWBSH*
| UWBSH | DWBSH | ||||
|---|---|---|---|---|---|
| Fraction | Mr | IC50 (mg/ml) | Fraction | Mr | IC50 (mg/ml) |
| UWBSH-I | <10 kDa | 0.065 ± 0.004a | DWBSH-I | <10 kDa | 0.248 ± 0.02a |
| UWBSH-II | <5 kDa | 0.046 ± 0.01b | DWBSH-II | <5 kDa | 0.198 ± 0.05a |
| UWBSH-III | <2 kDa | 0.003 ± 0.002c | DWBSH-III | <2 kDa | 0.130 ± 0.08a |
*Values are mean ± SD of three independent experiments
Means denoted with different superscript letters within the same column indicate significant statistical differences (P < 0.05) among three types of fractions
The ACE inhibitory activity of the UWBSH varied with the molecular weight (MW) distribution, and the UWBSH-III with MW below 2 kDa showed the most potent ACE inhibitory activity. The IC50 value of UWBSH-III was increased two folds compared with the value of UWBSH-I. This result shows that majority of the peptides are of low molecular mass corresponding to relatively short-chain peptides with greater ACE inhibitory potency. This is in agreement with a previous crystallographic study which revealed that the active site of ACE cannot accommodate large peptide molecules (Natesh et al. 2003). The IC50 value of the DWBSH-III was also increased two fold compared with the value DWBSH-I.
Ultrafiltration is typically used to separate proteins based on their molecular weight. The primary mechanism for separation using an ultrafiltration membrane is to separate small particles and dissolved molecules based on molecular size. The asymmetric structure of the membrane causes molecules larger than the molecular weight cut off (MWCO) to be retained, but not bound, on the surface of the membrane while molecules smaller than the MWCO pass through the membrane substructure.
In this study, Papain contributed to a high degree of hydrolysis due to its specific and effective enzymatic action. Ultrafiltration was found to be a proficient method to evaluate ACE inhibitory activity of Papain-derived winged bean seed hydrolysates (Marczak et al. 2003). ACE inhibitory peptides derived from food protein mainly consisted of low-molecular weight peptides with a MW below 1,500 Da (Oshima et al. 1979). The bioactive potential of low-molecular weight peptides is high because small peptides can be absorbed in the intestine without being decomposed by digestive enzymes and then reach target sites in the body. According to a study by Wu and Ding (2001), soybean which is quite similar with winged bean seed in having ACE inhibitory activity comprised of oligopeptides with 2–8 amino acid residues. The results of DH, Fig. 1 (IA and IB) of the hydrolysates in this study indicated that the hydrolysates had a suitable molecular weight for such a property. The ACE inhibitory activity of UWBSH and DWBSH observed in this work is, thus, quite promising especially when compared to activities reported for some other food protein hydrolysates (Marczak et al. 2003; Vermeirssen et al. 2004). However, it is understandable that UWBSH-III offered a higher IC50 value compared with DWBSH-III suggesting that undefatted winged bean seed are better raw material for producing potential food protein hydrolysate inhibitors.
Conclusions
Papain was found to be the most efficient protease for production of undefatted winged bean seed hydrolysate with potent ACE inhibitory activity (IC50, 0.064 mg mL−1) compared to defatted winged bean seeds (IC50, 0.249 mg mL−1). Further fractionation of papain-derived UWBSH showed that UWBSH-III exhibited strongest ACE inhibitory activity with IC50 at 0.003 mg mL−1. Although further studies needs to be conducted before the use of bioactive peptides from winged bean seeds protein, the results obtained in this study might contribute to the development of a novel functional ACE inhibitory biopetide-based functional food. Furthermore, with the growing consumer interest for whole foods, identification of avenues for use of winged bean seeds hydrolysates could be promising. Besides, verification of antihypertensive activity of the tested hydrolysates using some in vivo models is strongly recommended for future studies.
Acknowledgments
The authors would like to thank Ministry of Higher Education (MOHE), Malaysia for the financial support through the FRGS project no. 03-5523655-11801 awarded to Prof. Dr. Nazamid Saari.
References
- Adler-Nissen J. Enzymatic hydrolysis of food proteins. London: Elsevier Applied Science Publishers; 1986. [Google Scholar]
- Akıllıoğlu HG, Karakaya S. Effects of heat treatment and in vitro digestion on the angiotensin converting enzyme inhibitory activity of some legume species. Eur Food Res Technol. 2009;229(6):915–921. doi: 10.1007/s00217-009-1133-x. [DOI] [Google Scholar]
- AOAC . Official methods of analysis, vol 187. 14. Washington: Association of Official Analytical Chemists; 1984. [PubMed] [Google Scholar]
- Barbana C, Boye JI. Angiotensin I-converting enzyme inhibitory properties of lentil protein hydrolysates: determination of the kinetics of inhibition. Food Chem. 2011;127(1):94–101. doi: 10.1016/j.foodchem.2010.12.093. [DOI] [Google Scholar]
- Bedroche J, Yust MM, Giron-Calle J, Alaiz M, Millan F, Vioque J. Utilisation of chickpea protein isolates for production of peptides with angiotensin I-converting enzyme (ACE)-inhibitory activity. J Sci Food Agric. 2002;82:960–965. doi: 10.1002/jsfa.1126. [DOI] [Google Scholar]
- Boye JI, Roufik S, Pesta N, Barbana C. Angiotensin I-converting enzyme inhibitory properties and SDS-PAGE of red lentil protein hydrolysates. LWT Food Sci Technol. 2010;43(6):987–991. doi: 10.1016/j.lwt.2010.01.014. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. AnBio. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Černý K, Kordylas M, Pospíšil F, Švábenský O, Zajíc B. Nutritive value of the winged bean (Psophocarpus palustris Desv.) Br J Nutr. 1971;26(02):293–299. doi: 10.1079/BJN19710035. [DOI] [PubMed] [Google Scholar]
- Cheung HS, Cushman DW. Inhibition of homogeneous angiotensin-converting enzyme of rabbit lung by synthetic venom peptides of Bothrops jararaca. Biochim Biophys Acta (BBA) Enzymol. 1973;293(2):451–463. doi: 10.1016/0005-2744(73)90352-5. [DOI] [PubMed] [Google Scholar]
- Cheung HS, Feng-Lai W, Ondetti MA, Sabo EF, Cushman DW. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J Biol Chem. 1980;255:401–407. [PubMed] [Google Scholar]
- Chopra M, Scott N, McMurray J, McLay J, Bridges A, Smith W, Belch J. Captopril: a free radical scavenger. Br J Clin Pharmacol. 2012;27(3):396–399. doi: 10.1111/j.1365-2125.1989.tb05384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claydon AA. Review of the nutritional value of the winged bean Psophocarpus tetragonolobus (L.) D.C., with special reference to P. papua, New Guinea. Sci New Guinea. 1975;3(2):103–114. [Google Scholar]
- Damrongsakkul S, Ratanathammapan K, Komolpis K, Tanthapanichakoon W. Enzymatic hydrolysis of rawhide using papain and neutrase. J Ind Eng Chem. 2008;14(2):202–206. doi: 10.1016/j.jiec.2007.09.010. [DOI] [Google Scholar]
- Ekpenyong TE, Borchers RL. Amino acid profile of the seed and other parts of the winged bean. Food Chem. 1982;9(3):175–182. doi: 10.1016/0308-8146(82)90095-4. [DOI] [Google Scholar]
- Ficara E, Rozzi A, Cortelezzi P. Theory of pH–stat titration. Biotechnol Bioeng. 2003;82(1):28–37. doi: 10.1002/bit.10541. [DOI] [PubMed] [Google Scholar]
- Garcia V, Palmer J. Proximate analysis of five varieties of winged beans, psophocarpus tetragonolobus (L.) DC*. Int J Food Sci Technol. 1980;15(5):469–476. doi: 10.1111/j.1365-2621.1980.tb00965.x. [DOI] [Google Scholar]
- Gernaey K, Bogaert H, Vanrolleghem P, Massone A, Rozzi A, Verstraete W. A titration technique for on-line nitrification monitoring in activated sludge. Water Sci Technol. 1998;37(12):103–110. doi: 10.1016/S0273-1223(98)00342-4. [DOI] [Google Scholar]
- Ghadge PN, Shewalkar SV, Wankhede D (2010) Effect of processing methods on qualities of instant whole legume: pigeon pea (Cajanus cajan L.). Agric Eng Int CIGR J
- Gu RZ, Li CY, Liu WY, Yi WX, Cai MY. Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmo salar L.) skin. Food Res Int. 2011;44(5):1536–1540. doi: 10.1016/j.foodres.2011.04.006. [DOI] [Google Scholar]
- Humiski LM, Aluko RE. Physicochemical and bitterness properties of enzymatic pea protein hydrolysates. J Food Sci. 2007;72(8):605–611. doi: 10.1111/j.1750-3841.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- Hyun CK, Shin HK. Utilization of bovine blood plasma proteins for the production of angiotensin I converting enzyme inhibitory peptides. Process Biochem. 2000;36(1):65–71. doi: 10.1016/S0032-9592(00)00176-X. [DOI] [Google Scholar]
- Junfeng F, Saito M, Tatsumi E, Lite LI. Preparation of angiotensin I-converting enzyme inhibiting peptides from soybean protein by enzymatic hydrolysis. Food Sci Technol Res. 2003;9(3):254–256. doi: 10.3136/fstr.9.254. [DOI] [Google Scholar]
- Kapel R, Chabeau A, Lesage J, Riviere G, Ravallec-Ple R, Lecouturier D, Wartelle M, Guilochon D, Dhulster P. Production in continuous enzymatic membrane reactor, of an anti-hypertensive hydrolysate from an industrial alfalfa white protein concentrate exhibiting ACE inhibitory and opiod activities. Food Chem. 2006;98:120–126. doi: 10.1016/j.foodchem.2005.05.062. [DOI] [Google Scholar]
- Kim J, Whang J, Kim K, Koh J, Suh H. Preparation of corn gluten hydrolysate with angiotensin I converting enzyme inhibitory activity and its solubility and moisture sorption. Process Biochem. 2004;39(8):989–994. doi: 10.1016/S0032-9592(03)00205-X. [DOI] [Google Scholar]
- Kristinsson HG, Rasco BA. Biochemical and functional properties of Atlantic Salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. J Agric Food Chem. 2000;48(3):657–666. doi: 10.1021/jf990447v. [DOI] [PubMed] [Google Scholar]
- Lever AF, Hole DJ, Gillis CR, McInnes GT, Meredith PA, Murray LS, Reid JL. Is cancer related to hypertension or to its treatment? Hypertens Res-Clin E. 1999;21:937–946. doi: 10.3109/10641969909061022. [DOI] [PubMed] [Google Scholar]
- Li G-H, Le G-W, Shi Y-H, Shrestha S. Angiotensin I–converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr Res. 2004;24(7):469–486. doi: 10.1016/j.nutres.2003.10.014. [DOI] [Google Scholar]
- Li GH, Le GW, Shi YH, Shrestha S. Angiotensin-I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmocological effects. Nutr Res. 2004;24:469–486. doi: 10.1016/j.nutres.2003.10.014. [DOI] [Google Scholar]
- Lim TK (2012) Edible medicinal and non-medicinal plants. Edible medicinal and non-medicinal plants 2:867–878. doi:10.1007/978-94-007-1764-0
- Marczak ED, Usui H, Fujita H, Yang Y, Yokoo M, Lipkowski A. New hypertensive peptides isolated from rapeseed. Peptides. 2003;24:791–798. doi: 10.1016/S0196-9781(03)00174-8. [DOI] [PubMed] [Google Scholar]
- Mark KS, Davis TP. Stroke: development, prevention and treatment with peptidase inhibitors☆. Peptides. 2000;21(12):1965–1973. doi: 10.1016/S0196-9781(00)00346-6. [DOI] [PubMed] [Google Scholar]
- Massone A, Gernaey K, Rozzi A, Verstraete W (1998) Measurement of ammonium concentration and nitrification rate by a new titrimetric biosensor. Water Environ Res: 343–350
- Menotti A, Kromhout D, Blackburn H, Fidanza F, Buzina R, Nissienen A. Food intake patterns and 25-year mortality from coronary heart disease: cross-cultural correlations in the Seven Countries Study. The Seven Countries Study Research Group. Eur J Epidemiol. 1999;15(6):507–515. doi: 10.1023/A:1007529206050. [DOI] [PubMed] [Google Scholar]
- Natesh R, Schwager SLU, Sturrock ED, Acharya KR. Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature. 2003;421(6922):551–554. doi: 10.1038/nature01370. [DOI] [PubMed] [Google Scholar]
- National Research Council AP. The winged bean: a high-protein crop for the tropics. 2. Washington: National Academy Press; 1981. [Google Scholar]
- Oshima G, Shimabukuro H, Nagasawa K. Peptide inhibitors of angiotensin I-converting enzyme in digests of gelatin by bacterial collagenase. Biochim Biophys Acta (BBA) Enzymol. 1979;566(1):128–137. doi: 10.1016/0005-2744(79)90255-9. [DOI] [PubMed] [Google Scholar]
- Pedroche J, Yust MM, Girón-Calle J, Alaiz M, Millán F, Vioque J. Utilisation of chickpea protein isolates for production of peptides with angiotensin I–converting enzyme (ACE)–inhibitory activity. J Sci Food Agric. 2002;82(9):960–965. doi: 10.1002/jsfa.1126. [DOI] [Google Scholar]
- Pospisil F, Karikari S, Boamah-Mensah E. Investigation of winged bean in Ghana. World Crops. 1971;23(5):260–264. [Google Scholar]
- Pripp AH, Isaksson T, Stepaniak L, Sørhaug T, Ardö Y. Quantitative structure activity relationship modelling of peptides and proteins as a tool in food science. Trends Food Sci Technol. 2005;16(11):484–494. doi: 10.1016/j.tifs.2005.07.003. [DOI] [Google Scholar]
- Ravindran G, Palmer JK, Gajameragedara SM. Seed polysaccharides of some winged bean varieties. J Agric Food Chem. 1989;37(2):327–329. doi: 10.1021/jf00086a011. [DOI] [Google Scholar]
- Rozan P, Kuo YH, Lambein F. Free amino acids present in commercially available seedlings sold for human consumption. A potential hazard for consumers. J Agric Food Chem. 2000;48(3):716–723. doi: 10.1021/jf990729v. [DOI] [PubMed] [Google Scholar]
- Serveau C, Juliano L, Bernard P, Moreau T, Mayer R, Gauthier F. New substrates of papain, based on the conserved sequence of natural inhibitors of the cystatin family. Biochimie. 1994;76(2):153–158. doi: 10.1016/0300-9084(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Han XQ, Synowiecki J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus) Food Chem. 1995;53(3):285–293. doi: 10.1016/0308-8146(95)93934-J. [DOI] [Google Scholar]
- Shin ZI, Yu R, Park SA, Chung DK, Ahn CW, Nam HS, Kim KS, Lee HJ. His-His-Leu, an angiotensin I converting enzyme inhibitory peptide derived from Korean soybean paste, exerts antihypertensive activity in vivo. J Agric Food Chem. 2001;49(6):3004–3009. doi: 10.1021/jf001135r. [DOI] [PubMed] [Google Scholar]
- Suh HJ. Isolation of angiotensin I converting enzyme inhibitory peptide from soybean hydrolysate. Food Sci Biotechnol. 2000;9(6):378–381. [Google Scholar]
- Takahashi N, Nishibe H. Some characteristics of a new glycopeptidase acting on aspartylglycosylamine linkages. J Biochem. 1978;84(6):1467–1473. doi: 10.1093/oxfordjournals.jbchem.a132270. [DOI] [PubMed] [Google Scholar]
- Vermeirssen V, Van Camp J, Verstraete W. Fractionation of angiotensin I converting enzyme inhibitory activity from pea and whey protein in vitro gastrointestinal digests. J Sci Food Agric. 2004;85(3):399–405. doi: 10.1002/jsfa.1926. [DOI] [Google Scholar]
- Wharton CW. The structure and mechanism of stem bromelain. Evaluation of the homogeneity of purified stem bromelain, determination of the molecular weight and kinetic analysis of the bromelain-catalysed hydrolysis of N-benzyloxycarbonyl-L-phenylalanyl-L-serine methyl ester. Biochem J. 1974;143(3):575. doi: 10.1042/bj1430575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesekara I, Qian ZJ, Ryu B, Ngo DH, Kim SK. Purification and identification of antihypertensive peptides from seaweed pipefish (Syngnathus schlegeli) muscle protein hydrolysate. Food Res Int. 2011;44(3):703–707. doi: 10.1016/j.foodres.2010.12.022. [DOI] [Google Scholar]
- Wu J, Ding X. Hypotensive and physiological effect of angiotensin converting enzyme inhibitory peptides derived from soy protein on spontaneously hypertensive rats. J Agric Food Chem. 2001;49(1):501–506. doi: 10.1021/jf000695n. [DOI] [PubMed] [Google Scholar]
- Yang Y, Tao G, Liu P, Liu J. Peptide with angiotensin I-converting enzyme inhibitory activity from hydrolyzed corn gluten meal. J Agric Food Chem. 2007;55(19):7891–7895. doi: 10.1021/jf0705670. [DOI] [PubMed] [Google Scholar]
- Yust MM, Pedroche J, Giron-Calle J, Alaiz M, Millan F, Vioque J. Production of ACE inhibitory peptides by digestion of chickpea legumin with alcalase. Food Chem. 2003;81:363–369. doi: 10.1016/S0308-8146(02)00431-4. [DOI] [Google Scholar]